Abstract
Functional disability is a major concern in patients with rheumatoid arthritis (RA). This retrospective study investigated the risk factors for vertebral fractures (VFs) in postmenopausal RA patients and determined the impact of VFs on functional status. Data from a cohort of 200 postmenopausal RA patients in a single hospital registry were analyzed. Demographic and clinical data, imaging data from spine radiographs, and bone mineral density (BMD) data were collected from the patients at baseline and at the final visit (a mean of 2.9 years after the first visit). Risk factors for incident VFs and their impact on the modified health assessment questionnaire (mHAQ) were analyzed. Twenty-eight patients (14 %) developed new VFs (NVFs). Logistic regression analysis adjusted for age, BMI, and disease duration revealed that daily dose of prednisolone, femoral neck BMD, use of active vitamin D3, and use of a bisphosphonate at baseline were factors associated with NVF, with odds ratios (95 % confidence interval) of 1.27 (1.05–1.54), 0.94 (0.91–0.97), 0.34 (0.13–0.89), and 0.31 (0.12–0.82), respectively. Patients with NVF exhibited worse mHAQ scores and a greater increase in mHAQ scores from baseline compared with those without NVF. In conclusion, incident VFs were associated with reduced functional status in postmenopausal patients with RA. It is important to prevent VFs to maintain the functional status of RA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is defined as a chronic systemic disease characterized by proliferative synovitis, and it results in severe joint destruction. The prevalence of RA in Japan is estimated at 1 % of the population, which is almost the same as in Western populations (0.5–1.0 %) [1, 2]. Inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α), as well as proteinases such as matrix metalloproteinases, are abundantly expressed in synovial tissues of RA patients and are involved in joint destruction [3]. In severe RA, persistent inflammation leads to generalized osteoporosis as well as local osteoporosis, which increases the risk of fragility fractures.
In RA, functional disability is a major concern because it not only restricts a patient’s activities of daily living but also reduces work capability, resulting in a substantial economic burden [4–6]. There are several studies concerning risk factors associated with the functional status of RA patients [7–11]. However, there are few studies assessing the relationship between fractures and functional status of RA patients. We investigated the influence of vertebral fractures (VFs) on the functional status of postmenopausal RA patients, and determined the risk factors for occurrence of VF.
Materials and methods
Patients
The subjects were selected from a single hospital registry of RA patients (National Hospital Organization Sagamihara Hospital). Two hundred postmenopausal RA patients who had complete records of demographic characteristics, clinical data such as disease activity score in 28 joints (DAS-28) and modified health assessment questionnaire (mHAQ), bone mineral density (BMD), and spine radiographs were eligible for this study. All patients fulfilled the American College of Rheumatology 1987 revised classification criteria for RA. The data were collected at baseline and at the final visit (a mean of 2.9 years after the first visit). All patients gave written informed consent, and the study was approved by the local ethics committee of Sagamihara Hospital.
Assessment of VFs in thoracic and lumbar spine
Lateral view radiographs of thoracic and lumbar spine were obtained from all the patients. VFs were diagnosed using the criteria of the Japanese Society for Bone and Mineral Research: the ratio of vertebral height at the mid and the anterior (or posterior) region was less than 0.8, and/or the ratio of the vertebral height at the anterior and posterior region was less than 0.75. New VFs (NVFs) were defined as follows: the vertebral height at the final assessment decreased by over 15 % or 4 mm compared with baseline. The films were independently investigated and assessed by three expert clinicians (H.F., J.T., and N.J.).
Information on the existence of back pain was also obtained from all patients. Clinical (symptomatic) VF was defined as VF with back pain, and asymptomatic VF as VF without back pain.
Measurements of bone mineral density
BMD measurements of the hip (femoral neck) and the lumbar spine (L1–4, anterior–posterior) were performed by trained technicians using dual-energy x-ray absorptiometry (QDR-4500, Hologic Co., Japan).
Data collection
Data were collected twice at a mean ± standard deviation (SD) interval of 2.9 ± 0.52 years. Baseline data were obtained from 2002 to 2005, and the final assessment was performed from 2005 to 2008. Clinical data included disease duration, age at menopause, length of time after the menopause, prescribed daily dose of prednisolone (PSL), use of bisphosphonates (etidronate disodium, alendronate or risedronate), calcium, and active vitamin D3 (alfacalcidol or calcitriol). Rheumatology experts examined the tender joint count (28 joints), the swollen joint count (28 joints), and existence of joint replacement (total hip or knee joint replacement), and the patient’s and investigator’s global assessments of disease activity were measured on a 100-mm visual analog scale. DAS was computed using the 28-joint count. Self-reported physical disability was assessed by mHAQ score.
Statistical analysis
Demographic characteristics, clinical data, and BMD measurements were compared between NVF(−) and NVF(+) groups at baseline using 2-sided t-tests for continuous variables and chi-squared tests for categorical variables. The possible predictors of NVF were subsequently entered into a logistic regression analysis. The inclusion criteria for independent variables in the logistic regression analysis were those variables with a p value <0.10 in the univariate analysis to identify the variables that might be associated with NVF. Statistical analyses were performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). To assess which factor was statistically significant, the residuals which represented the difference between the observed value of the variables and the predicted value were analyzed.
Results
Baseline characteristics
Table 1 presents the demographic and clinical characteristics of 200 patients at baseline. The mean age of the patients was 61.5 ± 6.5 years, disease duration was 15.0 ± 10.1 years, and postmenopausal period was 12.1 ± 7.3 years. The proportion of rheumatoid factor-positive patients was 73.0 %. The classification and the stage of RA were as follows: Steinbrocker’s classification, class 1, 60 patients (30.0 %); class 2, 117 patients (58.5 %); class 3, 23 patients (11.5 %), and class 4, no patient (0.0 %); Steinbrocker’s stage 1, 37 patients (18.5 %); stage 2, 38 patients (19.0 %); stage 3, 37 patients (18.5 %), and stage 4, 88 patients (44 %). Mean mHAQ score was 0.55 ± 0.53 and mean DAS-28 score was 3.82 ± 1.08. Fifty-three patients (26.5 %) had low disease activity (DAS-28 ≤3.2), 124 patients (62.0 %) had moderate disease activity (3.2 < DAS-28 ≤ 5.1), and 23 patients (11.5 %) had high disease activity (DAS-28 >5.1). The number of patients who had joint replacement surgery in the lower extremities (total hip or knee joint replacement) was 32 (16.0 %).
Mean BMD was 0.787 ± 0.152 g/cm2 at the lumbar spine, and 0.576 ± 0.137 g/cm2 at the femoral neck. Osteoporosis, defined as a T-score ≤−2.5 SD, was identified at the lumbar spine in 40.5 % of patients and at the femoral neck in 32.5 %. A previous VF was detected in 36 patients (18.0 %) at baseline.
New vertebral fractures
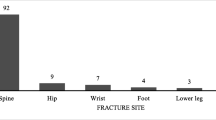
Twenty-eight patients (14 %) had NVFs during the observation period, and the prevalence of VF at the final visit was 27.5 % (55 patients) (Table 1). Of the 28 patients with NVF, 17 patients had one fracture, and 11 patients had two or more fractures. In total, 47 of 3,400 vertebrae (1.4 %) were fractured, 16 (34.0 %) being thoracic fractures and 31 (66.0 %) lumbar fractures. The annual incidence of NVF was 4.8 per 100 patients. Thirteen of 28 (46.4 %) NVF patients had asymptomatic NVF, while 15 patients (53.6 %) had clinical (symptomatic) NVF. Thirty-nine of 55 (70.9 %) VF patients at the final assessment had asymptomatic VF, while 16 patients (29.1 %) had clinical VF (p < 0.001). In patients treated with PSL ≥5 mg/day, the prevalence of asymptomatic VF was significantly higher than in those with symptomatic VF (30.7 and 8.0 %, respectively, p < 0.001). Fractures were most commonly identified in the mid-thoracic and the thoracolumbar regions, and the distribution of fractured vertebrae was similar to that reported in osteoporosis patients (Fig. 1).
The baseline characteristics were compared between NVF(−) and (+) groups (Table 1). There were no statistically significant differences in age, height, weight, BMI, disease duration, postmenopausal duration, rheumatoid factor positivity, proportion of PSL users, proportion of bisphosphonate users, calcium users, active vitamin D3 (alphacalcidol) users, C-reactive protein level, erythrocyte sedimentation rate, serum bone alkaline phosphatase, urine type I collagen cross-linked N-telopeptides, and the proportion of patients who underwent joint replacement surgery. Significant differences were observed in the daily dose of PSL, mHAQ scores, DAS-28 scores, and prevalent VF. The mean daily doses of PSL were 2.9 ± 2.5 and 4.2 ± 2.5 mg/day, mHAQ scores were 0.52 ± 0.53 and 0.73 ± 0.53, and DAS-28 scores were 3.75 ± 1.09 and 4.23 ± 0.96 in the NVF(−) and (+) groups, respectively. The prevalence of VF at baseline was 15.7 and 32.1 %, respectively. Mean final DAS-28 score was 3.6 ± 1.1 and 4.2 ± 1.2 (p < 0.05), and final BMD at the femoral neck was 0.573 ± 0.124 and 0.500 ± 0.111 (p < 0.01) in the NVF(−) and (+) groups, respectively.
Logistic regression analysis adjusted for age, BMI, and disease duration using variables whose p-value was less than 0.10 by univariate analysis revealed that dose of PSL (mg/day), femoral neck BMD, use of vitamin D3, and use of a bisphosphonate at baseline were factors associated with NVF, with odds ratios (95 % confidence interval) of 1.27 (1.05–1.54), 0.94 (0.91–0.97), 0.34 (0.13–0.89), and 0.31 (0.12–0.82), respectively (Table 2). Patients with higher DAS-28 score had a greater tendency to suffer NVF, with no statistical significance (odds ratio 1.45, p = 0.075).
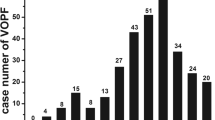
When the patients were subdivided into four groups based on the use of PSL and on DAS-28, the incidence of NVF was lowest in patients with low disease activity (DAS-28 <4.0) and receiving lower doses of PSL (<5 mg/day) (p < 0.01), while the incidence in patients with lower disease activity (DAS-28 <4.0) receiving higher doses of PSL (≥5 mg/day) was the highest of all the groups (p < 0.05). There was no significant difference in the incidence of NVF between patients with high disease activity (DAS-28 ≥4.0) receiving higher doses of PSL (≥5 mg/day) and those receiving lower doses of PSL (<5 mg/day) (p = 0.426) (Fig. 2).
Influence of NVFs on functional status
Mean mHAQ scores of NVF(−) and NVF(+) groups at the final assessment were 0.54 ± 0.56 and 0.88 ± 0.56, respectively, with a significant difference (p = 0.003). Patients with NVF at L3–5 exhibited significantly poorer final mHAQ scores than NVF(−) patients (p < 0.05) (Fig. 3a). Patients with NVF also exhibited a greater increase in mHAQ score from baseline compared with those without NVF (p < 0.05) (Fig. 3b). We also examined the influence of NVF in patients without baseline VF, and found that the mHAQ scores in these patients were significantly higher than in NVF(−) patients with no baseline VF (0.86 ± 0.63 and 0.48 ± 0.53, respectively, p < 0.01).
Impact of vertebral fractures on mHAQ scores. a Effect of a new vertebral fracture on final mHAQ score. b Effect of a new vertebral fracture on the change in mHAQ score from baseline. c Effect of asymptomatic and clinical new vertebral fractures on mHAQ score. * and **, significantly different; *p < 0.05, **p < 0.01
The mean mHAQ score in the group with NVF at the L3–5 level (without baseline VF) was significantly higher than in the group with no NVF (0.90 ± 0.69 and 0.48 ± 0.53, respectively, p < 0.05). The mean final mHAQ score in the clinical NVF(+) group was significantly higher than in the NVF(−) group (0.91 ± 0.69 and 0.54 ± 0.56, respectively, p < 0.05), while the mean mHAQ score (0.79 ± 0.42) in patients with asymptomatic NVF(+) was higher than in the NVF(−) group, with no significant difference (Fig. 3c).
Discussion
Several studies have reported that the risk of VF in RA patients is higher than that in primary osteoporosis patients. The prevalence of VF was reported as 15–36 %, and the annual incidence of NVF was around 4.0 per 100 patients [12–19]. Spector et al. [20] reported that the rate of VF in women with RA was over twice that of controls. Arai et al. [21] reported that the prevalence of VF in Japanese RA patients was 21 %, while that in age-matched healthy women was 5 %. We found that 14 % of the postmenopausal RA patients in our study developed NVF during 2.9 years (mean) of observation, and the prevalence of VFs was 27.5 % in total, which confirmed the high risk of VFs in RA patients, although we did not compare the prevalence with control patients.
Although a statistically significant difference was not found for disease activity in the logistic regression analysis, there was a tendency for DAS-28 to be higher in the NVF(+) group, and this also affected the incidence of NVF associated with BMD (existence of osteoporosis). El Maghraoui et al. [13] also reported that the prevalence of VF was increased in patients with higher DAS score. Dirven et al. [12] reported that disease activity over time was higher in patients with VFs. This may be because high levels of proinflammatory cytokines such as TNF-α and IL-6 in RA patients with high disease activity may lead to deleterious bone quality, resulting in greater susceptibility to VF.
In contrast to previous studies, age was not significantly associated with NVF incidence. When patients were categorized into three age groups (≤59, 60–69, and ≥70 years old), the percentage of bisphosphonate users was highest in the ≥70-year-old group (46.9, 56.8, and 70.8 %, respectively), which may affect the incidence of NVF.
Although PSL use is a risk factor for NVF, the occurrence of NVF in patients with high disease activity (DAS-28 ≥4.0) was lower in PSL ≥5 mg treatment group than in PSL <5 mg treatment group, although there was no significant difference (Fig. 2). We speculated that, in spite of its deleterious effects on bone, PSL use has some beneficial effects on preventing VFs in these patients by controlling disease activity. Consistent with our results, Ghazi et al. reported that the prevalence of VF was inversely related to the use of steroids, and Dirven et al. reported that there was no association between VFs and steroid use [12, 17, 21–23].
The efficacy of drug treatment against VF has been well established in osteoporosis patients [24–29]. Although we did not find a significant difference in the occurrence of NVF between the groups with or without bisphosphonate treatment in our study (9.8 and 18.4 %, respectively, p = 0.08), there was a tendency for patients using bisphosphonates, in particular alendronate and risedronate, to suffer NVF less frequently than those without bisphosphonate treatment (data not shown). However, the sample size in each category was too small to draw a definite conclusion. In addition, the duration of bisphosphonate treatment was not recorded.
Importantly, the patients with NVF exhibited significantly worse final mHAQ scores and a greater increase in mHAQ compared with those without NVF, indicating that NVFs decrease the functional status of RA patients. Furthermore, we found that a NVF without a baseline VF affected patient functional status similar to a NVF with a previous VF.
There are few studies that have investigated the relationship between VF and functional status of RA patients. Dirven et al. reported that functional ability over time was worse in patients with VFs than in patients without VFs, but they did not examine VFs at baseline. Furuya et al. [30] demonstrated that the risk of VF increased by 2.42 for each 1-point increase in Japanese HAQ scores, which is consistent with our results. These results clearly demonstrate the importance of VF prevention in maintaining good functional status in RA patients. Our data also suggest that fracture of the lower lumbar spine had a greater impact on health-related quality of life (QOL). Previous studies in the general population which investigated the association between the site of vertebral deformity and QOL reported similar results [31–33].
The incidence of asymptomatic VF was significantly higher than that of clinical, symptomatic VF in patients treated with PSL. This result is consistent with a previous study [14]. Functional status was poorer in patients with clinical VF or asymptomatic VF than in patients without VF, but clinical VF affected the mHAQ score more than asymptomatic VF. Because the difference was found in the analysis of both NVF and total VF, it is likely that the clinical symptom itself also contributes to a decrease in functional status.
There are several limitations to our study. First, this was a retrospective cohort study performed in one hospital. In addition, only 200 patients in the registry were eligible for the study, mainly because of the incompleteness of the data. In this study we analyzed NVF regardless of the existence of baseline VF because we thought it clinically essential to include data of previous VF in the investigation of incidental NVF. In addition, a larger number of patients is needed to perform additional sub-analysis. Furthermore, the duration of bisphosphonate treatment was not recorded, as mentioned above. Future prospective studies with more systematic data collection are required to draw definite conclusions.
In conclusion, we demonstrated that the incidence of NVF was a risk factor for worsening functional status in postmenopausal patients with RA. In addition, the dose of PSL, BMD, active vitamin D3 use, and bisphosphonate use were strongly associated with the occurrence of NVF. Disease activity was also an important factor. To maintain a good QOL in RA patients, it is essential to prevent VFs by treating osteoporosis as well as reducing RA disease activity.
References
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S (2013) Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. doi:10.1007/s10165-013-0863-6
Tanaka S (2007) Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am J Nephrol 27:466–478
Fautrel B, Guillemin F (2002) Cost of illness studies in rheumatic diseases. Curr Opin Rheumatol 14:121–126
Furneri G, Mantovani LG, Belisari A, Mosca M, Cristiani M, Bellelli S, Cortesi PA, Turchetti G (2012) Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin Exp Rheumatol 30:S72–S84
Tanaka E, Inoue E, Mannalithara A, Bennett M, Kamitsuji S, Taniguchi A, Momohara S, Hara M, Singh G, Yamanaka H (2010) Medical care costs of patients with rheumatoid arthritis during the prebiologics period in Japan: a large prospective observational cohort study. Mod Rheumatol 20:46–53
Adachi JD, Adami S, Gehlbach S, Anderson FA Jr, Boonen S, Chapurlat RD et al (2010) Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin Proc 85:806–813
Welsing PM, van Gestel AM, Swinkels HL, Kiemeney LA, van Riel PL (2001) The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum 44:2009–2017
Sokka T, Kankainen A, Hannonen P (2000) Scores for functional disability in patients with rheumatoid arthritis are correlated at higher levels with pain scores than with radiographic scores. Arthritis Rheum 43:386–389
Wolfe F (1999) Determinants of WOMAC function, pain and stiffness scores: evidence for the role of low back pain, symptom counts, fatigue and depression in osteoarthritis, rheumatoid arthritis and fibromyalgia. Rheumatology (Oxford) 38:355–361
Breedveld FC, Han C, Bala M, van der Heijde D, Baker D, Kavanaugh AF, Maini RN, Lipsky PE (2005) Association between baseline radiographic damage and improvement in physical function after treatment of patients with rheumatoid arthritis. Ann Rheum Dis 64:52–55
Dirven L, van den Broek M, van Groenendael JH, de Beus WM, Kerstens PJ, Huizinga TW, Allaart CF, Lems WF (2012) Prevalence of vertebral fractures in a disease activity steered cohort of patients with early active rheumatoid arthritis. BMC Musculoskelet Disord 13:125
El Maghraoui A, Rezqi A, Mounach A, Achemlal L, Bezza A, Ghozlani I (2010) Prevalence and risk factors of vertebral fractures in women with rheumatoid arthritis using vertebral fracture assessment. Rheumatology (Oxford) 49:1303–1310
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, Giorgino R, Moro L, Giustina A (2006) High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39:253–259
Ursum J, Britsemmer K, van Schaardenburg D, Lips PT, Dijkmans BA, Lems W (2009) High prevalence of vertebral deformities in elderly patients with early rheumatoid arthritis. Ann Rheum Dis 68:1512–1513
Baskan BM, Sivas F, Alemdaroglu E, Duran S, Ozoran K (2007) Association of bone mineral density and vertebral deformity in patients with rheumatoid arthritis. Rheumatol Int 27:579–584
Ghazi M, Kolta S, Briot K, Fechtenbaum J, Paternotte S, Roux C (2012) Prevalence of vertebral fractures in patients with rheumatoid arthritis: revisiting the role of glucocorticoids. Osteoporos Int 23:581–587
Orstavik RE, Haugeberg G, Mowinckel P, Hoiseth A, Uhlig T, Falch JA, Halse JI, McCloskey E, Kvien TK (2004) Vertebral deformities in rheumatoid arthritis: a comparison with population-based controls. Arch Intern Med 164:420–425
Vis M, Haavardsholm EA, Boyesen P, Haugeberg G, Uhlig T, Hoff M, Woolf A, Dijkmans B, Lems W, Kvien TK (2011) High incidence of vertebral and non-vertebral fractures in the OSTRA cohort study: a 5-year follow-up study in postmenopausal women with rheumatoid arthritis. Osteoporos Int 22:2413–2419
Spector TD, Hall GM, McCloskey EV, Kanis JA (1999) Risk of vertebral fracture in women with rheumatoid arthritis. BMJ 306:558
Arai K, Hanyu T, Sugitani H, Murai T, Fujisawa J, Nakazono K, Kondo N, Endo N (2006) Risk factors for vertebral fracture in menopausal or postmenopausal Japanese women with rheumatoid arthritis: a cross-sectional and longitudinal study. J Bone Miner Metab 24:118–224
Orstavik RE, Haugeberg G, Uhlig T, Falch JA, Halse JI, Hoiseth A, Lilleas F, Kvien TK (2003) Vertebral deformities in 229 female patients with rheumatoid arthritis: associations with clinical variables and bone mineral density. Arthritis Rheum 49:355–360
Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, Di Munno O, Frediani B, Cantatore F, Pellerito R, Bartolone S, La Montagna G, Adami B (2000) A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol 27:2582–2589
Harris ST, Watts NB, Jackson RD, Genant HK, Wasnich RD, Ross P, Miller PD, Licata AA, Chestnut CH III (1993) Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. Am J Med 95:557–567
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Fujita T, Orimo H, Inoue T, Kaneda K, Sakurai M, Morita R et al (2007) Clinical effect of bisphosphonate and vitamin D on osteoporosis: reappraisal of a multicenter double-blind clinical trial comparing etidronate and alfacalcidol. J Bone Miner Metab 25:130–137
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Furuya T, Kotake S, Inoue E, Nanke Y, Yago T, Kobashigawa T et al (2007) Risk factors associated with incident clinical vertebral and nonvertebral fractures in Japanese women with rheumatoid arthritis: a prospective 54-month observational study. J Rheumatol 34:303–310
Suzuki N, Ogikubo O, Hansson T (2009) The prognosis for pain, disability, activities of daily living and quality of life after an acute osteoporotic vertebral body fracture: its relation to fracture level, type of fracture and grade of fracture deformation. Eur Spine J 18:77–88
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15:1384–1392
Cockerill W, Ismail AA, Cooper C, Matthis C, Raspe H, Silman AJ et al (2000) Does location of vertebral deformity within the spine influence back pain and disability? European Vertebral Osteoporosis Study (EVOS) Group. Ann Rheum Dis 59:368–371
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Omata, Y., Hagiwara, F., Nishino, J. et al. Vertebral fractures affect functional status in postmenopausal rheumatoid arthritis patients. J Bone Miner Metab 32, 725–731 (2014). https://doi.org/10.1007/s00774-013-0552-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-013-0552-8