Abstract
The aim of this study was to investigate the association of vertebral deformities developed as a result of osteoporosis in female patients with rheumatoid arthritis (RA) with bone mineral density (BMD) and disease activity parameters. In the study, 100 female patients with the diagnosis of RA and 56 healthy subjects were recruited. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and rheumatoid factor (RF) tests were performed and the number of swollen and tender joints, level of pain and health assessment questionnaire (HAQ) were recorded in order to evaluate disease activity. Anteroposterior and lateral thoracic and lumbosacral roentgenograms of all patients were taken for radiological examination and deformities of vertebrae were assessed. BMD measurements of patients were performed on vertebrae L1–4 of lumbar region and on total hip, femur neck, trochanter and Ward’s triangle of the right side. Vertebral deformity was established in 30% of RA patient group and 7.1% of control group and this was statistically significant. In the statistical analysis, no statistically significant difference was found between BMD measurements of RA and control groups. Patients with RA were divided into two subgroups with regard to using corticosteroids (CS) or not. Vertebral deformity was 32.4% in the subgroup using CS and 24.1% in the subgroup not using CS, and the difference was not statistically significant. There was a correlation between number of deformed joint and age and vertebral deformity incidence. RA is a risk factor on its own for the development of osteoporosis and vertebral deformity and this risk increases by age, excess number of deformed joints and severe course of disease. We think that precautions should be taken immediately to suppress the disease activity as well as to protect the quality and density of bone and to prevent the development of vertebral deformity and fracture while planning the treatment of patients with RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is the most common inflammatory arthritis affecting 0.5–1% of the general population worldwide and although RA is properly considered a disease of the joints, it can cause a variety of extra-articular manifestations [1]. One of the well known extra-articular manifestations of RA is osteoporosis [2]. Two types of osteoporosis, peri-articular and generalized, are seen in RA. It was demonstrated that drugs used in treatment of RA, including corticosteroids (CS), were associated with increased local inflammatory activity of peri-articular osteoporosis and decreased disease activity of generalized osteoporosis [3–5]. For the diagnosis of osteoporosis, to measure the bone mineral density (BMD), dual energy X-ray absorbsiometry (DEXA) method is commonly used. According to World Health Organization (WHO) criteria, values ≤ −2.5 SD of T score are considered as osteoporosis [4, 6, 7]. Many studies reported that along with osteoporosis prevalence of vertebral deformity was increased in RA [2, 8, 9]. Vertebral fractures are usually asymptomatic and may appear during normal daily activity, have an association with increased morbidity and mortality, and are risk predictors for development of additional osteoporotic fracture [10, 11].
This study investigated the association of vertebral deformities developed as a result of osteoporosis with BMD measurements and disease activity parameters in female patients with RA.
Materials and methods
One hundred female RA patients diagnosed according to the American College of Rheumatology (ACR) criteria [12] and 56 healthy subjects were recruited to the study. Patients with a disease influencing the bone metabolism, with high levels of parathormone (PTH), alkaline phosphatase (AP), and high calcium excretion in 24-h urine and patients that received drugs for the treatment of osteoporosis prior to the study were excluded. Before the study, all patients had physical examination thoroughly and the number of swollen, tender, and deformed joints and body mass index (BMI) were recorded. Subsequently, via laboratory analysis, complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), and biochemical analysis including BUN, creatinine, Na, K, Ca, P, AP, SGOT, SGPT, GGT, uric acid, total protein, and albumin were performed. Also, 24 h urine samples were obtained from all the patients for urinalysis.
All patients were questioned for menarche age, menopause age, smoking habits, estrogen treatment, use of CS, and disease modifying drugs.
General disease activity and pain were measured using visual analogue scale (VAS) by both the patient and the doctor. In addition, functional status of all patients was assessed using health assessment questionnaire (HAQ) [13].
Anteroposterior and lateral thoracic and lumbosacral roentgenograms of all patients were taken as radiological examination and deformities in thoracic and lumbar vertebrae were evaluated by a radiologist according to the semi-quantitative method described by Genant et al. [14]. In respect to this method, in anterior posterior or middle parts of vertebrae, 20–25% loss of height (grade 1), 25–40% loss of height (grade 2), >40% loss of height (grade 3) were considered as mild, moderate, and severe deformity, respectively.
BMD measurements of patients were performed on vertebrae L1–4 of lumbar region and on total hip, femur neck, trochanter, and Ward’s triangle of the right side using Hologic QDR 2000 DEXA device (Hologic Inc, Waltham, MA, USA). Results were recorded as gram per square centimeter.
All measurements of patients and healthy subjects were compared using the statistical software SPSS version 10.0 (SPSS Inc, Chicago, IL, USA). Definitive statistics were presented as mean ± SD. For inter-group comparisons “significance of difference between two independent groups test” was used and P < 0.05 was considered significant.
Results
A total of 100 female patients with RA were included in the study. About 60 female patients were in postmenopausal, 40 female patients were in premenopausal period, and their ages ranged from 24 to 76 years (mean 52.7 ± 12.2 years). About 56 healthy women were taken as the control group of which 34 of them were in postmenopausal and 22 of them were in premenopausal period, and their ages ranged from 29 to 70 years (mean 52.7 ± 13.9 years). Statistical analysis showed no significant difference in age between patients with RA and the control group (P > 0.05).
BMI values were 29.6 ± 5.3 kg/m2 in the patient group, 29.9 ± 5.4 kg/m2 in the control group, while menarche age was 13.8 ± 1.9 years in the patient group and 14.2 ± 1.5 years in the control group, and with the analysis no statistically significant difference was determined between the two groups (P > 0.05).
While mean menopausal age was 47.4 ± 0.5 years in the patient group, it was found 44.4 ± 8.5 years in the control group, but there was no statistically significant difference (P > 0.05). Rate of estrogen use was 6% in the patient group, 10.7% in the control group, and smoking rate was established to be 16 and 21.4%, respectively, but between patient and control groups no statistically significant difference was found (P > 0.05).
Former nonvertebral fracture incidence in patients with RA was 15 and 17.9% in the control group, but the difference between groups was not statistically significant (P > 0.05). The results are summarized in Table 1.
The mean BMD that was measured at the L1–4 region was 0.864 ± 0.184 g/cm2, at the total hip was 0.807 ± 0.163 g/cm2, at the femur neck was 0.749 ± 0.146 g/cm2, and at Ward’s triangle was 0.623 ± 0.197 g/cm2 in the patient group, and these values were 0.900 ± 0.173, 0.812 ± 0.163, 0.758 ± 0.142, and 0.625 ± 0.209 g/cm2 for the control group, respectively. Statistical analysis revealed no significant difference between the groups (P > 0.05). In the patient group, we observed 30% vertebral deformity rate where as in the control group this rate was 7.1% and the difference was statistically significant (P < 0.05). The results are given in Table 2.
RA patient group was reassessed for CS use by dividing into two subgroups. Thus, 71 patients with RA using CS were compared with 29 patients with RA not using CS in terms of demographical and clinical parameters. For the CS group daily dose of CS was 2.5–15 mg (mean 7.06 ± 2.31 mg), duration of treatment was 1–168 month (mean 35.45 ± 36.48 month) and the cumulative dose was 0.15–36 g (mean 7.48 ± 8.56 g) and no statistically significant difference was determined (P > 0.05). In patients using CS, BMD measurements were 0.853 ± 0.183 g/cm² at L1–4, 0.803 ± 0.154 g/cm² at the hip, 0.739 ± 0.134 g/cm² at femur neck, and 0.611 ± 0.172 g/cm2 at Ward’s triangle, while in the group not using CS, BMD values were established as 0.894 ± 0.187, 0.820 ± 0.187, 0.776 ± 0.172 and 0.655 ± 0.248 g/cm2, respectively, but between two groups no statistically significant difference was observed (P > 0.05). When groups were evaluated with regard to vertebral deformity, although a higher rate of deformity was observed in the group using CS (32.4%) when compared with the group not using CS (24.1%), the difference was not statistically significant (P > 0.05). Results are summarized in Table 3.
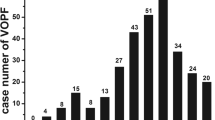
As the group of patients with RA was reassessed, vertebral deformity was observed in 30 patients. Evaluation of the vertebral deformity between the regions of dorsal 4 to lumbar 5 by the Genant method revealed grade 1 deformity in 66 vertebrae (85.71%), grade 2 deformity in 7 vertebrae (9.09%), and grade 3 deformity in 4 vertebrae (5.19%). When we examined the patients individually we observed only 1 deformity in 9 patients (30%), 2 deformities in 6 patients (20%), and 3 or more deformities in 15 patients (50%). Most of the deformities were at mid-thoracic and thoraco-lumbar regions (Fig. 1). The statistical analysis showed that RA patients with vertebral deformity were significantly older and had more deformed joints than the RA patients without deformity (P < 0.001 and 0.05, respectively). In contrast, no statistically significant difference was determined with respect to BMD measurements between the two patient groups (P > 0.05). Results are summarized in Table 4.
We observed only grade 1 vertebral deformities in 7.1% of the healthy control group.
Discussion
Osteoporosis is one of the well known extra-articular complications of RA and it is shown that these patients have an increased risk of vertebral and hip fracture [2]. Particularly, it is important to determine vertebral deformities developing after vertebral fractures caused by osteoporosis [15] as newly conforming deformity risk increases 5–12 folds in association with number of former deformities [11]. These deformities increase morbidity and mortality, and bring along additional osteoporotic fracture risk [10].
In a previous study, it has been reported that in patients with RA, vertebral deformities were seen more frequently compared to control group [8]. In our study also, a significantly higher rate of vertebral deformity was established in patients with RA. In the patient group, among the scanned 1,400 vertebrae, 77 of them were found to have vertebral deformity. In previous studies, Orstavik et al. reported higher rates of grade 2 and 3 vertebral deformity in RA patients [8, 10]. It has been suggested that osteoporosis observed in RA patients were closely related to age, sex and functional disability of the patient, dose and duration of the CS treatment and activity and duration of the rheumatic disease [2, 16–18]. Low incidence of grade 2 and 3 deformities, in our study, is likely because mean disease duration of 8.93 years is significantly shorter than the mean disease durations, which are 16.6 and 16.7 years, in the other studies.
CS used in order to suppress the symptoms of inflammation in RA may also have an influence on bone metabolism [19]. In patients using CS, osteoporosis is reported to develop related to hyperparathyroidism secondary to decrease of intestinal calcium absorption from the intestines and increase of renal calcium excretion, inhibition of osteoblastic function and growth factors and decrease of concentrations of sex hormones [20]. Previously, in the study by Lems et al. [11] that investigated the prevalence of vertebral deformity in patient with RA, they determined increased deformity incidence in patients with RA both using and not using CS. Nijs et al. [21] reported that, however, CS use in patients with RA increased risk of vertebral deformity and symptomatic fracture; this effect could not attain significant levels. In our study, 71% of patients with RA were using CS and when compared with the patients not using CS in terms of demographic and clinical parameters, no statistically significant difference was found between these two groups. In contrast, when we compared the BMD measurements, although a reduction in lumbar and hip regions as well as an elevation in vertebral deformity incidence was determined in patients with RA, these values could not reach statistically significant levels. Disease activity in patients with active RA is associated with increased generalized loss of bone mass probably because of increased release of bone-resorbing cytokines, such as interleukin-1, interleukin-6, tumor necrosis factor α and interferon-γ [21]. Although osteoporosis develops in peripheral joints in early period, longer time is required for development of generalized osteoporosis. In our study, we did not find any significant difference between patient subgroups using and not using CS with disease duration. Etiologies of osteoporosis in RA patients are multifactorial and besides disease duration, reduced physical activity caused by pain, inflammation and deformity are also important factors. Currently, no consensus has been obtained on whether low-dose steroid use in patients with RA is a risk factor for generalized OP [22]. While low-dose CS treatment may produce a negative effect on bones, in patients with high disease activity, suppression of inflammation and increasing physical activity, as well as mobilization of the RA patient may prevent the development of osteoporosis [3].
Previous studies reported that patients with RA who have vertebral deformity were older and that presence of vertebral deformity was associated with number of deformed joints [10, 23]. Both generalized osteoporosis and joint erosion in patients with RA is considered as a result of an increase in osteoclastic activity [21]. However, we performed no radiographic joint damage scoring in our study, and previous studies reported that number of deformed joints could also be utilized as an alternative scoring that indicated the joint damage [24]. Our study also found a significant association between age and vertebral deformity and number of deformed joints in patients with RA. In patients who have a high number of deformed joints, besides more severe course of the disease, development of functional disability in earlier period leading the patients indispensably to live a more sedentary life may have increased development of osteoporosis and vertebral deformity risk.
Although there is a close relation between BMD and vertebral deformity in postmenopausal osteoporosis, such a relation cannot be mentioned in osteoporosis secondary to inflammatory diseases or CS use [8]. Orstavik et al. [23] emphasized that RA patients with low BMD values particularly had tendency to develop vertebral deformity. Another study reported that RA patients showed a higher rate of vertebral deformity independent from BMD value and CS use [8]. Peel et al. [9] determined no significant difference of BMD in RA patients using CS with or without vertebral deformity. We also found no significant association with lumbar and femoral regions BMD and presence of vertebral deformity. These results suggest that unlike postmenopausal osteoporosis, different etiopathogenetic mechanisms play a role in the development of both osteoporosis and vertebral deformity in patients with RA, so different prevention and treatment principles should be carried out.
In conclusion, RA is a risk factor on its own for development of osteoporosis and vertebral deformity and this risk increases more by older age, excess number of deformed joints, and severe course of disease. So, we think that for protecting the quality and density of bone and to prevent the development of vertebral deformity and fractures, precautions should be taken immediately to suppress the disease activity while planning the treatment of patients with RA.
References
Firestein GS (2005) Rheumatoid arthritis. In: Harris ED, Budd RC, Firestein GS, Genovese MC, Sergent JS, Ruddy S, Sledge CB (eds) Kelley’s textbook of rheumatology, 7th edn, vol 2. Philadelphia, Pennsylvania, pp 996–1042
Haugeberg G, Uhlig T, Falch JA et al (2000) Bone mineral density and frequency of osteoporosis in female patients with RA, results from 394 patients in Oslo County Rheumatoid Arthritis Register. Arthritis Rheum 43:522–530
Hansen M, Florescu A, Stoltenberg M et al (1996) Bone loss in rheumatoid arthritis. Scand J Rheumatol 25:367–376
Harrison BJ, Huctchinson CE, Adams J et al (2002) Assessing periarticular bone mineral density in patients with early psöriatic arthritis or rheumatoid arthritis. Ann Rheum Dis 61:1007–1011
Laan RFJM, van Riel PLCM, van de Putte LBA (1992) Bone mass in patients with rheumatoid arthritis. Ann Rheum Dis 51:826–832
Lems WF, Dijkmans BAC (1998) Should we look for osteoporosis in patients with rheumatoid arthritis. Ann Rheum Dis 57:325–327
Haugeberg G, Orstavik RE, Uhlig T et al (2002) Clinical decision rules in rheumatoid arthritis: do they identify patients at high risk for osteoporosis? Testing clinical criteria in a population based cohort of patients with rheumatoid arthritis recruited from the Oslo Rheumatoid Arthritis Register. Ann Rheum Dis 61:1085–1089
Orstavik RE, Haugeberg G, Mowinckel P et al (2004) Vertebral deformities in rheumatoid arthritis—a comparison to population based controls. Arch Int Med 164:420–425
Peel NF, Moore DJ, Borroington NA et al (1995) Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis 54:801–806
Orstavik RE, Haugeberg G, Uhlig T et al (2003) Vertebral deformities in 229 female patients with RA. Associations with clinical variables and bone mineral density. Arthritis Rheum 49:355–360
Lems WF, Jahangier ZN, Raymakers J et al (1997) Methods to score vertebral deformity in patients with rheumatoid arthritis. Br J Rheum 36:220–224
Arnett FC, Edworthy SM, Bloch DA et al (1998) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–320
Kirwan JR, Reeback JS (1986) Stanford health assessment questionnaire modified to assess disability in British patients with rheumatoid arthritis. Br J Rheum 25:206–209
Genant HK, Wu CY, van Kuijk C et al (1993) Vertebral fracture assessement using a semiquantitative technique. J Bone Min Res 8:1137–1148
Ismail AA, Cooper C, Felsaberg D et al (1999) The European vertebral osteoporosis study group. The number and type of vertebral deformities. Epidemiological characteristics and relation to back pain and height loss. Osteoposis Int 9:206–213
Kelly CA, Bartholomev P, Lapwoth A et al (2002) Peripheral bone density in patients with RA and factors which influence it. Eur J Int Med 13:423–427
Forslind K, Keller C, Svensson B et al (2003) Reduced bone mineral density in early rheumatoid arthritis is associated with radiological joint damage at baseline and after 2 years? J Rheumatol 30:2590–2596
Inaba M, Nuigata N, Goto H et al (2003) Preferential reductions of periarticular trabecular bone component in ultradistal radius and of calcaneous ultrasonography in early-stage RA. Osteoporosis Int 14:683–687
Orstavik RE, Haugeberg G, Uhlig T et al (2004) Quantitative ultrasound and bone mineral density: discriminatory ability in patients with rheumatoid arthritis and controls with and without vertebral deformities. Ann Rheum Dis 69:945–951
Stein CM, Pincus T (2001) Glucocorticoids. In Ruddy S, Harris ED, Sledge CB (eds) Kelley’s textbook of rheumatology, 6th edn, vol1. Philadelphia, Pennsylvania, pp 823–840
de Nijs RNJ, Jacobs JWG, Bijlsma JWJ et al (2001) Prevalence of vertebral deformities and symptomatic vertebral fractures in corticosteroid treated patients with rheumatoid arthritis. Rheumatology 40:375–1383
Haugeberg G, Orstavik RE, Kvien TK (2003) Effects of rheumatoid arthritis on bone. Curr Opin Rheumatol 15:469–475
Orstavik RE, Haugeberg G, Uhlig T et al (2005) Incidence of vertebral deformities in 255 female rheumatoid arthritis patients measured by morphometric X-ray absorptiometry. Osteoporosis Int 16:35–42
Orces CH, Del Rincon I, Abel MP et al (2002) The number of deformed joints as a surrogate measure of damage in rheumatoid arthritis. Arthritis Rheum 47(1):67–72
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Başkan, B.M., Sivas, F., Alemdaroğlu, E. et al. Association of bone mineral density and vertebral deformity in patients with rheumatoid arthritis . Rheumatol Int 27, 579–584 (2007). https://doi.org/10.1007/s00296-007-0323-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0323-8