Abstract
Based on ISO 17034 and ISO Guide 35, a new series of matrix reference materials for 23 trace elements in 2 mg/mL gold solutions with five concentration levels (0, 1, 5, 10 and 20) ng/mL were developed, respectively. High-purity gold CRM GBW02793 with purity of 99.9995 % used as raw material was dissolved and then doped with multi-element solutions to prepare the candidate RMs. For ICP-MS measurements, matrix-matched and internal standard calibrations were studied and evaluated. The limits of detection (LoDs) ranged from 0.002 to 0.35 ng/mL. The satisfactory spike recoveries from 96 % to 108 % were obtained, demonstrating the measurement trueness. The ICP-MS method was also applied in the homogeneity and stability study. The statistical analysis suggested that the elements were well distributed by measuring 11 units with duplicate analysis for each. Besides, no significant trends were observed in the long-term stability test at room temperature for 12 months or in the short-term stability test at 60 ℃ and − 20 ℃ for 7 days. Through collaborative characterization by eight expert laboratories, the RM at each concentration level was certified for the mass fractions of 23 elements, respectively. All applied measurement methods in the characterization were further validated by using CRM ERM-EB507. The measurement results from all laboratories showed great consistency, and the overall mean values which were consistent with the target values were used as the certified values. Additionally, uncertainties arising from inhomogeneity (ubb), instability (us) and value assignment (uchar) were comprehensively combined. The developed RMs would ensure reliable and traceable analytical results of trace elements in fine gold and gold jewelry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-purity gold (Au) is increasingly used in the electronic industrial field [1, 2] because of its unique chemical and physical properties, such as outstanding resistance to corrosion and oxidation, high thermal and electrical conductivity and excellent solder-wetting properties [3,4,5,6,7]. The purity of gold considerably affects its properties [8, 9]. With the development of electronic chips toward increased component density, the requirements for tolerance of defects and heat conductivity are becoming more strict. Gold components with purity of 99.999 % are advantageous for their excellent reliability and heat conductivity, ensuring the chip’s performance. Compared with 99.99 % purity gold, the 99.999 % purity gold has significantly improved solderability and stability [1]. In addition, the environment friendliness [3] (in comparison with lead solder) makes 99.999 % high-purity gold a preferred choice. In the Chinese national standard for high-purity gold, i.e., GB/T 25933–2010 [10], 21 inorganic elements were strictly limited, with limited values of 1 mg kg−1 or 2 mg kg−1. Therefore, analyzing trace elements in high-purity gold is essential for the quality control of electronic devices.
Matrix interference has been a challenge for the accurate analysis of trace elements. To eliminate or reduce the matrix interferences, the generally used matrix removal techniques prior to detection are based on the solvent extraction [11,12,13,14,15] and reductive deposition methods [16,17,18]. It not only reduces the matrix interferences, but also improves the detection limits. However, the tedious and complicated matrix separation procedures are time-consuming and may result in element contamination of Na, Mg, etc., or element losses in the co-extraction or co-deposition procedure. Apart from matrix separation, accurate and rapid methods like standard addition [19, 20], matrix match and internal standard [21] methods are also used to reduce matrix effect. Compared with ICP-OES and AAS, ICP-MS [19,20,21] is a preferred spectrometry technique owing to its multi-element detection capability, high sensitivity and low detection limit. The standard method for trace elements quantification in gold is also based on ICP-MS (GB/T 25934.2). Direct analysis of high-purity gold is feasible, because ICP-MS could tolerate approximately 0.2 % (dilution 500-fold for high-purity Au) of the total dissolved solids (TDS) and thus avoid blockage of the sample cone [22].
However, although a maximum level and a standard method are in force, no certified reference materials (CRMs) of trace elements in gold solution are commercially available to date. CRMs are commonly used for analytical method validation, instrument calibration and value assignment. The certified values assigned in CRMs are traceable to the International System of Units (SI) [23], and thus, the use of CRMs provides comparable and traceable analytical results [24, 25].
In this work, based on ISO 17034 [26] and ISO Guide 35 [27], five matrix reference materials (RMs) for the analysis of trace elements in gold solution with analytes concentrations of (0, 1, 5, 10 and 20) ng/mL were developed, respectively. Since the RMs for each concentration level used the same measurement and estimation methods, only the results of level 20 ng/mL were discussed for length. Each RM (except for level 0 ng/mL) was certified for 23 elements in accordance with GB/ T 25933.2. A total of eight expert labs were invited to the certification. The homogeneity and stability tests were carried out for each element. The uncertainties were evaluated comprehensively by combining the uncertainty of inhomogeneity, instability and value assignment.
Materials and methods
Instrumentation
A total of eight labs who had rich experiments of RM development and had been demonstrated the technical competence of certification were invited for the certification. Inductively coupled plasma quadrupole mass spectrometry (ICP-QMS) and high resolution inductively coupled plasma sector field mass spectrometry (HR-ICP-SFMS) were used. The instruments used by eight labs are summarized in Table 1. For ICP-QMS, collision/reaction cell was commonly used to eliminate or reduce polyatomic spectral interferences caused by matrix element and discharge gas Ar. For HR-ICP-SFMS equipped with a double focusing magnetic sector mass spectrometer of reversed Nier-Johnson geometry, the spectral interferences could be eliminated using medium-resolution (MR) or high-resolution (HR) mode. The selected resolution modes for HR-ICP-MS analysis are listed in Table 2 for different elements.
Analytical balance XP204 from Mettler Toledo (d = 0.1 mg, Switzerland) was used. A DMA 35 type densimeter from Anton–Paar (Graz, Austria) was used for density measurement. All bottles used in the preparation of Au solutions were purchased from Nalgene (Thermo Fisher, Waltham, MA, USA), and the materials of the used beaker, 10 L stock solution bottle and 60-mL bottle were PFA (perfluoroalkoxy), PP (polypropylene) and HDPE (high-density polyethylene), respectively.
Reagents and samples
Raw materials for preparation
High-purity gold CRM GBW02793 with purity of (99.9995 ± 0.0002) % from NIM was used to prepare the candidate RMs. The 23 impurity elements of concern in GBW02793 were mostly less than 0.05 mg kg−1, except for Na ((0.85 ± 0.5) mg kg−1), Al ((0.85 ± 0.5) mg kg−1), Cr ((0.11 ± 0.04) mg kg−1), Fe ((0.29 ± 0.09) mg kg−1), Cu ((0.12 ± 0.04) mg kg−1), Ag ((0.36 ± 0.11) mg kg−1) and Pt ((0.078 ± 0.156) mg kg−1). Multi-element standard solution (GNM-M304347-2013, 100 μg ml−1) was purchased from Guobiao (Beijing) Testing & Certification Co., Ltd. (GBTC, Beijing, China), and single-element standard solution (1000 μg ml−1) of Rh (ICP-46H-1) from AccuStandard (New Haven, CT, USA) and Pd (IV-AAPD1), Te (IV-AATE1), Ir (IV-AAIR1) and Pt (IV-AAPT1) from Inorganic Ventures (IV, Christiansburg, VA, USA) were purchased to prepare multi-element stock solution I which was used as dopants for candidate RMs preparation. The solution I containing 23 elements with a concentration of approximately 1 mg/kg in 10% HNO3 was prepared gravimetrically by weighing multi-element standard solution GNM-M304347-2013 and single-element standard solution of Rh, Pd, Te, Ir and Pt in a 250 mL quartz volumetric flask. The mass fraction of 23 elements and the other impurities were checked by using ICP-MS to avoid gross errors. Ultra-pure HNO3 (55 %, m/m) from Tama Chemicals (Kanagawa, Japan), ultra-pure HCl (30%, m/m) from Merck (Darmstadt, Germany) and purified de-ionized water (18.2 MΩ cm−1) prepared using a Millipore purification system (Burlington, MA, USA) were used for candidate RMs and stock solution preparation.
Primary standards for certification
Single-element standard solution of Na, Mg, Al, Mn, Se, Rh, Pd, Sn, Sb, Te, Pt and Bi with a concentration of approximately 10 mg g−1 or 1 mg g−1 (SRM series) from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA), single-element standard solution of Ti, Cr, Fe, Ni, Cu, Zn, As, Ag, Cd and Pb with a concentration of approximately 1000 μg ml−1 (GBW series) from NIM and multi-element standard solutions containing Ir with a concentration of 10 μg g−1 (GBW (E) 082430) from NIM were used as primary standards in the candidate RM characterization procedure to ensure the metrological traceability. The primary standards used for measurements from all eight labs are summarized in Table S1 (see Electronic Supplementary Material, ESM). Single-element standard solutions of Sc (GBW08684, 1000 μg mL−1) from NIM and Cs (ICP-12N-0.1X-1, 1000 μg mL−1) as well as Re (ICP-45W-0.01X-1, 100 μg mL−1) from AccuStandard were used as internal standards. To achieve multi-element analysis, calibration standard stock solution II containing 22 elements and internal standard stock solution III containing three elements (Sc, Cs and Re) were prepared by diluting the mentioned above single-element standard solutions, respectively. For the two stock solutions, the concentration of elements was 1 mg/kg and the acidity was 10 % HNO3. To avoid cross-contamination and gross errors, 25 elements in both stock solutions were checked using ICP-MS.
Gold CRMs for method validation
CRM ERM-EB507 of alloying elements in white gold was purchased from Federal Institute for Materials Research and Testing (BAM, Berlin, Germany) for method validation and quality assurance. The CRM as a blind sample was used to demonstrate technical competence of participating labs before certification.
Preparation of candidate reference materials
To eliminate surface contamination, GBW02793 was ultrasonically cleaned sequentially by methyl alcohol for 3 min, 50 % (v/v) aqua regia with heating at 120 ℃ for 3 min, de-ionized water thrice and methyl alcohol twice for 1 min each. After that, the cleaned Au was dried by blowing using high-purity nitrogen. All vessels used in the preparation of candidate RMs were cleaned by soaking them for more than a week using 10 % HNO3 and rinsing them using de-ionized water.
Approximately 60 g cleaned Au was accurately weighed using analytical balance XP204 in a 1-L PFA beaker with cover, and then, 300 mL 50 % (v/v) aqua regia was added. The samples were dissolved and heated for 3–4 h at 90 ℃ on a hot plate, through which the gold stock solution with Au concentration of approximately 20.0 mg g−1 in 20 % (v/v) aqua regia was prepared. After that, the completely dissolved Au solutions were transferred to a 5 L PFA bottle and 1200 mL 50 % (v/v) aqua regia and de-ionized water were added to 3000 g.
Finally, five candidate RMs containing 23 impurity elements with a concentration of approximately (0, 1, 5, 10 and 20) ng/mL in 2 mg mL−1 Au solution with 2 % (v/v) aqua regia were prepared by adding approximately 500 g gold stock solution and about (0, 5, 25, 50 and 100) g multi-element stock solutions I, respectively, into a 10-L FLPE (fluoride-layer polyethylene) bottle, followed by adding de-ionized water to approximately 5000 mL at 20 °C ± 2 ℃. The density of candidate RMs was determined by a densimeter to be 1.007 g mL−1 at 20 °C ± 2 ℃. Finally, the prepared candidate RMs were bottled in 60-mL HDPE (high-density polyethylene) vials.
Selection of operating parameters, isotopes and resolution (collision/reaction) mode
The HR-ICP-SFMS was optimized at lab 1, with operating parameters summarized in Table S2 (see SEM). Specific isotopes and resolutions were selected to avoid isobaric and polyatomic interferences. Except for As, medium-resolution (MR, about 4000) mode was used. To eliminate the interferences from 40Ar35Cl on 75As, the mass resolution should be higher than 7781 and a high-resolution (HR, about 10,000) mode was required. For ICP-QMS, collision/reaction cells were generally used to minimize polyatomic interferences. At lab 3, different gas modes, i.e., no gas (STD, standard), O2 (DRC, dynamic reaction cell) and He (KED, kinetic energy discrimination) modes, were employed. For ICP-QMS analysis, due to the interference of 40Ar12C, 35Cl17O, 40Ar17O and 40Ar35Cl, the KED mode for 52Cr and 57Fe and the DRC mode for 75As were used. The summarized isotopes and resolution (collision/reaction) modes for HR-ICP-SFMS and ICP-QMS are listed in Table 2. For the other labs, the selection of isotopes and resolution (collision/reaction) was very similar, except for Fe analysis for which some labs (2, 8) used the 56Fe combine with KDE mode.
ICP-MS methods used by participants
Due to the high concentration of Au, the direct analysis (DA) of candidate RMs would cause heavy matrix effect and signal drift. Generally, matrix effect in ICP-MS can lead to the suppression of analytes signals in high matrix concentration samples relative to that in an aqueous solution [28]. To compensate for the matrix effect, matrix separation (SM), standard addition (SA) or matrix-matched (MM) calibration were always necessary. SA method could be used independently, whereas SM and MM should be combined with external calibration (EC). In addition to matrix interference, the high concentration may also result in the deposition of sample on cones, potentially causing signal drift over time. Simple dilution (SD) or internal standard (IS) could be used to solve the signal drift problems. The isotope dilution mass spectrometry (ID-MS) method was not used for any lab for reasons of cost and inability to analyze monoisotopic elements, although it can provide superior measurement trueness and precision compared to other calibration strategies [29, 30].
Sample preparation methods and calibration strategies employed by the participants are summarized in Table 3. Labs 2, 3 and 5 employed the SA method, and the others used the MM-EC method. In lab 3, the sample was diluted tenfold to reduce the signal drift. In labs 2 and 7, the alternating determination of sample and standard was carried out to check the signal drift, although the IS was not used. Labs 2 and 8 used ICP-OES method after the separation of matrix (SM-ICP-OES) for only method validation purpose.
Homogeneity and stability study
The homogeneity study assesses the distribution of the trace elements to be certified in the units bottled. For this purpose, 11 units were selected from the candidate RMs in a random stratified sample picking scheme. Two independent sub-samples from each unit were chosen. A total of 22 samples (11 units × 2 sub-samples) for all 23 elements were analyzed randomly by the validated HR-ICP-MS method, the same as the characterization method at NIM. The overall sample homogeneity was assessed by one-way analysis of variance (ANOVA). According to ISO Guide 35 [27], the F test was carried out and the samples were considered homogeneous if the F test values were smaller than those of the critical values at 95 % confidence interval.
In this work, long-term stability and short-term stability were assessed for all the 23 elements to be certified. For long-term stability, candidate RMs placed at room temperature were assessed on a periodic basis for 12 months and will be monitored continuously in the future. Two units were randomly selected, and each unit was analyzed for three times repeatedly by HR-ICP-MS at a predetermined time of (0, 1, 3, 6 and 12) months. The classic stability study was conducted for long-term stability assessment, and the samples were analyzed under reproducibility conditions. The short-term stability was checked by storing the selected candidate RMs at − 20 ℃ and 60 ℃ for (0, 1, 3 and 7) days. After the respective period, all units were stored at room temperature on the assumption that no instability occurred at this temperature. At the end of the 7-day-period, all sample units were analyzed simultaneously by HR-ICP-MS under repeatability conditions as an isochronous stability study [31]. For statistical analysis, regression analysis as recommended by ISO Guide 35 [27] was employed.
Characterization and value assignment
The certified values were assigned through an inter-laboratory collaborative determination program, with eight participating laboratories. The labs were selected based on their approved expertise in trace impurity elements analysis in rare and precious materials. Each lab received two bottles of candidate RMs and was asked to provide four values, with two independent replicates from each bottle. The labs were free to choose analytical methods for their measurement. In addition, the participants received gold solution of CRM ERM-EB507 (dilution 50,000-fold using 10 % HCl) with known mass fractions of Ag, Cu, Zn and Ni to analyze together with the candidate RMs for method validation. The arithmetic mean values derived from participating labs were assigned as the certified value after discarding the outliers according to the Grubbs criterion.
Results and discussion
ICP-MS method evaluation and validation: matrix effect, signal drift, precision, linearity, limits of detection and trueness
The characterization method was evaluated and validated at NIM and other labs, covering aspects of the matrix effect, signal drift, precision, linearity of calibration curves, limits of detection (LoD) and trueness. The matrix effect and signal drift were effectively corrected using matrix-matched and internal standards (Sc, Cs and Re) calibration, respectively. The measurement trueness was estimated based on the recovery experiment, CRM validation and validation with different methods, respectively. The recovery experimental was carried out at lab1, the CRM validation was done by all eight labs, and the comparison with different methods was made by lab 2 and lab 8. Although the method evaluation including matrix effect, signal drift, etc., was done at NIM (lab1) only, it was very similar among the labs.
Matrix effect
The matrix effect was evaluated by comparing the measured intensity signals (in cps) of analytes in Au solution (2 mg mL−1 Au + 2 % (v/v) aqua regia) with those in aqueous (2 % (v/v) aqua regia) solution. The curves of Cr in different solutions, for example, were compared, and obvious signal suppression in high concentration Au solution was observed, as shown in Fig. 1. For all elements of concern, the signals were suppressed by about 40 %–70 %. It was also found that the smaller the mass number, the more severe the matrix suppression. Therefore, for the accurate measurement, the correction of matrix effect was necessary.
Signal drift and precision
The signal drift was also investigated by observing the signal changes of elements in Au solutions through 1.5 h continuous measurement. Results showed that the intensities of Al and Bi, representing low and high mass numbers, reduced significantly with analysis time, as shown in Fig. 2. It was probably because of the deposition of Au samples on the skimmer cone, indicating the necessity to use internal standard (IS). By using suitable IS, the signal drift was corrected effectively. For Al, the RSD (n = 91) reduced from 11.7 % to 2.3 % and for Bi from 7.0 % to 2.7 %. In the process of the homogeneity test, 22 sub-samples were measured consecutively within the shortest time possible to reduce instrument drift, and the RSDs (n = 22) of 22 measurements with and without using IS were compared. As shown in Fig. 2, after IS calibration, the RSDs were reduced from approximately 5 % to 1 %. The good precision (RSD ~ 1%) was satisfactory for the homogeneity test, stability test and value assignment for the candidate RMs.
Linearity, limits of detection and recovery
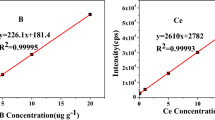
The linearity of calibration curves was assessed by using the linear correlation coefficient R, and the results of all elements of concern are listed in Table 4. It showed that for each of the 23 elements, the R was greater than 0.999. The good linearity was considered satisfactory for accurate analysis. LoD is an essential performance parameter for an analysis method, especially for the analysis of trace elements. High-purity gold solution with a concentration of 2 mg mL−1 in 2 % (v/v) aqua regia derived from GBW02793 was used as blank solutions to determine LoDs of elements of concern according to the 3s (n = 11) criterion, with results shown in Table 4. It showed that except for Na, Fe, Zn, Se and Sn, the LoDs were less than 0.1 ng/mL which could meet the need for trace analysis. For the method validation, the measurement trueness based on spike recovery was assessed. Three spiked samples (adding 20 ng/mL) were prepared, and the recoveries of all elements were measured. As shown in Table 4, the spiked recoveries were in the range of 96.0 % to 108.2 %, indicating good trueness of the analytical method.
Method validation
To demonstrate the technical competence of participating labs and to validate the ICP-MS method, a gold CRM ERM-EB507 was measured by all participants before the certification. The solid CRM ERM-EB507 was dissolved by aqua regia, diluted 50,000-fold gravimetrically using 10% HCl, and then distributed as a blind sample to participating labs. The labs were asked to dilute the received CRM solution 20 – 150 fold before the measurement to keep the mass fraction of certified Ag, Cu, Ni and Zn at the concentration of approximately (1–20) ng/mL. The results of all participants are listed in Table 5. The analytical results by the established ICP-MS methods showed good agreement with the certified values of ERM-EB507 within the uncertainty intervals for most labs. The relatively large deviation of the results from lab 2 and lab 6 cannot be attributed to system error from the developed methods because many uncertainty sources were not included, such as the standard solution, curve linearity and weighing. Actually, for the result in bold, the relative deviation (RD) between the measured and certified values was just about 2 %–4 %, which was considered relatively small and reasonable for ICP-MS analysis. It should be noted that the use ERM-EB507 for method validation had limitations due to the difference of element-to-gold ratio between ERM-EB507 and the developed CRM in this work. The lack of completely matrix-matched and element-matched CRM makes it a compromise choice for the use of gold CRM ERM-EB507.
Further, two approaches based on different principles were compared in lab 2 and lab 8 to verify the ICP-MS method. The arithmetic mean values of trace elements in the candidate RMs deviated from all labs by ICP-MS were recognized as the reference values with which the average results using ICP-OES method after the separation of matrix (SM-ICP-OES) in lab 2 and lab 8 were compared, as listed in Table 6. According to the standard method GB/T 25934.1 [11], the organic solvent ethyl acetate was used for the matrix separation. A bottle (about 40 mL) of candidate RM was extracted and then concentrated to 10 mL for ICP-OES analysis. The experiment for each lab was repeated three times to get the mean values. The average values of measured mean values from lab 2 and lab 8 by using SM-ICP-OES and the corresponding two times of standard deviation (2SD) are listed in Table 6 for comparison. The rigorous uncertainty evaluation was not given by lab 2 and lab 8, so the 2SD was used as the approximation of expanded uncertainty. Even though a good agreement was obtained within the uncertainty intervals in most cases, the results of Ti and Bi showed relatively large deviations. Moreover, the uncertainty of SM-ICP-OES was relatively large for most elements due to the considerable differences of the analytical results from lab 2 and lab 8, as shown in Fig. S1 (see ESM). In fact, according to the standard method GB/T 25934.1, to obtain a good recovery and meet the requirements of ICP-OES detection, extracting a total of 5 g Au and at least 1000 ng impurities from the Au solutions would be suitable. However, since the impurity contents in this experiment were extremely low, although a whole bottle (40 mL) of solutions was used, only 0.08 g Au and about 800 ng impurities were extracted even at the highest concentration level of 20 ng/mL. Therefore, avoiding the element loss and contamination was challenging, resulting in poor recovery. Given this, the results derived from ICP-OES were not included in the value assignment.
Homogeneity and stability testing
For all analytes (23 elements), homogeneity was assessed in lab 1 in accordance with ISO Guide 35 [27]. The results were evaluated by using ANOVA, and the measured and statistical results of Bi as an example are shown in Fig. 3 and Table 7. The statistical results showed that F was less than Fcrit(= 2.85), which demonstrated that no significant difference was observed between within- and among-bottles. The F values for all elements ranging from 0.38 to 2.35 (approximately 1 in most cases) were all less than the Fcrit critical value (2.85). Therefore, the candidate RMs were thought homogeneous for all elements at 95% confidence interval, indicating that no contamination was introduced during the preparation of the candidate reference materials.
The short-term stability and long-term stability of RMs or CRMs should be assessed to set the storage, packing and transport conditions and determine the expiry date. Stability implied that all analytes should remain unchanged within the stated uncertainties over the period of validity of the certificate. In this work, for each of the 23 analytes, the long-term and short-term stability studies were carried out based on the Student’s t test. The existence of 1.5%(v/v) HCl in the candidate RM may result in the change of concentration of Ag over time, so more attention was paid to it in the stability studies. The measured mass fraction of Ag is described graphically in Fig. 4. It showed that whether in the long-term or the short-term study, no obvious trends during the predetermined storage were observed, demonstrating that the stability was acceptable. Actually, complexation reaction equilibrium of AgCl + Cl− ⇌ [AgCl2]+ existed in the solution, so it exhibited good stability. The variations could be attributed to the random error of the measurement, and it was obvious that the results were not out of the uncertainty range. In accordance with ISO Guide 35 [27] and t test, a regression line for each analyte was fitted by plotting the measurement results against time, and statistical tendency analysis was carried out. The slope β1 of the regression line was compared with the product of the standard deviation of the slope s(β1) multiplied by the critical t value at 95% confidence interval and n-2 degrees of freedom t0.95,n-2 (4.30 for short stability and 3.18 for long stability). The detailed data are listed in Table 8. The uncertainties (us) derived from long-term or short-term instability were listed, and for each type instability uncertainty ults or usts was used, shown in Table 9. It showed that the results of |β1| were all less than their respective t0.95,n-2·s(β1) values, demonstrating no significant slope for either long-term or short-term stability. Even though the candidate RM exhibited good stability under different conditions, we still recommended shipping and using it under specified conditions (room temperature) to avoid unknown problems. The long-term stability will be further monitored at regular intervals.
Plots of stability of Ag element: a long-term stability at room temperature for 12 months; b short-term stability at 60 ℃ for 7 days; c short-term stability at -20 ℃ for 7 days. Error bars represent the standard deviation of six measurements (two samples and three replicate analysis for each). The solid red line and the dash lines represent the certified value and expanded uncertainty (k = 2), respectively
Value assignment
A collaborative characterization program was conducted by eight participating laboratories that have been demonstrated technical competence. All data were thoroughly inspected for technical outliers first and subsequently tested for statistical outliers according to Grubbs criterion at 95 % confidence level. The tested results showed that no outlying values were detected. The normal distribution of results from all labs was then demonstrated by using the Shapiro–Wilk criterion. Therefore, the certified values of trace elements in the gold solutions were assigned based on the arithmetic mean values of all results submitted by participants. As an example, the results of Cu element from all laboratories are depicted graphically in Fig. 5, and the detailed values are listed in Table S3 (see SEM). The certified value of Cu in the candidate RM was assigned to be 19.3 ng/mL by our calculating the mean of all participants’ means. For all elements, the overall mean value and participants’ means are listed in Table S4 (see SEM), and the certified values were assigned. The RSDs of analytical results from 8 labs ranged from 1.9 % to 5.9 % for all elements, indicating good agreements between labs. The analytical values from various labs ranged from 18.5 to 20.5 for most elements, and the overall mean values had good agreements with the target values.
Plots of measurement mean results from all participants for Cu as an example in gold solution with their corresponding standard deviations (n = 4). The red solid line represents the mean value (19.3 ng/mL) of all laboratory means, and the expanded uncertainty (k = 2) is indicated by the dashed lines
Uncertainty estimation
According to ISO Guide 35 [27] and The Guide to the Expression of Uncertainty in Measurement (GUM) [32], the uncertainties of the candidate RMs were estimated systematically and comprehensively. It could be expressed as the following equation:
where uc is the combined uncertainty, k is the coverage factor at 95% confidence level (k = 2), uchar is the uncertainty related to the value assignment, ubb is the uncertainty associated with the sample inhomogeneity, and usts and ults are the uncertainties that resulted from short-term instability and long-term instability, respectively. The uchar associated with value assignment was estimated by combining uncertainties from multiple laboratories measurements, standard solutions (CRMs), weighing, standard curve linearity and blanks.
The detailed uncertainty evaluation methods, processes and calculations are summarized in Table 9 using element Ir as an example. For the uncertainty of value assignment, the uncertainty of measurement from multiple laboratories accounted for the largest in most cases, as shown in Table 9. The irrespective uncertainty contributions for all elements can be found in Table S5 (see SEM). The certified values and expanded uncertainties of all analytes are summarized in Table 10. The expanded uncertainties were approximated 5 % for all elements, which could meet the needs for accurate determination.
Conclusions
A new series of matrix reference materials of trace elements in gold solutions (Au = 2 mg/mL at 2 % (v/v) aqua regia) with elemental concentration of (0, 1, 5, 10, 20) ng/mL were developed, respectively. For each concentration level, 23 impurity elements in accordance with GB/T 25934.2 were characterized and the certified values were assigned. The characterization was carried out based on a collaborative measurement program involving eight laboratories that have been demonstrated technical competence. The matrix-matched ICP-MS with IS calibration method was developed and validated by using the recovery experiment and CRM ERM-EB507. The homogeneity and short-term as well as long-stability stability were tested by using the validated HR-ICP-MS method. It was proven to be sufficiently homogeneous and stable for all certified elements. Uncertainties of the RMs were comprehensively estimated by combining uncertainty contributions from value assignment, inhomogeneity and instability. The CRMs would be a useful tool in validating new analytical methods and for ensuing reliable and traceable measurement results of trace elements in high-purity gold.
References
Tan Z, Chen J, Wen M, Wang C, Guo J, Xu Y, Shen Y, Guan W (2019) Review on study of high purity gold sputtering target material used in electronics industry. Precious Metals. 40(2):83–87 ((in Chinese))
Zhang J, Xie H, Yang A, Zhu Y, Deng Z, Chen D (2015) Status and prospect of preparative methods for high-purity gold. Precious Metals 36(3):81–86 ((in Chinese))
Holliday R, Goodman P (2002) Going for gold [gold in electronics industry]. IEE Rev 48(3):15–19
Han T, Nag A, Afsarimanesh N, Akhter F, Liu H, Sapra S, Xu Y (2019) Gold/polyimide-based resistive strain sensors. Electronics 8(5):565
Fitzpatrick C, Olivetti E, Miller TR, Roth R, Kirchain R (2015) Conflict minerals in the compute sector: estimating extent of tin, tantalum, tungsten, and gold use in ICT products. Environ Sci Technol 49(2):974–981
Kim Y, Kim H, Yoo HJ (2009) Electrical characterization of screen-printed circuits on the fabric. IEEE Trans Adv Packag 33(1):196–205
Shamkhalichenar H, Bueche CJ, Choi JW (2020) Printed circuit board (PCB) technology for electrochemical sensors and sensing platforms. Biosensors 10(11):159
Kinneberg DJ, Williams SR, Agarwal DP (1998) Origin and effects of impurities in high purity gold[J]. Gold Bulletin 31(2):58–67
Iizuka Y, Kawamoto A, Akita K, Daté M, Tsubota S, Okumura M, Haruta M (2004) Effect of impurity and pretreatment conditions on the catalytic activity of Au powder for CO oxidation. Catal Lett 97(3):203–208
GB/ T 25933–2010, High Puirty Gold[S]. Beijing, China Standards Press, 2010.(in Chinese)
GB/ T 25934.1–2010, Methods for Chemical Analysis of High Puirty Gold-Part 1: Ethyl Acetate Extraction Seperation-Inductively Coupled Plasma-Atomic Emission Spectrometry-Determination of Impurities Elements Contents[S]. Beijing, China Standards Press, 2010. (in Chinese)
GB/ T 25934.3–2010, Methods for Chemical Analysis of High Puirty Gold-Part 3: Ethylether Extraction Seperation-Inductively Coupled Plasma-Atomic Emission Spectrometry-Determination of Impurities Elements Contents[S]. Beijing, China Standards Press, 2010. (in Chinese)
Ivanova E, Jordanov N, Havezov I, Stoimenova M, Kadieva S (1990) AAS determination of trace elements in high-purity gold after matrix separation by solvent extraction. Fresenius J Anal Chem 336(6):501–502
Sun DD, Cao XY (2001) determination of impurity elements in high purity gold using ICP-AES method after matrix extraction and separation. Guang pu xue yu Guang pu fen xi Guang pu 21(6):849–851
Jung BH, Park YY, An JW, Kim SJ, Tran T, Kim MJ (2009) Processing of high purity gold from scraps using diethylene glycol di-N-butyl ether (dibutyl carbitol). Hydrometallurgy 95(3–4):262–266
Ivanova E, Havesov I, Berndt H, Schaldach G (1990) ET-AAS determination of traces of As, Sb and Sn in pure gold after matrix separation with hydrazine. Fresenius J Anal Chem 336(4):320–321
Lincoln AJ, Kohler JC (1964) Spectrographic Determination of Trace Impurities in High-Purity Gold[M]//Developments in Applied Spectroscopy. Springer, Boston, MA, pp 265–278
Karadjova I, Arpadjan S, Jordanova L (2000) Determination of trace metals in high purity gold. Fresenius J Anal Chem 367(2):146–150
Xie H, Huang K, Nie X, Fu L (2009) Determination of trace elements in high purity gold by high resolution inductively coupled plasma mass spectrometry. J Wuhan Univ Technol-Mater Sci Ed 24(4):608–612
GB/ T 25934.2–2010, Methods for Chemical Analysis of High Puirty Gold-Part 2: Inductively Coupled Plasma Mass Spectrometry-Standard Enter Emendation-Inner Standard Method-Determination of Impurities Elements Contents[S]. Beijing, China Standards Press, 2010. (in Chinese)
Wang X, Gong C, Yang S, Wang H (2017) Determination of trace impurity elements in high purity gold jewelry by internal standard method using ICP-MS[J]. Gold 38(5):79–82 ((in Chinese))
Yang L, Nadeau K, Meija J, Grinberg P, Pagliano E, Ardini F, Mester Z (2018) Inter-laboratory study for the certification of trace elements in seawater certified reference materials NASS-7 and CASS-6. Anal Bioanal Chem 410(18):4469–4479
International Organization for Standardization (2017) ISO/IEC 17025, General requirements for the competence of testing and calibration laboratories. ISO, Geneva, Switzerland
Isabel T, Marc DL, Erik VB (2004) Trends in quality in the analytical laboratory. I. Traceability and measurement uncertainty of analytical results. TrAC Trends Anal Chem. 23(7):480–90
International Organization for Standardization (2007). ISO/IEC Guide 99 International vocabulary of metrology-basic and general concepts and associated terms (VIM), 3rd edition. Geneva, Switzerland
International Organization for Standardization (2016) ISO 17034, General requirements for the competence of reference material producers. ISO, Geneva, Switzerland
International Organization for Standardization (2017) ISO Guide 35, Reference materials-guidance for characterization and assessment of homogeneity and stability. ISO, Geneva, Switzerland
Agatemor C, Beauchemin D (2011) Matrix effects in inductively coupled plasma mass spectrometry: a review. Anal Chim Acta 706(1):66–83
De Bièvre XP (1994) Isotope dilution mass spectrometry in trace element analysis in biological specimens. Elsevier, Amsterdam
Yang L, Sturgeon RE (2009) High accuracy and precision isotope dilution mass spectrometry: an application to the determination of Mo in seawater. J Anal At Spectrom 24:1327–1335
Lamberty A, Schimmel H, Pauwels J (1998) The study of the stability of reference materials by isochronous measurements. Fresenius J Anal Chem 360:359–361
International Organization for Standardization (2008), ISO/IEC Guide 98-3 Evaluation of measurement data - Guide to the expression of uncertainty in measurement. ISO, Geneva, Switzerland
Acknowledgements
Financial support by the National key R&D program of China (project 2019YFF0217101) is gratefully acknowledged. We also thank Ms Jiao Hui for English edition of the whole manuscript.
Author information
Authors and Affiliations
Contributions
Jianying Zhang did the experments for method development of CRM characterization and CRM candidacte preparation, and also wrote the main manuscript; Yanjie Cui and Tao Zhou did the expriments for CRM prapration preparation; Jinzhuan Wang, Zanfang Zhu, Fangmei Liu, Guangyang Xiao, Jing Yu, Xinlei Wang, Boling Huang, Chun Li and Xingjiao Huang did the expriments for the CRM characterization.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Cui, Y., Zhou, T. et al. Development of Certified Reference Materials of Trace Elements in Gold Solution (GBW02797–GBW02800). Accred Qual Assur 28, 299–310 (2023). https://doi.org/10.1007/s00769-023-01557-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-023-01557-8