Abstract
Cytochrome c (cyt c) forms oligomers by domain swapping. It exchanges the C-terminal α-helical region between protomers, and the Met80‒heme iron bond is perturbed significantly in domain-swapped oligomers. The peroxidase activity of cyt c increases by Met80 dissociation from the heme iron, which may trigger apoptosis. This study elucidates the effect of the Met80 heme coordination on cyt c domain swapping by obtaining oligomers for both wild-type (WT) and M80A human cyt c by an addition of ethanol to their monomers, followed by lyophilization and dissolution to buffer, and investigating their dimer properties. The absorption and circular dichroism spectra of WT and M80A cyt c exhibited similar changes upon dimerization, indicating that Met80 does not affect the oligomerization process significantly. According to differential scanning calorimetric measurements, Met80 coordination to the heme iron had an effect on the stabilization of the monomer (ΔH = 16 kcal/mol), whereas no large difference was observed between the dimer-to-monomer dissociation temperatures of WT and M80A cyt c (61.0 °C). The activation enthalpy values were similar and relatively large for the dissociation of both WT and M80A cyt c dimers (WT, 120 ± 10 kcal/mol; M80A, 110 ± 10 kcal/mol), indicating that the dimers suffered large structural changes upon dissociation to monomers independent of the Met80 coordination to the heme iron. These results indicate that cyt c domain swapping may occur regardless of the Met80 coordination, whereas the monomer is stabilized by Met80 but the domain-swapped dimer structure and stability are less affected by the Met80 coordination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytochrome (cyt) c is a heme protein that shuttles electrons from the cytochrome bc 1 complex to cytochrome c oxidase in mitochondria. Cyt c also plays a key role in apoptosis, where it is released to the cytosol when permeabilization of the mitochondrial outer membrane occurs [1, 2]. Cyt c contains three long α-helices, and its heme is bound to the polypeptide chain by covalent bonds with two cysteine residues thorough their sulfur atoms. Histidine (His18) and methionine (Met80) are coordinated to the heme iron of cyt c in its native state [3, 4], whereas its coordination structure has been shown to change under different conditions [5,6,7]. When cyt c interacts strongly with cardiolipins in the membrane, interactions between the N- and C-termini are apparently broken with Met80 dissociated from the heme iron [8]. Cyt c also triggers apoptosis by an increase in peroxidase activity upon interaction with cardiolipin [9]. The peroxidase activity enhancement of cyt c by the interaction with cardiolipin has been attributed to the dissociation of Met80 from the heme ion [10]. The Met80 heme ligand has been shown to swing out of the heme crevice and be replaced by a water molecule in the crystal structure of the trimethyllysine 72-to-alanine mutant of iso-1-cyt c, enhancing peroxidase activity compared to the wild-type (WT) protein [11]. Met80 of cyt c has also been shown to dissociate from the heme iron at alkaline pH, and instead, Lys72, Lys73, or Lys79 coordinates to the heme iron [12,13,14,15].
We have previously shown that horse cyt c forms polymers by successive domain swapping [16]. Domain swapping is a phenomenon where one molecule exchanges its domain or secondary structural element with another molecule [17,18,19,20]. From our results, in the crystal structure of dimeric horse cyt c (Fig. 1), as well as that of trimeric horse cyt c, the C-terminal helices were replaced by the corresponding domains of other cyt c molecules. The Met80–heme bond was perturbed significantly and the peroxidase activity increased for dimeric horse cyt c compared to its monomer [16, 21]. Residual absorbance was observed at ~695 nm in the absorption spectrum of domain-swapped dimeric horse cyt c, indicating conformation flexibility that permits stabilization of the dimer via the Met80–heme bond [16]. Cyt c was found to domain swap by treatment with ethanol [16], whereas it was also shown to domain swap when refolding from its guanidinium ion-induced unfolded state [22]. The hydrophobic interaction between the N- and C-terminal α-helices at the early stage of folding was shown to be important for the domain swapping [22]. A certain amount of cyt c molecules interacted as oligomers in its molten globule state, whereas the domain-swapped dimer of cyt c was obtained by refolding from its molten globule state [23].
Protein and active site structures of horse cyt c. a Monomer and b dimer protein structures, and c monomer and d dimer active site structures are shown (PDB codes: monomer, 1HRC; dimer, 3NBS). The green and light blue regions in the dimer correspond to different protomers. The carbon atoms of the hemes are shown in pale colors. The water molecule coordinating to the hemes is shown as a red sphere. The sulfur atoms of the heme axial Met ligand and heme-linked Cys are shown in yellow. The nitrogen atoms of the hemes and heme axial His ligands are shown in blue
Many other c-type cyts have been shown to domain swap [24,25,26,27,28]. For example, Pseudomonas aeruginosa (PA) cyt c 551 exhibits a folding structure similar with mammalian cyt c, but is smaller (82 amino acids) than mammalian cyt c, while horse and human cyt c have the same amino acid length (104 amino acids) and a relatively large similarity in amino acid sequence (Table S1). In PA cyt c 551, His16 and Met61 are coordinated to the heme iron [29]. PA cyt c 551 exchanges the region containing the N-terminal α-helix and heme between molecules for its dominant domain-swapped dimer instead of exchanging the C-terminal region between molecules [25]. In dimeric PA cyt c 551, Met61 is coordinated to the heme iron, but the coordinating Met originates from the other protomer to which the heme belongs. Concerning the domain swapping ability, the secondary structures have been shown to be perturbed by mutating the heme-coordinating Met to Ala (M61A), and the oligomerization ability by domain swapping decreased significantly for PA M61A cyt c 551 compared to that of the WT protein, where the decrease in domain swapping ability was attributed to the perturbation in the secondary structures by the removal of Met61 [25]. However, the major swapping regions between cyt c and PA cyt c 551 are different, and the effect of Met80 heme coordination on cyt c domain swapping remains unclear, whereas Met80 dissociation from the heme iron is believed to strongly affect the cyt c release to cytosol and the initiation of apoptosis. Additionally, the replacement of heme-coordinating Met69 with Ala for Thermus thermophiles cyt c 552, a thermostable c-type cyt, has been shown to affect its tertiary structure but has almost no effect on the secondary structures [30]. In this study, we replaced Met80 with Ala (M80A) in oxidized (ferric) human cyt c, and elucidated the effect of Met80 coordination on domain swapping by investigating the formation and stability of oligomers.
Materials and methods
Protein expression and purification
Amino acid substitution of Met80 was performed by PCR-based in vitro mutagenesis of the original plasmid vector using M80A-F and M80A-R primers (Table S2). Mutated DNA was purified using the KOD-Plus-Mutagenesis kit (Toyobo). DNA sequencing was carried out with the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Inc., Foster City, CA, USA) and an ABI 3100 Avant generic analyzer (Applied Biosystems, Inc.). Recombinant WT and M80A cyt c were overexpressed using E. coli Rosetta 2(DE3) pLysS cells (Novagen). Purification of WT and M80A human cyt c were performed according to the methods described elsewhere [13, 31]. The masses of WT and M80A cyt c were checked by MALDI-TOF mass measurements with an Autoflex II mass spectrometer (Bruker Daltonics) using sinapinic acid as a matrix in a linear mode.
Preparation of oligomers
WT and M80A human cyt c oligomers were prepared by the reported method except for an addition of 65% (v/v) ethanol [16]. The cyt c solution was centrifuged, and the obtained precipitate was subsequently lyophilized. The lyophilized precipitate was redissolved in 10 mL of 50 mM potassium phosphate buffer, pH 7.0. After incubation of the obtained cyt c solution at 37 °C for 40 min, the solution was filtrated, and dimeric cyt c was purified by gel filtration chromatography (HiLoad 26/60 Superdex 75, GE Healthcare) several times using a fast protein liquid chromatography (FPLC) system (BioLogic DuoFlow 10, Bio-Rad, CA, USA) with 50 mM potassium phosphate buffer, pH 7.0, at 4 °C. The purified cyt c dimers were used immediately after purification.
The oxidized WT and M80A human cyt c solutions were analyzed by size exclusion chromatography (SEC; column: Superdex 75 30/100 GL, GE healthcare) using the FPLC system (BioLogic DuoFlow 10, Bio-Rad) with 50 mM potassium phosphate buffer, pH 7.0, at 4 °C. The peak areas in the elution curves of the size exclusion chromatograms were obtained by least-square fitting the peaks with Gaussian curves using the software, Igor Pro 6.0 (WaveMetrics, Portland). The stabilities of oxidized dimeric WT and M80A cyt c were investigated by incubation of the purified dimers (heme, 50 μM) in 50 mM potassium phosphate buffer, pH 7.0, containing 500 μM ferricyanide at 52–60 °C.
Optical absorption and circular dichroism measurements
Optical absorption spectra of oxidized WT and M80A human cyt c were measured with a UV-2450 spectrophotometer (Shimadzu) using a 1-cm path length quartz cell at 25 °C. The extinction coefficients of oxidized monomeric and dimeric M80A cyt c were determined with the pyridine hemochrome method [32]. The pH titration of oxidized M80A cyt c was performed from pH 8.74 to 2.75 by a continuous addition of a small amount of 1.0 M HCl to cyt c in 50 mM potassium phosphate. The dilution of cyt c solution with 1.0 M HCl was less than 6% at the final point (pH 2.75), and the intensity of each absorption spectrum was calibrated according to its cyt c concentration change by the dilution. Absorbance at 398.5 nm (isosbestic point between His/H2O 6-coordinate and His 5-coordinate species; pKa = 3.8 [33]) was plotted against pH, and the pKa value was determined by least-square fitting the data to the Henderson–Hasselbalch equation using the software, Igor Pro 6.0.
Circular dichroism (CD) spectra of oxidized WT and M80A cyt c were measured with a J-725 CD spectropolarimeter (Jasco, Japan) using a 0.1-cm path length quartz cell at room temperature.
Differential scanning calorimetric measurements
Differential scanning calorimetry (DSC) thermograms of oxidized WT and M80A human cyt c (heme, 100 µM) in their monomeric and dimeric forms were measured using VP-DSC (MicroCal, GE Healthcare) at a scan rate of 1 °C/min in 50 mM potassium phosphate buffer, pH 7.0.
Kinetic analysis
After incubation of purified dimeric WT cyt c or dimeric M80A cyt c in 50 mM sodium phosphate buffer, pH 7.0, for various times at 52, 55, 58, and 60 °C, the monomer and dimer amounts were analyzed by SEC (column: Superdex 75 30/100 GL, GE healthcare; monitoring wavelength, 410 nm) using a FPLC system (BioLogic DuoFlow 10, Bio-Rad) with the same buffer at 4 °C. The peak areas of the monomer and dimer in the elution curves of the chromatograms were obtained by least-square fitting the peaks with Gaussian curves using Igor Pro 6.0. The dimer amount was plotted against incubation time at each temperature, and the rate constant (k) was obtained by least-square fitting the data with an exponential curve using Igor Pro 6.0.
Results
Oligomerization
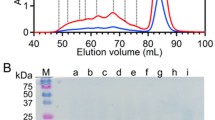
WT and M80A cyt c in 50 mM potassium phosphate buffer, pH 7.0, were treated with 65% (v/v) ethanol, lyophilized, and dissolved in the same buffer. The Fe‒Met80 bond is apparently broken by an addition of ethanol [34], although the precise effect of ethanol on cyt c domain swapping is still unclear. By analyzing the ethanol-treated cyt c with SEC, several peaks were detected in the chromatograms (Fig. 2). Monomeric and dimeric cyt c peaks were observed at about 12.8 and 11.4 mL, respectively, in the elution curves. In addition to these peaks, higher order oligomers were detected up to the exclusion limit (MW, 100,000) of the column. A similar amount of high-order oligomers has been observed by successive domain swapping of horse cyt c on ethanol treatment [16], whereas only small amounts of high order oligomers have been detected for PA cyt c 551 [25] and Hydrogenobacter thermophilus (HT) cyt c 522 [24]. The C-terminal region is swapped in horse cyt c [16], whereas the N-terminal region is mainly swapped in PA cyt c 551 [25] and HT cyt c 552 [24]. High order oligomers have been generated by treatment with ethanol for horse cyt c, owing to the long flexible hinge loop (Thr78‒Ala83) [16]. These results indicate that human cyt c domain swapped the C-terminal region successively by treatment with ethanol, similar to the case of horse cyt c domain swapping.
Size exclusion chromatograms of oxidized human cyt c. Chromatograms of WT (red) and M80A (blue) cyt c after treatment of the monomers with ethanol are shown. After cyt c (200 μM) was incubated in the presence of 65% (v/v) ethanol at 25 °C, the precipitates were lyophilized and subsequently dissolved to buffer at 4 °C. The intensities of the curves are normalized by the monomer absorbance. Measurement conditions: column: Superdex 75 10/300 GL; flow rate: 0.5 mL∕ min; monitoring wavelength: WT, 410 nm; M80A, 406 nm; solvent: 50 mM potassium phosphate buffer; pH: 7.0; temperature: 4 °C
Absorption spectra
The Soret band of oxidized WT human cyt c blue-shifted from 410 to 407 nm by the dimerization (Fig. 3a), similar to the shift observed in the optical absorption spectra of oxidized horse cyt c by its dimerization [16]. The extinction coefficient of oxidized monomeric WT human cyt c was determined as 9.93 × 104 M−1 cm−1 at 410 nm and that of the dimer as 1.14 × 105 M−1 cm−1 at 407 nm by the pyridine hemochrome method [32]. The 695-nm absorption band of cyt c has been commonly used for assessments of conformational transitions in cyt c associated with the disruption of the Fe–Met80 bond. The intensity of the 695-nm band was decreased by the dimerization of oxidized WT human cyt c, suggesting that the Fe–Met80 bond was disrupted in the dimer. The Soret band of dimeric WT human cyt c was observed at 406 nm with another absorption band at ~529 nm and a shoulder peak at ~562 nm, where similar bands have been observed in the spectrum of dimeric horse cyt c [16]. These spectral properties resemble those of the hydroxide-bound form of Met-depleted cyt c [35, 36], indicating that Met80 was dissociated from the heme and a hydroxide ion was bound to the heme for dimeric WT human cyt c.
Absorption spectra of oxidized a WT and b M80A human cyt c. Absorption spectra of monomeric (black) and dimeric (WT red; M80A blue) cyt c are shown. Expansion of fifty times for the absorption in the 650–800 nm regions is also shown. The y-axes represent the extinction coefficients. Measurement conditions: cyt c concentration: 10 μM (heme); buffer: 100 mM potassium phosphate buffer; pH: 7.0; temperature: 25 °C
For oxidized M80A human cyt c, the Soret band was observed at 406 nm in the monomer spectrum and shifted to 404 nm in the dimer spectrum, and the 695-nm band was missing in the monomer and dimer spectra due to the lack of Met80 (Fig. 3b). The extinction coefficients of oxidized monomeric and dimeric M80A human cyt c were determined to be 1.14 × 105 (at 406 nm) and 1.27 × 105 M−1 cm−1 (at 404 nm), respectively. External ligands have been shown to bind to the heme iron in heme-coordinating Met-depleted cyt c [37, 38], as well as domain-swapped horse cyt c [39]. Since absorption band was observed at 625 nm in oxidized dimeric M80A human cyt c—indicative of a high-spin ferric heme—a water molecule may be bound to the heme iron in the M80A cyt c dimer [35, 36, 40]. The pKa value for water/hydroxide ion coordination to the heme in oxidized monomeric M80A human cyt c was obtained as 6.3 ± 0.2 (Fig. S1), which was slightly higher than that reported for M80A iso-1 cyt c (pKa = 5.62) [36]. The pKa value of M80A human cyt c indicates that a hydroxide ion is mainly coordinated to the heme iron at neutral pH. However, a water molecule may be coordinated to the heme iron in its dimer, due to the widening of the heme site in the domain-swapped dimer.
CD spectra
The CD spectra of oxidized M80A human cyt c exhibited similar changes to those observed in the spectra of oxidized WT human cyt c upon dimerization. The intensity of the negative peak at 208 nm in the CD spectrum increased for the dimer compared to that of its monomer, concomitant with a decrease in intensity of the positive peak around 195 nm (Fig. 4). These results indicate that M80A cyt c suffered a similar secondary structural change to WT cyt c on dimerization. Moreover, these changes were similar with the spectral changes previously observed for dimerization of horse cyt c [16], strongly suggesting that human cyt c forms a dimer by the same mechanism as horse cyt c.
CD spectra of oxidized a WT and b M80A human cyt c. CD spectra of monomeric (black) and dimeric (WT red; M80A blue) cyt c are shown. The protein concentrations were calculated from the intensities of the Soret bands. Measurement conditions: sample concentration: 10 μM (heme); buffer: 50 mM potassium phosphate buffer; pH: 7.0; temperature: room temperature
Differential scanning calorimetry measurements
The enthalpy changes (ΔH) of the dimer dissociation to monomers for oxidized WT and M80A human cyt c and their dissociation temperatures (T m) were obtained by DSC measurements (Fig. 5). The ΔH value for the dissociation of oxidized dimeric M80A cyt c to monomers increased to −14 ± 2 kcal/mol compared to that of oxidized dimeric WT cyt c (−30 kcal/mol). The T m of oxidized dimeric WT and M80A human cyt c were both obtained as 61.0 ± 0.5 °C, which is a similar value to the dissociation temperature of dimeric horse cyt c to monomers (58.0 °C) [16]. The ΔH value of oxidized dimeric human WT cyt c was obtained as −30 ± 2 kcal/mol, which was not largely different from that of oxidized dimeric horse cyt c (−40 ± 2 kcal/mol) [16].
Differential scanning calorimetry thermograms of oxidized a WT and b M80A human cyt c. Thermograms of monomeric (black) and dimeric (WT red; M80A blue) cyt c are depicted. Measurement conditions: Sample concentration: 100 μM (heme); scan speed, 1 °C/min; buffer: 50 mM potassium phosphate buffer; pH: 7.0
Kinetics of dimer dissociation
The k values at each temperature (52, 55, 58, and 60 °C) obtained for dimeric WT and M80A human cyt c are listed in Tables S3 and S4. The activation enthalpy (ΔH ‡) and activation entropy (ΔS ‡) for the dissociation of oxidized dimeric human cyt c to monomers were obtained by the Eyring plot of ln(k/T) vs. the reciprocal of temperature (Fig. 6). From the plot, the ΔH ‡ and ΔS ‡ for the dissociation of oxidized dimeric WT cyt c were obtained as 120 ± 10 kcal/mol and 310 ± 10 cal/(mol K), respectively, and those of oxidized dimeric M80A cyt c as 110 ± 10 kcal/mol and 290 ± 20 cal/(mol K), respectively. These values for WT and M80A cyt c were similar, indicating that Met80 does not have a large effect on the activation energy of dimer dissociation, although the monomer is stabilized at about ΔH = −16 kcal/mol by the Met80 coordination to the heme iron (Fig. 5). It is noteworthy that the activation energy is relatively large, indicating that the protein suffers a large structural change upon dissociation of the dimer to monomers.
Plots of ln (k/T) vs. reciprocal of temperature for dissociation of oxidized dimeric WT (red) and M80A (blue) human cyt c. The linear lines are obtained by least-square fitting the plots. Reaction conditions: Sample concentration: 10 μM (heme); solvent: 50 mM potassium phosphate buffer; pH: 7.0; temperature: 52, 55, 58, and 60 °C
Discussion
The number of elucidated domain-swapped structures of proteins has been increasing [18,19,20, 41,42,43], including heme proteins [16, 27, 44,45,46,47,48]. The active site structure with His and Met coordinated to the heme iron is apparently conserved after the dimerization for most of the c-type cyts, although the swapping regions are different (N-terminal regions for PA cyt c 551 [25] and HT cyt c 552 major dimer [24]; C-terminal regions for HT cyt c 552 minor dimer [26] and Aquifex aeolicus cyt c 555 [28]). However, Met80 dissociates from the heme iron for dimeric horse cyt c [16], where the easier Met dissociation compared to other c-type cyts may be necessary for the increase in its peroxidase activity [21] and apoptosis initiation [9]. In this study, M80A cyt c domain swapped even though there was no Met for coordination to the heme iron, demonstrating that cyt c may domain swap regardless of the Met coordination (Fig. 2b). These results are consistent with the previously reported character that the hydrophobic interaction between the N- and C-terminal α-helices at the early stage of folding is important for cyt c domain swapping [22].
The loop containing the heme-ligating Met is more mobile and less stable in horse cyt c compared to PA cyt c 551 [49, 50]. The Met–heme coordination of horse cyt c is disrupted at mild denaturing conditions [51] or alkaline pH [52], whereas that of PA cyt c 551 is conserved until the protein is almost completely unfolded [53]. However, a certain amount of domain-swapped oligomers was detected in this study for the M80A mutant of human cyt c (Fig. 2), but in the previous work, the oligomer amount decreased significantly for PA cyt c 551 by the Met61 mutation to Ala [25]. The secondary structures did not change significantly for cyt c by the Met80 mutation, whereas they have been shown to change for PA cyt c 551 by the Met61 mutation [25]. These results indicate that the stability of the protein secondary structures has a larger effect on domain swapping compared to the Met‒heme bond stability against denaturing agents or pH. Although the monomer of human cyt c was destabilized by the removal of heme-coordinating Met80, oligomers were formed for the M80A mutant by treatment with ethanol (Fig. 2), presumably because cyt c unfolds when it forms domain-swapped oligomers [22].
The ΔH values for the dissociation of oxidized dimers to monomers were −14 and −30 kcal/mol for oxidized dimeric M80A and WT cyt c, respectively (Fig. 5). The increase of 16 kcal/mol in the ΔH value for dimeric M80A cyt c compared to dimeric WT cyt c may correspond to the Met dissociation from the heme iron in the monomer for M80A cyt c, since the ΔH value of met coordination to the heme iron has been estimated to be −18 kcal/mol [54]. These results indicate that coordination of Met80 to the heme iron has a certain effect on the stabilization of the monomer. However, the C-terminal α-helix may also have an effect on the stabilization of the monomer, since dimeric M80A cyt c exhibited a negative ΔH value of −14 kcal/mol by the dimer dissociation. The Thr78–Lys87 amino acids of the Ω loop including the hinge region (Thr78–Ala83) of horse cyt c form hydrogen bonds to the rest of the protein in a relatively broad region [16]. The hydrogen bonds at Lys72(Nζ)/Met80(O), Lys72(Nζ)/Phe82(O), Lys79(N)/Hem(O2D), and Lys79(Nζ)/Thr47(O) (<3.2 Å between heavy atoms) are detected in the monomer but not in the dimer. All these residues are conserved between human and horse cyt c, except for the 47th amino acid, which is Thr47 and Ser47 for horse and human cyt c, respectively (Table S1). Formation of these hydrogen bonds may contribute to the negative ΔH value upon dissociation of dimeric cyt c to monomers.
In conclusion, this study indicates that Met80 stabilizes the monomer structure by Met80 coordination to the heme iron, whereas the domain-swapped dimer structure is not affected significantly by Met80. The activation enthalpy and activation entropy for dimer dissociation did not change significantly between WT and M80A cyt c, indicating that the domain-swapped dimer stability is less affected by the Met80 coordination. Protein secondary structure stability has been shown to be an important factor for domain swapping in cyt c. A proper understanding of the effect of Met‒heme coordination on domain swapping in cyt c may not only elucidate its domain swapping character but also provide useful information for constructing artificial protein complexes by domain swapping.
Abbreviations
- CD:
-
Circular dichroism
- Cyt:
-
Cytochrome
- DSC:
-
Differential scanning calorimetry
- FPLC:
-
Fast protein liquid chromatography
- HT:
-
Hydrogenobacter thermophilus
- ΔH :
-
Enthalpy change
- ΔH ‡ :
-
Activation enthalpy
- PA:
-
Pseudomonas aeruginosa
- K :
-
Rate constant
- ΔS ‡ :
-
Activation entropy
- SEC:
-
Size exclusion chromatography
- T m :
-
Dissociation temperature
- WT:
-
Wild-type
References
Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR (2005) Science 310:66–67
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cell 91:479–489
Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E (1971) J Biol Chem 246:1511–1535
Bushnell GW, Louie GV, Brayer GD (1990) J Mol Biol 214:585–595
Simon M, Metzinger-Le Meuth V, Chevance S, Delalande O, Bondon A (2013) J Biol Inorg Chem 18:27–38
Lin YW, Wang J (2013) J Inorg Biochem 129:162–171
Hannibal L, Tomasina F, Capdevila DA, Demicheli V, Tortora V, Alvarez-Paggi D, Jemmerson R, Murgida DH, Radi R (2016) Biochemistry 55:407–428
Hanske J, Toffey JR, Morenz AM, Bonilla AJ, Schiavoni KH, Pletneva EV (2012) Proc Natl Acad Sci USA 109:125–130
Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG (2005) Nat Chem Biol 1:223–232
Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE (2006) Biochemistry 45:4998–5009
McClelland LJ, Mou TC, Jeakins-Cooley ME, Sprang SR, Bowler BE (2014) Proc Natl Acad Sci USA 111:6648–6653
Rosell FI, Ferrer JC, Mauk AG (1998) J Am Chem Soc 120:11234–11245
Pollock WB, Rosell FI, Twitchett MB, Dumont ME, Mauk AG (1998) Biochemistry 37:6124–6131
Assfalg M, Bertini I, Dolfi A, Turano P, Mauk AG, Rosell FI, Gray HB (2003) J Am Chem Soc 125:2913–2922
Assfalg M, Bertini I, Turano P, Mauk AG, Winkler JR, Gray HB (2003) Biophys J 84:3917–3923
Hirota S, Hattori Y, Nagao S, Taketa M, Komori H, Kamikubo H, Wang Z, Takahashi I, Negi S, Sugiura Y, Kataoka M, Higuchi Y (2010) Proc Natl Acad Sci USA 107:12854–12859
Bennett MJ, Choe S, Eisenberg D (1994) Proc Natl Acad Sci USA 91:3127–3131
Liu Y, Eisenberg D (2002) Protein Sci 11:1285–1299
Rousseau F, Schymkowitz JW, Itzhaki LS (2003) Structure 11:243–251
Gronenborn AM (2009) Curr Opin Struct Biol 19:39–49
Wang Z, Matsuo T, Nagao S, Hirota S (2011) Org Biomol Chem 9:4766–4769
Parui PP, Deshpande MS, Nagao S, Kamikubo H, Komori H, Higuchi Y, Kataoka M, Hirota S (2013) Biochemistry 52:8732–8744
Deshpande MS, Parui PP, Kamikubo H, Yamanaka M, Nagao S, Komori H, Kataoka M, Higuchi Y, Hirota S (2014) Biochemistry 53:4696–4703
Hayashi Y, Nagao S, Osuka H, Komori H, Higuchi Y, Hirota S (2012) Biochemistry 51:8608–8616
Nagao S, Ueda M, Osuka H, Komori H, Kamikubo H, Kataoka M, Higuchi Y, Hirota S (2015) PLoS One 10:e0123653
Ren C, Nagao S, Yamanaka M, Komori H, Shomura Y, Higuchi Y, Hirota S (2015) Mol BioSyst 11:3218–3221
Miyamoto T, Kuribayashi M, Nagao S, Shomura Y, Higuchi Y, Hirota S (2015) Chem Sci 6:7336–7342
Yamanaka M, Nagao S, Komori H, Higuchi Y, Hirota S (2015) Protein Sci 24:366–375
Matsuura Y, Takano T, Dickerson RE (1982) J Mol Biol 156:389–409
Behera RK, Nakajima H, Rajbongshi J, Watanabe Y, Mazumdar S (2013) Biochemistry 52:1373–1384
Wang ZH, Lin YW, Rosell FI, Ni FY, Lu HJ, Yang PY, Tan XS, Li XY, Huang ZX, Mauk AG (2007) ChemBioChem 8:607–609
Berry EA, Trumpower BL (1987) Anal Biochem 161:1–15
Yeh SR, Han SW, Rousseau DL (1998) Accounts Chem Res 31:727–736
Hirota S, Ueda M, Hayashi Y, Nagao S, Kamikubo H, Kataoka M (2012) J Biochem 152:521–529
Lu Y, Casimiro DR, Bren KL, Richards JH, Gray HB (1993) Proc Natl Acad Sci USA 90:11456–11459
Silkstone GG, Cooper CE, Svistunenko D, Wilson MT (2005) J Am Chem Soc 127:92–99
Bren KL, Gray HB, Banci L, Bertini I, Turano P (1995) J Am Chem Soc 117:8067–8073
Banci L, Bertini I, Bren KL, Gray HB, Sompornpisut P, Turano P (1995) Biochemistry 34:11385–11398
Nugraheni AD, Nagao S, Yanagisawa S, Ogura T, Hirota S (2013) J Biol Inorg Chem 18:383–390
Brill AS, Williams RJ (1961) Biochem J 78:246–253
Bennett MJ, Sawaya MR, Eisenberg D (2006) Structure 14:811–824
Janowski R, Kozak M, Jankowska E, Grzonka Z, Grubb A, Abrahamson M, Jaskolski M (2001) Nat Struct Biol 8:316–320
Newcomer ME (2002) Curr Opin Struct Biol 12:48–53
Nurizzo D, Silvestrini MC, Mathieu M, Cutruzzola F, Bourgeois D, Fulop V, Hajdu J, Brunori M, Tegoni M, Cambillau C (1997) Structure 5:1157–1171
Crane BR, Rosenfeld RJ, Arvai AS, Ghosh DK, Ghosh S, Tainer JA, Stuehr DJ, Getzoff ED (1999) EMBO J 18:6271–6281
Czjzek M, Létoffé S, Wandersman C, Delepierre M, Lecroisey A, Izadi-Pruneyre N (2007) J Mol Biol 365:1176–1186
Nagao S, Osuka H, Yamada T, Uni T, Shomura Y, Imai K, Higuchi Y, Hirota S (2012) Dalton Trans 41:11378–11385
Silva MA, Lucas TG, Salgueiro CA, Gomes CM (2012) PLoS One 7:e46328
Bai Y, Sosnick TR, Mayne L, Englander SW (1995) Science 269:192–197
Russell BS, Zhong L, Bigotti MG, Cutruzzola F, Bren KL (2003) J Biol Inorg Chem 8:156–166
Russell BS, Melenkivitz R, Bren KL (2000) Proc Natl Acad Sci USA 97:8312–8317
Hong XL, Dixon DW (1989) FEBS Lett 246:105–108
Yamamoto Y, Terui N, Tachiiri N, Minakawa K, Matsuo H, Kameda T, Hasegawa J, Sambongi Y, Uchiyama S, Kobayashi Y, Igarashi Y (2002) J Am Chem Soc 124:11574–11575
George P, Glauser SC, Schejter A (1967) J Biol Chem 242:1690–1695
Acknowledgements
We thank Mr. Leigh McDowell, Nara Institute of Science and Technology, for his advice on manuscript preparation. This work was partially supported by Grants-in-Aid for Scientific Research from JSPS (Category B, No. JP26288080, S.H.; Challenging Exploratory Research, No. JP15K13744, S.H.; Scientific Research on Innovative Areas, No. JP16H00839, S.H.; Young Scientists B, No. JP16K17935, S.N.), Natural Science Foundation of Sichuan Province of China (No. 11ZB029, Z.W.), Open Project of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (No. CSPC2011-7-2, Z.W.), and Doctoral Fund of China West Normal University (No. 07B011, Z.W.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hirota, S., Yamashiro, N., Wang, Z. et al. Effect of methionine80 heme coordination on domain swapping of cytochrome c . J Biol Inorg Chem 22, 705–712 (2017). https://doi.org/10.1007/s00775-017-1446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-017-1446-3