Abstract
Dy, Ho, Er, Tm, and Sc mono-element solution certified reference materials (CRMs) with the certified value of 983.3 μg g−1 were developed with high-purity lanthanide oxides by using a novel purity characterization strategy. In the purity characterization process, complexometric titration was first employed to acquire the total metal ion concentration reacting with EDTA. Twenty-seven non-lanthanide impurities were measured by an external ICP-MS method with three multi-element calibration solution CRMs as calibrants. To avoid REO(H)+ interference from the main lanthanide matrix, two strategies namely LA-ICP-MS and MD-ICP-MS were optimized and used for the measurement of 15 rare earth impurities. The purity of lanthanide oxide material was obtained by subtracting the 42 impurities from the total metal ions reacting with EDTA. After purity characterization, the solution CRMs were prepared with a gravimetric method, and the CRM values were verified with corresponding NIST rare earth solution SRMs. It was shown that 15 units with duplicate analysis are enough to demonstrate the homogeneity of these candidate reference materials. The statistical results also showed no significant trends in stability tests for 24 months. The final uncertainties of the CRMs were evaluated by combining uncertainty contributions including the sample characterization and gravimetric preparation (uchar), between-bottle homogeneity (ubb), and stability (us). The relative expanded uncertainties of the five CRMs are 0.5%. These CRMs are primarily intended for use in the measurement and calibration procedures of lanthanide analysis in environmental and geological areas. Most importantly, the purity characterization strategy of this study will provide a new idea for the certification of high-purity and mono-element solution reference materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth element (REE) is one of a set of 17 chemical elements in the periodic table, specifically the 15 lanthanides, as well as scandium and yttrium. Lanthanide compounds, because of their valuable magnetic, catalytic, and optical properties [1], are widely used as catalysts or additives to materials for industry [2] and medicine, astrophysical materials [3], and as a doping agent in the optical industry [4]. In recent years, it was also noticed that some lanthanide bifunctional metal chelating agents were used to label peptides or proteins intended for protein quantification in life science [5,6,7]. As a result, there is increasing interest for developing rapid and sensitive methods for the determination of rare earth elements.
With the increasing application area of REEs, analytical techniques of lanthanides have been developing significantly. Earlier analysis of rare earth elements was mainly by chemical titration; however, the enrichment process was complex and the detection limit was not good. Most importantly, interferences from adjacent lanthanides were very serious. Therefore, the titration can only acquire the total amount of lanthanide and cannot distinguish the impurities with similar titration complexation constant. Of various analytical techniques, neutron activation method (NAA) [8], inductively coupled plasma mass spectrometry (ICP-MS) [9], and inductively coupled plasma optical emission spectrometry (ICP-OES) [10, 11] have proven to be the most effective and extensively used techniques for the REE determination. Although these techniques made lanthanide analysis faster and more precise, quality assurance through the use of CRMs is still very crucial to ensure the traceability of analytical procedures [12]. A survey of currently available CRMs certified for lanthanide identified a number of products. Seventeen mono-elemental lanthanide solutions from NIST were produced, and the concentration of the lanthanide was approximate 10 mg g−1. La, Ce, Sm, Eu, Yb, Lu, and Y mono-element standard solution CRMs (GBW08651-08657) prepared from high-purity lanthanide oxide were developed more than 20 years ago in National Institute of Metrology, China.
In the development of lanthanide CRMs, full purity characterization of starting material is very challenging. The CRMs of NIST were prepared from high-purity lanthanide metal. However, the metal materials are not only very difficult to acquire, but also are very reactive and prone to be oxidized in purity characterization procedures. Another strategy is starting from lanthanide oxide. However, many other lanthanide impurities, mainly from the associated lanthanide minerals, were usually naturally present in the high-purity lanthanide oxides. Because of the similar chemical properties and spectral interferences of the impurities, it was very challenging to distinguish the impurities from the high-purity lanthanide for most analytical techniques. Therefore, most high-purity lanthanide oxide products only give the purity value with the total lanthanide oxide, not the specific targeted lanthanide element. Previously, in the development of REE CRMs, the purity of the raw lanthanide oxide was only characterized by chemical titration and quadruple ICP-MS semi-quantitative analysis [13]. However, when determining the REE impurities by ICP-MS, severe REO(H)+ interference induced by REE matrix would be encountered. As a result, the lanthanide impurities of raw REE materials cannot be accurately measured and subtracted.
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), sample introduction to ICP-MS with dry aerosols, can largely reduce the interferences from polyatomic ions injected by solution and is a highly accepted method for the determination of major, minor, and trace elements in solids [14,15,16]. However, in the determination of impurities in REE oxides by LA-ICP-MS, because of the shortage of matrix-matched CRMs, accurate determination of trace impurities is very difficult [17]. Additionally, membrane desolvator (MD) coupled with ICP-MS, by reducing the production of polyatomic ions with membrane desolvation, is also considered to be an effective approach for trace rare earth impurities in high-purity lanthanides [18].
In this study, Dy, Ho, Er, Tm, and Sc mono-element solution CRMs with the certified value of 1000 μg mL−1 were developed with high-purity lanthanide oxides by using a novel purity characterization strategy. In purity characterization process, complexometric titration was first employed to acquire the total metal ion concentration reacting with EDTA. The total metal ions reacting with EDTA include three contributions: the main lanthanide element, 15 rare earth impurities, and the other 27 non-rare earth elements. In the certification of 27 non-rare earth impurities, ICP-MS with three multi-element calibration solution CRMs (GBW(E)082429~082431, with 70 elements) as external calibrants was employed. For the rare earth impurities, to avoid REO(H)+ interference from main lanthanide matrix, two strategies namely LA-ICP-MS and MD-ICP-MS were optimized and used for the measurement of 15 rare earth impurities. The purity of lanthanide oxide material was obtained by subtracting the 42 impurities from the total metal ions. After purity characterization, the solution CRMs were prepared with gravimetric method, and the CRM values were verified with corresponding NIST rare earth solution SRMs (SRM 3115a, 3116a, 3123a, 3148a, and 3160a). The relative expanded uncertainties of Dy, Ho, Er, Tm, and Sc CRMs are 0.5%.
Experimental
Instrumentation
A CETAC LSX-213 system (CETAC Technologies, USA) and U6000AT+ Membrane Desolvator system (CETAC Technologies, Omaha, NE, USA) coupled to quadrupole ICP-MS (Agilent 7700x) was used for LA-ICP-MS and MD-LA-ICP-MS analyses. Optimization of ICP-MS was carried out with a normal tuning solution (10 ng g−1 Be, In, Bi). LA-ICP-MS was optimized by SRM610 glass sample for signal sensitivity and stability. The optimum instrumental parameters used for LA, MD, and ICP-MS are given in Table 1. Two electronic balances (XP205, d = 0.01 mg; XP64000L, d = 1 g, Mettler Toledo, Switzerland) were used for sample weighing in titration and CRM preparation process. The lanthanide oxide raw materials were pressed with tablet compressing machine (SYP-30TS, XIN NUO LIHUA Company, China) for LA-ICP-MS analysis. Deionized and purified water (18 MΩ·cm, Millipore Milli-Q-Plus water purifier, USA) were used in purity characterization experiments and CRM preparation process.
Reagents and standards
The lanthanide oxides (Dy2O3, Ho2O3, Er2O3, Tm2O3, Sc2O3) with high purity (total REO > 99.9~99.99%) were obtained from Minmetals (Beijing) Research Institute, China. ICP-MS tuning standard solution (GBW(E)130242) and four multi-element solutions (GBW(E)082428~082431) were all certified reference materials obtained from National Institute of Metrology, China. Ethylene diamine tetraacetic acid disodium (EDTA) (GBW06102) were certified by constant–current coulometry with Primary Chemical Purity Standard in National Institute of Metrology, China [19]. Dy, Ho, Er, Tm, and Sc mono-element standard solution (SRM 3115a, 3116a, 3123a, 3148a, and 3160a) from NIST were used for value verification procedures. Ascorbic acid, ammonium hydroxide, hexamethylenetetramine, xylenol orange, boric acid, and HCl were all GR purity and obtained from Beijing Chemical Regents Institute, China. HNO3 and HCl with metal-oxide-semiconductor (MOS) purity from Merck were used after sub-boiled purification.
Preparation of candidate materials
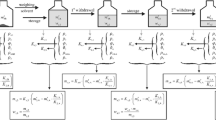
In the sample preparation for purity characterization, the high-purity lanthanide oxides were heated in muffle furnace under 900 °C for 6 h, and then cooled and preserved in a vacuum dryer. A certain amount of lanthanide oxide was accurately weighted in the beaker and approximately 200 mL 1:1 HCl was added. The solutions were heated and dissolved for approximate 30 min, and cooled down to room temperature. The stock solutions were diluted with sub-boiled HCl and deionized water to the concentration of 1000 μg mL−1 with 10% HCl matrix. After purity characterization, the Dy, Ho, Er, Tm, and Sc mono-element solution CRMs were prepared as the same procedure as that for purity characterization. The detailed procedure for sample preparation is as shown in Fig. 1. By gravimetrically weighing candidate CRM solution in a 2-L volumetric flask, the density of all the five candidate CRMs was determined to be 1.0018 g mL−1 at 20 ± °C.
Purity characterization of lanthanide oxides
Qualitative analysis of lanthanide oxides
Mixture of 0.2 g lanthanide oxide raw and 0.8 g boric acid was grinded in agate mortar for 20 min. The mixed powder was pressed with the tablet compressing machine at 20 MPa for 30 s. The isotopes of 163Dy+, 165Ho+, 166Er+, 169Tm+, and 45Sc+ of the pressed tablet samples were measured by LA-ICP-MS for qualitative analysis. The results showed that more than 1010 cps of the measured isotopes were observed, which proved the main lanthanide matrix of the raw material.
Total metal ion measurement by complexometric titration
Ten milliliters of prepared Dy CRM solution (target concentration 1000 mg L−1) for purity characterization was weighed in a conical flask; 50 mL deionized water, 0.2 g ascorbic acid, and 3 mL 1:1 ammonium hydroxide were added, and the pH was adjusted to 5.0~5.5. Drops of 0.3% xylenol orange and 5 mL hexamethylenetetramine with the concentration of 1 mol L−1 were added, and the color of the mixture solution was violet. EDTA standard solution with the concentration of 0.01 mol L−1 was used for titration. Cautiously and dropwise added when approaching end point, the total amount of used EDTA solution was approximate 5 mL. Fourteen parallel titrations of Dy candidate CRM were carried out by two separate experimenters, and the experimental procedures of Ho, Er, Tm, and Sc were similar with that of Dy.
Measurement of non-lanthanide impurities by ICP-MS
The impurities in candidate Dy, Ho, Er, Tm, and Sc CRM solutions were first semi-quantified by ICP-MS. By using the semi-quantification methods inherited in Agilent 7700x ICP-MS software, the impurities from Li to U in periodical table were semi-quantified. The solutions with the concentration of 1000 μg mL−1 were directly introduced to ICP-MS without dilution and the matrix lanthanide element was not detected in order to avoid signal overflowing.
After semi-quantification, 27 non-lanthanide elements with similar complex constant to lanthanides, including Ti, Zr, V, Cr, Fe, Co, Ni, Pd, Cu, Zn, Cd, Hg, Al, In, Tl, Mn, Pb, Bi, Th, Sc, Y, Ga, Pt, Sn, Sb, Hf, and U, were accurately measured by using external calibration ICP-MS. The candidate CRM solutions were introduced to ICP-MS without dilution, and five parallel samples of each candidate CRM solution were analyzed.
Measurement of rare earth impurities by LA-ICP-MS
Rare earth impurities of the pressed lanthanide tablets were determined by LA-ICP-MS. Five lines were scanned of each lanthanide tablet, and the signals of rare earth impurities of each tablet were averaged from the five lines. The concentration of each impurity was acquired by total signal normalization with sensitivity factor correction. The laser ablation conditions, including laser energy, spot size, scan rate, and frequency, were fully optimized in the experiment. To investigate the sensitivity factor (SF) of each lanthanide, 14 high-purity lanthanide oxides were doped in boric acid with different proportions and pressed to tablet. The lanthanide impurity concentrations of these in-house prepared calibrants were 0.005, 0.010, 0.020, and 0.030 g g−1. A pure boric acid tablet was prepared to calculate the limit of detection (LOD, 3 s of blank) and limit of quantification (LOQ, 10s of blank) of LA-ICP-MS.
Measurement of rare earth impurities by MD-ICP-MS
The CRM solutions with the concentration of 1000 μg mL−1 were directly introduced to MD-ICP-MS without dilution. The membrane desolvator conditions were optimized and listed in Table 1. Seventeen lanthanide multi-element CRMs (GBW(E)082428) were used as external calibrants, and five parallel samples were analyzed of each candidate CRM. As a blank sample, 10% HCl was used to calculate the LOD and LOQ of MD-ICP-MS.
Value verification by NIST lanthanide SRMs
After CRM preparation, the values of the candidate CRMs were verified by ICP-MS. The NIST SRMs (SRM 3115a, 3123a, 3116a, 3160a, and 3148a) were diluted to 2, 4, 8, and 16 ng g−1 as external calibrants. The candidate CRMs were also diluted to 10 ng g−1 before introduction to ICP-MS. Five parallel sub-units were measured of each candidate CRM.
Homogeneity and stability tests
Fifteen units were selected randomly from 1000 bottles of each candidate CRM, and two samples were tested independently from each unit. In the homogeneity and stability experiments, the CRM solution was diluted to 10 ng g−1 by accurate weighing and measured by ICP-MS. The analysis of 30 duplicate samples of each lanthanide was arranged in a randomized order, and the analysis was completed within the shortest time possible to minimize errors arising from instrumental bias. The overall sample homogeneity was assessed by a one-way analysis of variance (ANOVA). Samples were considered homogeneous if the F test values were smaller than those of the critical values at 95% confidence interval [20].
The stability was checked throughout the storage period at room temperature in 24 months. Five independent sub-samples of one bottle were analyzed for the determination of all the elements at 0, 4, 8, 12, 16, 20, and 24 months. The method for stability test was the same to that of homogeneity test, and the regression analysis was recommended in ISO Guide 35:2006 [20].
Results and discussions
Purity characterization of Dy2O3, Ho2O3, Er2O3, Tm2O3, and Sc2O3
Results of total metal cations by complexometric titration
Complexometric titration is a form of accurate analysis in which the formation of a colored complex is used to indicate the end point of a titration. Complexometric titrations are particularly useful for the determination of a mixture of different metal ions in solution. EDTA forms an octahedral complex with most 2+ metal cations, M2+, in aqueous solution. The main reason that EDTA is used so extensively in the titration of metal cation solutions is that the formation constant for most metal cation EDTA complexes is very high, which is very suitable for lanthanide analysis. In this work, by using complexometric titration, the total metal cation concentration (including the matrix lanthanide cations and some impurities) reacting with EDTA could be acquired, and the results were shown in Table 2.
The RSDs of titration results were less than 0.2%, which were good enough for total metal cation measurement. The uncertainty contribution of EDTA titration included standard deviation of EDTA titration (0.04~0.05%), uncertainty of EDTA CRM reagent (0.006%), uncertainties from weighing (0.03%), uncertainty from molar mass of elements (0.005%), and uncertainty from end point of indicator (0.02%). The standard uncertainty of EDTA titration was calculated from all these contributions according to EURACHEM [21].
However, it should be noticed that the metal cations reacting with EDTA include not only the main matrix lanthanide elements but also the impurities with similar titration complexation constant. In this study, the impurities were divided into non-lanthanide impurities and lanthanide impurities, and different measurement approaches were employed to measure them separately.
Results of non-lanthanide impurities by ICP-MS
ICP-MS semi-quantitative data can give the approximate content of each impurity, and find major impurities needed to be accurately quantified. In this study, the candidate Dy, Ho, Er, Tm, and Sc CRM solutions were first semi-quantified by ICP-MS. The Dy, Ho, Er, Tm, and Sc semi-quantification results showed that the contents of B, Na, Si, Al, and Ca contributed from 95.9 to 99.8% of all the metal cations. Since the complexation constants of B, Na, Si, and Ca (pH 5.0~5.5) are from 1.6 to 4.2 which are much lower than those of lanthanides (from 15.5 to 23.1), the B, Na, Si, and Ca cations do not react with EDTA in titration experiment [22]. However, because the complexation constant of Al is 16.3, it should be accurately measured and subtracted in the following experiments [23]. The complexation constant of lanthanide impurities is also very similar with the matrix which will be accurately measured by LA/MD-ICP-MS. In a word, considering the semi-quantification results and complexation constant data, 27 non-lanthanide elements including Ti, Zr, V, Cr, Fe, Co, Ni, Pd, Cu, Zn, Cd, Hg, Al, In, Tl, Mn, Pb, Bi, Th, Sc, Y, Ga, Pt, Sn, Sb, Hf, and U and 14 lanthanide elements including La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu were selected for accurate measurement and subtracted in subsequent mass balance calculation.
The 27 non-lanthanide cations were measured by external calibration ICP-MS, and the results were shown in Table 3. The total non-rare earth impurity concentrations of raw lanthanide oxides were from 0.00101 to 0.00136 g g−1. The standard uncertainty of each impurity included standard deviation of ICP-MS measurement, uncertainty of calibrant preparation, and uncertainty from standard curve linearity. And the total standard uncertainties of Dy, Ho, Er, Tm, and Sc were calculated from the standard uncertainty of each impurity.
Optimization of LA/MD-ICP-MS conditions
LA-ICP-MS, sample introduction to ICP-MS with dry aerosols, can largely reduce the interferences from polyatomic ions injected by solution. For example, the interference of 164DyH+ for 165Ho, 156DyO+ for 172Yb, and 168ErH+ for 169Tm could be significantly reduced. Therefore, LA-ICP-MS is much more suitable for lanthanide impurity analysis of lanthanide oxides. However, because of the shortage of matrix-matched lanthanide oxide CRMs for LA-ICP-MS, accurate quantification of impurities in lanthanide oxide is particularly challenging. Membrane desolvator preserves the high sample transport efficiency of the ultrasonic nebulizer and greatly reduces solvent loading into the ICP, thus alleviating solvent interferences. In this study, since the rare earth impurities were very important in mass balance subtraction, two approaches LA-ICP-MS and MD-ICP-MS were employed for rare earth impurity measurement. The rare earth impurity result by LA-ICP-MS was obtained from signal normalization with sensitivity factor correction.
To obtain satisfying results using LA-ICP-MS, a careful optimization of the measurement parameters is required (Table 1). Two crucial parameters, laser energy and scan speed, were considered in this experiment. In the bulk analysis by LA-ICP-MS, a higher laser energy can produce effective ample ablation and decrease the transformation time from solid particles to aerosols. In this work, the calculation of lanthanide impurities was deduced by total ion normalization, and the signals of lanthanide matrix need to be acquired. However, since the purity of lanthanide oxide sample is almost 99.9~99.99%, a much higher laser energy can make the signals of lanthanide matrix exceed the threshold of ICP-MS detector. Because 45Sc, 165Ho, and 169Tm are mono-isotope elements that can generate much higher signals, the laser energy of Sc, Ho, and Tm oxides was optimized to be 70% output and 100% output for Dy and Er. For an optimal compromise between signal sensitivity and stability, the scan speed employed is also a critical parameter. The fast scan speed may produce much more debris that deteriorate the signal stability. In this experiment, considering the signal sensitivity and precision, the scan speeds of 30, 40, and 50 μm s−1 were investigated and 40 μm s−1 seems to be the most appropriate one.
In the LA-ICP-MS experiment, because there was no matrix-matched lanthanide oxide CRMs, the lanthanide impurities were quantified by total signal normalization method with Eqs. 1 and 2.
where the indices x refer to the element of measured impurity, and M refers to the element of lanthanide matrix. The parameter C refers to the concentration of impurity, SF refers to the sensitivity factor of impurity element, I refers to the signal intensity of element, and b refers to isotope abundance of the measured element.
The results of SF of 14 lanthanide elements were shown in Fig. 2. Figure 2 showed that the SF of La was the highest, which was almost 3.6 times higher than that of the lowest Er. On the other hand, no obvious differences of SF were observed with the variation of impurity concentration. Therefore, the average of SF with different concentrations was used for impurity calculation in Eqs. 1 and 2.
Sensitivity factor of 14 lanthanide impurities. The calibrants in x-axis lanthanide were prepared by doping high-purity lanthanide oxides in boric acid with different proportions. The SF of each element in y-axis was calculated with Eq. 1
The limit of detection (LOD) and limit of quantification (LOQ) of each lanthanide (in Table 4) were also investigated to check if LA/MD-ICP-MS was sensitive enough to measure the impurities with very low concentrations.
In Table 4, the LODs and LOQs of MD-ICP-MS were slightly better than those of LA-ICP-MS because of enhanced analyte transport efficiency and reduced solvent loading to plasma. Even so, the LODs and LOQs of both the two techniques were within an order of magnitude, which can be used for determination of lanthanide impurities in high-purity lanthanide oxides.
Results of rare earth impurities by LA/MD-ICP-MS
In this work, two approaches were carried out to measure the 14 lanthanide impurities. The concentrations of all lanthanide impurities were higher than the LOD and LOQ of LA/MD-ICP-MS. Since the values from LA-ICP-MS were equivalent to those of MD-ICP-MS within the range of 50% of the average concentration, the average results of each impurity from the two methods were used as the impurity concentration. The standard uncertainty (u) of each impurity was evaluated to be 50% of the concentration [24]. Each rare earth impurity and the total impurity concentrations of Dy2O3, Ho2O3, Er2O3, Tm2O3, and Sc2O3 were shown in Table 5.
Purity results of Dy2O3, Ho2O3, Er2O3, Tm2O3, and Sc2O3
The purity of lanthanide oxides was acquired by subtraction non-lanthanide and lanthanide impurities from total metal cation titration results. A total of 42 impurities were considered, and the purity results were shown in Table 6. From Table 6, the concentrations of Dy, Ho, Er, Tm, and Sc in their oxide raw materials were 0.86555 ± 0.00098 g g−1, 0.86429 ± 0.00104 g g−1, 0.87132 ± 0.00124 g g−1, 0.86489 ± 0.00160 g g−1, and 0.64315 ± 0.00090 g g−1 (k = 2.0).
Results of value verification
ICP-MS has the advantages of high sensitivity and low background for rare earth measurement. Although the existence of oxide and hydride can produce polyatomic interferences, the signals of the matrix lanthanide element were much higher than those of interferences from impurities. Therefore, the values of candidate CRMs could be verified by ICP-MS with the NIST SRMs as calibrants. The verification results of Dy, Ho, Er, Tm, and Sc candidate CRMs were shown in Fig. 3 and were in good agreement with the certified values.
Homogeneity and stability results of candidate Dy, Ho, Er, Tm, and Sc CRMs
From the homogeneity test results (Table 7), no statistically significant heterogeneity was found. One-way ANOVA showed that the F test values are from 0.85 to 1.62 (relative standard deviation of 0.2–0.3%, respectively). All of them were smaller than the Fcritical value (2.42 at the 95% confidence interval with 14 degrees of freedom). These results indicate that the candidate lanthanide solution CRMs were homogeneous enough. The uncertainty of the sample inhomogeneity (ubb) was calculated using Eq. (3) [20] and listed in Table 7, which were from 0.7 to 1.2 mg L−1.
where CVb and CVw refer to the within variance and between variance of units that were derived from duplicate analysis of the 28 samples.
The long-term stability of candidate CRMs was evaluated for 24 months at room temperature. The stability was assessed on the basis of Student’s t test, and the uncertainty of long-term stability (us) is calculated using the following expression [25]: us = s(b1) · t. Results of us of the candidate CRMs are shown in Table 7, which were between 1.5 and 1.7 mg L−1. The long-term stability would be further monitored at regular intervals.
Certified values and uncertainty evaluation
After purity characterization, the solution CRMs were prepared with gravimetric method. Uncertainties of the candidate CRMs were calculated according to a modification GUM [25] and the recommendations given in ISO Guide 35:2006, using the equation:
where U is the expanded uncertainty of concentration of candidate CRMs, k is the coverage factor, and uCRM is the combined uncertainty. We used a coverage factor of 2, to provide a 95% confidence interval. The combined uncertainty uCRM was calculated as:
where ubb is the uncertainty associated with the between-bottle inhomogeneity; us is the uncertainty associated with the instability; uchar is the uncertainty associated with the gravimetric process, which includes the uncertainty from purity characterization, weighing of lanthanide raw material and solution, and density measurement. The certified values and uncertainties of Dy, Ho, Er, Tm, and Sc are shown in Table 8.
Conclusion
In this study, the development of Dy, Ho, Er, Tm, and Sc solution CRMs was presented. The certified values of the CRMs were given by gravimetric method after purity characterization of raw lanthanide oxide material. Complexation titration, ICP-MS, and LA/MD-ICP-MS were used in the purity characterization process. The CRMs were proven to be sufficiently homogeneous and stable for all the elements. The values of the CRMs were also verified by NIST lanthanide SRMs and good agreements were obtained. The relative expanded uncertainty of the candidate CRMs was estimated by combining uncertainty contributions including sample preparation, between-bottle homogeneity, and stability, and found to be 0.5%. These CRMs are primarily intended for use in the measurement and calibration procedures of lanthanide analysis in environmental and geological areas. Most importantly, the purity characterization strategy of this study will provide a new idea for the certification of high-purity and mono-element solution reference materials.
References
Wang K. Trace elements in life science. Beijing: China Metrology Publishing House; 1991.
Guo BS, Zhu WM, Xiong BK, Ji YJ, Liu Z, Wu ZM. Rare earths in agriculture. Beijing: China Agricultural Science and Technology Press; 1990.
Danilov NA, Krylov YS, Korpusov GV, Kostikova GV. Scintillators based on ytterbium chloride adducts with neutral organophosphorus extractants for detecting solar neutrino for low-energy neutrino spectroscopy experiment. Radiochemistry. 2003;45:128–33.
Sarkar SK, Rycyna RE, Lenkinski RE, Solleveld HA, Kinter LB. Yb-DTPA, a novel contrast agent in magnetic resonance imaging: application to rat kidney. Magn Reson Med. 1991;17:328–35.
Bomke S, Sperling M, Karst U. Organometallic derivatizing agents in bioanalysis. Anal Bioanal Chem. 2010;397:3483–94.
Tholey A, Schaumloffel D. Metal labeling for quantitative protein and proteome analysis using inductively-coupled plasma mass spectrometry. Trends Anal Chem. 2010;29:399–408.
Jörg B. Application of isotope dilution ICP–MS techniques to quantitative proteomics. Anal Bioanal Chem. 2010;397:3495–502.
Orvini E, Speziali M, Salvini A, Herborg C. Rare earth elements determination in environmental matrices by INAA. Microchem J. 2000;67:97–104.
Perna L, Bocci F, Aldave de las Heras L, De Pablo J, Betti M. Studies on simultaneous separation and determination of lanthanides and actinides by ion chromatography inductively coupled plasma mass spectrometry combined with isotope dilution mass spectrometry. J Anal At Spectrom. 2002;17:1166–71.
Parsons JG, Peralta-Videa JR, Tiemann KJ, Saupe GB, Gardea-Torresdey JL. Use of chemical modification and spectroscopic techniques to determine the binding and coordination of gadolinium(III) and neodymium(III) ions by alfalfa biomass. Talanta. 2005;67:34–45.
Galunin E, Alba MD, Santos MJ, Abrão T, Vidal M. Lanthanide sorption on smectitic clays in presence of cement leachates. Geochim Cosmochim Acta. 2010;74:862–75.
International Organization for Standardization. ISO/IEC 17025: general requirements for the competence of testing and calibration laboratories. 2nd ed. Geneva: International Organization for Standardization; 2005.
Li YQ, Wang DD. Development of rare earth solution CRMs. Acta Metall Sin. 2003;4:348–51.
Carter AK, Dussubieux L. Geologic provenience analysis of agate and carnelian beads using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS): a case study from Iron Age Cambodia and Thailand. J Archaeol Sci Rep. 2016;6:332–41.
Feng LX, Zhang D, Wang J, Shen DR, Li HM. A novel quantification strategy of transferrin and albumin in human serum by species-unspecific isotope dilution laser ablation inductively coupled plasma mass spectrometry (ICPMS). Anal Chim Acta. 2015;884:19–25.
Zhou H, Wang Z, Zhu Y. Quantitative determination of trace metals in high-purity silicon carbide powder by laser ablation inductively coupled plasma mass spectrometry without binders. Spectrochim Acta B At Spectrosc. 2013;90:55–60.
Limbeck A, Galler P, Bonta M, Bauer G, Nischkauer W, Vanhaecke F. Recent advances in quantitative LA-ICP-MS analysis: challenges and solutions in the life sciences and environmental chemistry. Anal Bioanal Chem. 2015;407:6593–617.
Han GJ, Wu X, Tong J. Determination of 14 trace rare earth impurities in high-purity CeO2 by inductively coupled plasma mass spectrometry with membrane desolvation. Journal of The Chinese Rare Earth Society. 2009;28:137–44.
Wu B, Chao JB. Assay of arsenic trioxide by high-precision coulometric titrimetry. Modern Scientific Instruments. 2009;3:69–72.
International Organization for Standardization. ISO guide 35: reference materials-general and statistical principles for certification. Geneva: International Organization for Standardization; 2006.
EURACHEM. Quantifying uncertainty in analytical measurement. Teddington: LGC; 1995.
Chemistry division of Hangzhou University. Handbook of Analytical Chemistry, 1st edition. Beijing:Chemical Industry Publishing House; 1980.
Meng F, Shi L. Rapid titrimetric determination of total rare earths contents in Pb-RE master alloy. Metall Anal. 2000;20:50–1.
Kipphardt H, Matschat R, Rienitz O, Schiel D, Gernand W, Oeter D. Traceability system for elemental analysis. Accred Qual Assur. 2006;10:633–9.
International Organization for Standardization. Evaluation of measurement data—guide to the expression of uncertainty in measurement. Geneva: ISO; 2008.
Funding
The authors received financial support from “National Key Research and Development Program” (No. 2017YFF0205402) and “National Natural Science Foundation” (No. 11475163).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with humans or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, L., Wu, B., Li, H. et al. Development of Dy, Ho, Er, Tm, and Sc mono-element standard solution certified reference materials (GBW08680-08684). Anal Bioanal Chem 411, 1365–1374 (2019). https://doi.org/10.1007/s00216-018-01567-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-01567-3