Abstract
The obesity epidemic among children has become a major public health issue, and the presence of childhood insulin resistance (IR) has been demonstrated prior to the onset of type 2 diabetes mellitus. However, it is unclear whether the metabolomic signature is associated with weight loss interventions in obese children with IR. Thirty-six obese children with IR were selected from the weight loss camp (Shenzhen Sunshine Xing Yada health Technology Co., LTD). Clinical parameters were collected before and after weight loss intervention. Targeted metabolomics of plasma samples was performed by ultra-performance liquid chromatography coupled to the tandem mass spectrometry, and principal component analysis, variable importance in projection, and orthogonal partial least squares discriminant analysis were used to obtain the differentially expressed metabolites. Pathway analysis was conducted with the Homo sapiens (HSA) sets in the Kyoto Encyclopedia of Genes and Genomes. We used machine learning algorithms to obtain the potential biomarkers and Spearman correlation analysis to clarify the association between potential biomarkers and clinical parameters. We found that clinical parameters and metabolite clusters were significantly changed in obese children with IR before and after weight loss intervention. Mechanistically, weight loss intervention significantly changed 61 metabolites in obese children with IR. Furthermore, 12 pathways were significantly changed. Moreover, the machine learning algorithm found 6 important potential biomarkers. In addition, these potential biomarkers were strongly associated with major clinical parameters. These data indicate different metabolomic profiles in obese children with IR after weight loss intervention, providing insights into the clinical parameters and metabolite mechanisms involved in weight loss programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The obesity epidemic among children and adolescents continues to worsen and remains a major public health issue in China (Pan et al. 2021) and around the world (Lister et al. 2023). Obesity in childhood correlates with a higher likelihood of encountering cardiovascular issues, like hypertension, ongoing inflammation, and insulin resistance. These factors can potentially lead to the progression of diabetes and heart diseases as one grows into adulthood (Bleich et al. 2018; McPhee et al. 2020).
IR is characterized as a state of decreased responsiveness of insulin-targeting tissues to high physiological insulin levels and is considered the pathogenic driver of numerous cardiovascular diseases, nonalcoholic fatty liver disease (NAFLD), and type 2 diabetes mellitus (T2DM) (Lee et al. 2022). T2DM can cause chronic kidney disease, retinopathy, lower limb amputation, myocardial infarction, stroke and other complications. IR observed in the pediatric population is a significant concern due to the established link between the early manifestation of this metabolic condition and a heightened susceptibility to microvascular complications (Huang et al. 2022).
The best approach to the prevention and treatment of IR and T2DM is lifestyle alteration. Numerous studies suggest that exercise can effectively reduce IR in obese children(Liu et al. 2020), (Elisa et al. 2016). Aerobic exercise could decrease the level of circulating branch-chain amino acids (BCAAs), which is the best metabolic predictor of improvement in insulin sensitivity in overweight humans (Glynn et al. 2015). Moreover, emerging studies have demonstrated that the plasma metabolome profile changes with resistance training and endurance training, revealing the exercise-induced changes in metabolites that may help explain the underlying mechanisms of physical exercise on metabolic health (Mardinoglu et al. 2018), (Grapov et al. 2019), (Short et al. 2019). In addition, in our previous study we found that weight loss intervention improved cardiometabolic health in children with metabolic syndrome (Liu et al. 2021). However, few studies have explored the effects of weight loss interventions on metabolites in obese children with IR. Thus, the aim of this study is to determine the metabolite changes in obese children with IR and to screen potential biomarkers for IR obesity phenotypes. On this basis, we explored the effects of weight loss intervention on the metabolic profile and the potential metabolic mechanism in obese children with IR.
Materials and methods
Experimental design and participant inclusion criteria
The research was designed as a comparative quasi-experiment. Children with obesity were defined as having a body mass index (BMI) that corresponded to the Chinese criteria (WS/T 586—2018). This standard was drafted by Peking University Institute of Child and Adolescent Health, China Center for Disease Control and Prevention, Institute of Nutrition and Health, Maternal and Child Health Center of the Chinese Center for Disease Control and Prevention (Supplementary Table 1). Obese participants (18 boys and 18 girls) aged 10–13 years were selected from the weight loss camp (Shenzhen, China) from June to September 2019. All participants and their parents signed informed consent forms. They can discontinue from this camp if they want. Besides, this study was approved by the Ethical Committee of the Guangzhou Sport University (No. 2018LCLL-008).
Basic clinical data were collected from all obese children, including height, body weight, gender, age and comorbidities (heart disease, kidney disease and hepatitis B, chronic illnesses). All participants were diagnosed without any of the above diseases and had no history of medication. There were thirty-six obese children diagnosed with IR. Obese children with a homeostasis model assessment insulin resistance (HOMA-IR) threshold of > 2.6 were identified in the IR group(Feroe et al. 2016),(Burrows et al. 2015). HOMA-IR was calculated using the following formula: \(\:HOMA-IR=\frac{FINs(\mu\:U/L)\ast\:FPG(mmol/L)}{22.5}\) (Liu et al. 2021).
During the 30-day extreme weight loss exercise intervention in weight loss camp, all subjects received the standard exercise combined with the diet control program. All subjects participated in two training sessions per day. Each daily training session began with a 30-minute general warm-up, followed by 80 min of exercise training at a maximal heart rate (HRmax) of 50-80%, and concluded with 10 min of relaxation. Dietary control for each group was designed based on the resting metabolic rates (RMR). Before the intervention, each participant’s RMR was measured by indirect calorimetry (Supplementary Table 2). The details of the intervention were described in a previous study (Liu et al. 2021).
To investigate the metabolomics characteristics of obese children with IR, we profiled plasma samples from the IR groups before and after weight loss intervention program (Fig. 1).
Clinical parameter measurements
Before eating in the morning, every participant’s height, weight, chest circumference (Chest C), waist circumference (WC), hip circumference (Hip C), waist to hip rate (WHR), thigh circumference (Thigh C) and calf circumference (Calf C) were measured. We used the body composition analyzer (T-SCAN PLUS, Korea) to measure body composition, including fat-free mass (FFM), skeletal muscle mass (SMM), body fat percentage (BFP), and fat mass (FM).
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by an electronic blood pressure monitor (OMRON HEM-1020, China). The levels of triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) in blood were analyzed by enzyme assays. Fasting plasma glucose (FPG) levels were determined using the glucose oxidase method (YeaSen, Cat: 60408ES60), while fasting insulin (FINS) levels were assessed through an enzyme-linked immunosorbent assay (Beyotime, Cat: PI608).
Targeted metabolomics profiling
The fasting plasma samples were collected via the antecubital vein with heparin sodium as an anticoagulant at the end of a 30-day extreme weight loss exercise program.
The plasma samples were allowed to stand for 30 min before being centrifuged at 4 °C for 10 min at 3000 g. Following centrifugation, the samples were rapidly frozen in liquid nitrogen and subsequently stored at − 80 °C. The targeted metabolomics of plasma samples was performed by Metabo-Profile (Shanghai, China). The sample preparation procedures were performed according to previously published methods with minor modifications (Xie et al. 2021). Briefly, 20 µL of plasma was mixed with 120 µL of methanol containing an internal standard (Supplementary Table 3) using a vortex, then centrifuged to extract the metabolites. All internal standards were commercially purchased as previous published methods.
Thirty microliters of supernatant was subjected to derivatization with 3-nitrophenylhydrazine (3-NPH) and N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide (EDC)·HCl (Sigma‒Aldrich, St. Louis, MO, USA). Subsequently, the derivatized samples were analyzed by ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC‒MS/MS) (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA, USA). All of the standards were obtained from Sigma‒Aldrich (St. Louis, MO, USA), Steraloids Inc. (Newport, RI, USA) and TRC Chemicals (Toronto, ON, Canada). The raw data files generated by UPLC‒MS/MS were processed using Targeted Metabolome Batch Quantification (TMBQ) software (v1.0, HMI, Shenzhen, Guangdong, China) to perform peak integration, calibration, and quantitation for each metabolite. The self-developed platform iMAP (v1.0, Metabo-Profile, Shanghai, China) was used for statistical analysis.
Quantification and statistical analysis
We used Shanghai Metabo-Profile (self-developed platform iMAP v1.0) for data analysis. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were also performed. Variable importance in projection (VIP) was obtained based on the OPLS-DA model. Differentially expressed metabolites (DEMs) were regarded as statistically significant when the VIP of the metabolite was > 1 and the p value was < 0.05.
Finally, pathway analysis was conducted with the Homo sapiens (HSA) sets by the Kyoto Encyclopedia of Genes and Genomes (KEGG). Support vector machine (SVM), random forest (RF), and logistic regression were used to establish a diagnostic model. Statistical algorithms were adapted from R studio’s widely used statistical analysis software packages (http://cran.r-project.org/).
Results
Effects of weight loss on the clinical characteristics of obese children
Multiple previous studies have shown that lifestyle modification can effectively improve physical condition. In this study, obese children with or without IR participated in 4-week lifestyle modification interventions. Body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), resting energy expenditure (REE), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FPG), fasting insulin (FINS), total cholesterol (TC), Triglycerides (TGs), homeostasis model assessment of insulin resistance (HOMA-IR) and HOMA insulin sensitivity (HOMA-IS), body weight (BW), chest circumference (Chest C), waist circumference (WC), hip circumference (Hip C), waist to hip ratio (WHR), thigh circumference (Thigh C), calf circumference (Calf C), fat mass (FM), fat-free mass (FFM), skeletal muscle mass (SMM), body fat percentage (BFP) were significantly decreased in children with IR after lifestyle modification (Fig. 2) (Table 1). No difference was observed in resting O2 and resting CO2 between before and after weight loss intervention (Table 1).
Plasma metabolomics patterns of IR children
To further explore the effects and mechanism of lifestyle modification on obese children with IR, Q300 metabolomics was used in this study. Our metabolomics results showed that the serum profile of IR children exhibits a wide range of deviations in plasma metabolite levels.
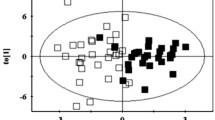
The differences between IR children before and after lifestyle modification were best described by orthogonal partial least squares discriminant analysis (OPLS-DA), with an R2 value of 0.896 and a Q2 value of 0.786 (Fig. 3A, B). Based on the OPLS-DA model results, a volcano plot was used to select the most important potential metabolites (Fig. 3C). Given that VIP > 1.0 and p < 0.05, 61 metabolites were identified (Supplementary Table 4). In addition, univariate statistical analysis (Student’s t test or Mann‒Whitney U test) was also used in IR children before and after lifestyle modification to identify significantly changed metabolites. We found that 36 metabolites were significantly increased and 59 metabolites were decreased in IR obese children after weight loss (Fig. 3D).
Plasma metabolome profiles of the obesity children with IR before and after weight loss intervention. (A) Principal component analysis (PCA) score plots; (B) Orthogonal projection to latent structures discriminant analysis (OPLS-DA) cross validation plot; (C) Volcano plots of OPLS-DA; (D) Volcano plots of univariate analysis
Furthermore, to identify potential metabolites that may play a critical role in weight loss of children with IR, intersection analysis of the differential metabolites from univariate statistics and multidimensional statistics was performed. VIP > 1 in multidimensional statistics and p < 0.05 and |log2FC| ≥ 0 in univariate statistics were used as the threshold values for potential metabolites selection in this analysis. There were 61 metabolites that matched the threshold value, as shown in Fig. 4; Table 2.
Pathways related to weight loss in children with IR
To understand the potential mechanism in children with IR obesity before and after weight loss, KEGG was used to analyze related metabolites. Of the 61 potential metabolites, 12 pathways (-ln(p) > 5) were significantly changed. The results of each of the 12 KEGG pathways were plotted to show the importance of the pathways (Fig. 5). The top 6 significant pathways were alanine, aspartate and glutamate metabolism; citrate cycle (TCA cycle); glycine, serine and threonine metabolism; aminoacyl-tRNA biosynthesis; and glyoxylate and dicarboxylate metabolism, which are involved in the process of IR.
Pathway analysis plot by the HSA set in KEGG. The first lap displays significantly 17 KEGG terms (FDR < 0.1) and the numbers of the metabolites corresponds to the outer lap. The second lap displays the number of the metabolites in the genome background and P values for enrichment of the differentially expressed metabolites. The third lap displays the ratio of the upregulated metabolites (deep purple) and downregulated metabolites (light purple). The fourth lap displays the enrichment factor of each KEGG term
Potential biomarkers
To reveal the potential biomarkers of weight loss in IR obese children, random forest (RF), support vector machine (SVM) and Boruta analyses were performed. Intersection analysis of the top 10 important metabolites of RF, SVM and Boruta analysis was performed. We obtained 6 important potential biomarkers, namely, citric acid, aconitic acid, tyrosine, serine, methylcysteine and glycine (Figs. 6 and 7).
Potential marker from prediction and diagnosis model. (A) Data results of feature selection by Boruta analysis; (B) Importance scores of top 10 important differential metabolites by random forest (RF); (C) Importance scores of top 10 important differential metabolites by support vector machine (SVM); (D) Venn plot of differential important metabolites
Changes in 6 most important potential biomarkers concentrations in obese children with IR following weight loss intervention. (A) Citric acid; (B) Aconitic acid; (C) Tyrosine; (D) Glycine; (E) Methylcysteine; (F) Serine. Data are presented as mean ± standard deviation. *p < 0.05 between corresponding groups. **p < 0.01 between corresponding groups
Associations of metabolites with major clinical parameters
Spearman correlation analysis revealed that metabolites were correlated with a range of clinical parameters. For example, serine, methylcysteine, glycine, aconitic acid and citric acid were mostly positively associated with HOMA-IR but inversely associated with HOMA-IS, BMI, body fat mass (BFM), BW, LDL-C, TGs, TC, diastolic pressure (DP), systolic pressure (SP) and REE. Tyrosine was mainly negatively associated with HOMA-IR and positively associated with HOMA-IS, BMI, BFM, fat percentage (PBF), BW, LDL-C, TGs, TC, DP, SP, WHR and REE (Fig. 8).
Discussion
In the current study, we outlined the metabolite features of obese IR children after weight loss. Moreover, we further demonstrated that serine, methylcysteine, glycine, aconitic acid, tyrosine and citric acid were the most significantly changed metabolites in the process of weight loss. Additionally, we demonstrated the association between 6 important metabolites and major clinical parameters.
Numerous studies have suggested that lifestyle modification is associated with variable effects on weight loss and body health (Wadden et al. 2020), (Smith et al. 2020) (Kushner and Ryan 2014) (Kushner 2018). In a previous study, we found that 4 weeks of weight loss intervention could reduce body weight and contributed to cardiometabolic health in children with metabolic syndrome (Liu et al. 2021). Consistent with these findings, in this study, we found that 4 weeks of weight loss intervention could effectively decrease body weight, BMI, HOMA-IR and body circumference in obese IR children (Fig. 2). These findings suggest that weight loss intervention is an effective method for weight loss in obese children, including those with IR and metabolic syndrome.
The significant alteration of the metabolome that occurs in obesity is related to wellbeing, and profiling can be used to identify clinically significant heterogeneity (Cirulli et al. 2019). Consistent with this viewpoint, our metabolome data showed that there was profound change of the metabolome in IR children before and after lifestyle modification (Fig. 3). In addition, univariate statistics and multidimensional statistics were used to obtain 61 important potential metabolites (Fig. 4). Based on these metabolites, 12 related pathways were enriched in children with IR obesity before and after weight loss invention (Fig. 5). Alanine, aspartate and glutamate metabolism plays an important role in the pathogenesis of metabolic syndrome (Sookoian and Pirola 2012). Consistent with this finding, metabolism of alanine, aspartate, and glutamate was also significantly changed in obesity children with IR following the intervention (Fig. 5). The TCA cycle is the foremost critical metabolic pathway that supplies energy to the body and is the ultimate common oxidative pathway for carbohydrates, fats and amino acids. The TCA cycle pathway has been associated with obesity, prediabetes and IR (Zhang et al. 2021) (Adams et al. 2009) (Muoio and Newgard 2008). Guasch et al.(Guasch-Ferré et al. 2020) found that TCA cycle-related metabolites (such as aconitate, citrate, isocitrate and malate) were significantly connected with type 2 diabetes risk in a population consuming the mediterranean diet. In line with these findings, our results revealed that the TCA cycle pathway was significantly changed in children with IR obesity before and after weight loss intervention (Fig. 5). Glycine metabolism is an important pathway involved in weight loss. Glycine concentrations in serum was increased after which loss(Adeva-Andany et al. 2018). As expected, our pathways analyses confirmed this concept. In addition, we found that glyoxylate and dicarboxylate metabolism and aminoacyl-tRNA biosynthesis were all involved in the weight loss process of children with IR obesity after lifestyle modification (Fig. 5). OuYang et al. (OuYang et al. 2020) found that high glucose intake altered glyoxylate and dicarboxylate metabolism in a prediabetes rat model. Lind et al. (Lind et al. 2022) found that aminoacyl-tRNA biosynthesis was related to insulin sensitivity and was a pathway that was highlighted between subjects with normal weight and obesity. These interesting results all support our findings that the above metabolic pathways are involved in the weight loss process of children with IR obesity.
To obtain the most important metabolites, RF, SVM and Boruta analyses were performed. Citric acid, aconitic acid, tyrosine, serine, methylcysteine and glycine were obtained in this analysis. Citric acid is an intermediate in the citric acid cycle, and due to its antioxidant properties, it is widely used as an excipient in pharmaceutical preparations. Muroyama et al. (Muroyama et al. 2003) found that a 12-week intervention with daily intake of thiamin, arginine, caffeine and citric acid significantly decreased serum TGs, abdominal visceral fat and percent body fat in healthy subjects with a high percentage body fat (> 25.0%). Zou et al. (Zou et al. 2020) found that the concentrations of citrate, aconitic acid and α-ketoglutarate were lower in skeletal muscle from severely obese women with type 2 diabetes than in lean nondiabetic subjects’ skeletal muscle. Similar with these results, we found that the concentrations of citrate and aconitic acid were significantly decreased in obese IR children after the weight loss program (Fig. 6). These results suggested that the lifestyle modification had good effects.
Serine is a nonessential amino acid but plays an important role in the metabolic processes that burn glucose and fatty acids for energy (Gao et al. 2018). Obese participants showed lower serine levels than those of lean controls (Fridman et al. 2021). Aging mice treated with serine had significantly decreased concentrations of IL-6, IL-1β and leptin in the serum compared with control mice (Zhou et al. 2018). In addition, serine is essential for the production of glycine (He et al. 2023), which is an important amino acid in cardiometabolic diseases. A deficiency in glycine intensifies the progression of obesity, hypercholesterolemia, hyperglycemia, and atherosclerosis. Conversely, glycine supplementation significantly ameliorates glycemic regulation, dyslipidemia, cardiovascular function, and hepatic steatosis (Rom et al. 2018). Plasma glycine levels are lower in IR individuals than in healthy individuals. However, after exercise, weight loss and metformin treatment, IR was improved, and the glycine concentration was increased (Adeva-Andany et al. 2018). Consistent with these results, we found that the concentrations of serine and glycine were significantly increased after weight loss intervention in obese children with IR (Fig. 7).
Methylcysteine is a bioactive substance found in garlic and human blood. Castro et al. found that diabetic rats treated with methylcysteine had significantly decreased blood glucose and NF-ΚB levels (Castro et al. 2021). In this study, we found that methylcysteine was significantly increased and was associated with HOMA-IR and HOMA-IS in children with IR obesity after weight loss intervention (Figs. 7 and 8). These findings are consistent with a previous report that the levels of metabolites related to S-methylcysteine were significantly increased after weight loss intervention in children and adolescents with severe obesity (Sohn et al. 2022).
Tyrosine is another important metabolite that is postive associated with HOMA before and after one year of lifestyle changes in obese children (Kretowski et al. 2016) (Hellmuth et al. 2016). Furthermore, Mohorko et al. (Mohorko et al. 2015) found that tyrosine had significant strong associations with BMI and waist circumference and was significantly higher in young adults with metabolic syndrome than in healthy young adults. Consistent with these reports, our results showed that tyrosine was significantly decreased and was associated with BMI and HOMA-IS in obese children with IR after weight loss intervention (Figs. 7 and 8).
While the present study provides valuable insights into the metabolomic responses to weight loss interventions in obese children with insulin resistance, several limitations must be considered when interpreting our findings. Firstly, our sample size, though adequate for the metabolomic analysis, may limit the generalizability of the results to broader populations. The relatively short duration of the intervention may not capture the long-term sustainability of weight loss or the stability of the metabolic changes observed. Additionally, the study’s design did not allow for differentiation between the effects of diet, exercise, or their combination on the metabolomic profiles. The potential influence of genetic predispositions and environmental factors on individual responses to the intervention was not explored, which could be crucial given the known heterogeneity in responses to weight loss strategies. Furthermore, the lack of a control group that did not receive the intervention limits our ability to attribute the observed changes solely to the weight loss program. Lastly, while we employed rigorous analytical methods, the complexity of metabolomic data and the multiple comparisons made increase the likelihood of type I errors. Future studies with larger cohorts, longer follow-up periods, and inclusion of genetic and environmental covariates could help overcome these limitations and provide a more comprehensive understanding of the metabolic changes associated with weight loss in obese children with insulin resistance.
Conclusion
Our findings indicate that there were significantly different metabolomic profiles in obese children with IR after weight loss intervention, providing insights into the clinical parameters and metabolite mechanisms involved in weight loss interventions.
Data availability
No datasets were generated or analysed during the current study.
References
Adams SH, Hoppel CL, Lok KH et al (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139:1073–1081. https://doi.org/10.3945/jn.108.103754
Adeva-Andany M, Souto-Adeva G, Ameneiros-Rodríguez E et al (2018) Insulin resistance and glycine metabolism in humans. Amino Acids 50:11–27. https://doi.org/10.1007/s00726-017-2508-0
Bleich SN, Vercammen KA, Zatz LY et al (2018) Interventions to prevent global childhood overweight and obesity: a systematic review. Lancet Diabetes Endocrinol 6:332–346. https://doi.org/10.1016/S2213-8587(17)30358-3
Burrows R, Correa-Burrows P, Reyes M et al (2015) Healthy Chilean adolescents with HOMA-IR ≥ 2.6 have increased cardiometabolic risk: Association with genetic, biological, and environmental factors. J Diabetes Res 2015(783296). https://doi.org/10.1155/2015/783296
Cirulli ET, Guo L, Leon Swisher C et al (2019) Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab 29:488–500e2. https://doi.org/10.1016/j.cmet.2018.09.022
de Castro VMD, Medeiros KC, de Lemos P et al (2021) S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue Cell 69:101483. https://doi.org/10.1016/j.tice.2020.101483
Elisa CM, Rodrigo SD, Alexandre KGP et al (2016) Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med 93:211–218. https://doi.org/10.1016/j.ypmed.2016.10.020
Feroe AG, Attanasio R, Scinicariello F (2016) Acrolein metabolites, diabetes and insulin resistance. Environ Res 148:1–6. https://doi.org/10.1016/j.envres.2016.03.015
Fridman V, Zarini S, Sillau S et al (2021) Altered plasma serine and 1-deoxydihydroceramide profiles are associated with diabetic neuropathy in type 2 diabetes and obesity. J Diabetes Complicat 35:107852. https://doi.org/10.1016/j.jdiacomp.2021.107852
Gao X, Lee K, Reid MA et al (2018) Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep 22:3507–3520. https://doi.org/10.1016/j.celrep.2018.03.017
Glynn EL, Piner LW, Huffman KM et al (2015) Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 58:2324–2335. https://doi.org/10.1007/s00125-015-3705-6
Grapov D, Fiehn O, Campbell C et al (2019) Exercise plasma metabolomics and xenometabolomics in obese, sedentary, insulin-resistant women: impact of a fitness and weight loss intervention. Am J Physiol Endocrinol Metabolism 317:E999–E1014. https://doi.org/10.1152/ajpendo.00091.2019
Guasch-Ferré M, Santos JL, Martínez-González MA et al (2020) Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, mediterranean diet, and type 2 diabetes. Am J Clin Nutr 111:835–844. https://doi.org/10.1093/ajcn/nqaa016
He L, Ding Y, Zhou X et al (2023) Serine signaling governs metabolic homeostasis and health. Trends Endocrinol Metab 34:361–372. https://doi.org/10.1016/j.tem.2023.03.001
Hellmuth C, Kirchberg FF, Lass N et al (2016) Tyrosine is Associated with insulin resistance in Longitudinal Metabolomic profiling of obese children. J Diabetes Res 2016(2108909). https://doi.org/10.1155/2016/2108909
Huang L, Wu P, Zhang Y et al (2022) Relationship between onset age of type 2 diabetes mellitus and vascular complications based on propensity score matching analysis. J Diabetes Invest 13:1062–1072. https://doi.org/10.1111/jdi.13763
Kretowski A, Ruperez FJ, Ciborowski M (2016) Genomics and metabolomics in obesity and type 2 diabetes. J Diabetes Res 2016:1–2. https://doi.org/10.1155/2016/9415645
Kushner RF (2018) Weight loss strategies for treatment of obesity: Lifestyle management and pharmacotherapy. Prog Cardiovasc Dis 61:246–252. https://doi.org/10.1016/j.pcad.2018.06.001
Kushner RF, Ryan DH (2014) Assessment and lifestyle management of patients with obesity: clinical recommendations from systematic reviews. JAMA 312:943–952. https://doi.org/10.1001/jama.2014.10432
Lee S-H, Park S-Y, Choi CS (2022) Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metabolism J 46:15–37. https://doi.org/10.4093/dmj.2021.0280
Lind L, Salihovic S, Sundström J et al (2022) Metabolic profiling of obesity with and without the metabolic syndrome: a multisample evaluation. J Clin Endocr Metab 107:1337–1345. https://doi.org/2022041907543995000
Lister NB, Baur LA, Felix JF et al (2023) Child and adolescent obesity. Nat Rev Dis Primers 9:24. https://doi.org/10.1038/s41572-023-00435-4
Liu J, Zhu L, Su Y (2020) Comparative effectiveness of high-intensity interval training and moderate-intensity continuous training for cardiometabolic risk factors and cardiorespiratory fitness in childhood obesity: a meta-analysis of randomized controlled trials. Front Physiol 11:214. https://doi.org/10.3389/fphys.2020.00214
Liu J, Zhu L, Liao J, Liu X (2021) Effects of extreme weight loss on cardiometabolic health in children with metabolic syndrome: a metabolomic study. Front Physiol 12:731762. https://doi.org/10.3389/fphys.2021.731762
Mardinoglu A, Gogg S, Lotta LA et al (2018) Elevated plasma levels of 3-hydroxyisobutyric acid are associated with incident type 2 diabetes. EBioMedicine 27:151–155. https://doi.org/10.1016/j.ebiom.2017.12.008
McPhee PG, Singh S, Morrison KM (2020) Childhood obesity and cardiovascular disease risk: working toward solutions. Can J Cardiol 36:1352–1361. https://doi.org/10.1016/j.cjca.2020.06.020
Mohorko N, Petelin A, Jurdana M et al (2015) Elevated serum levels of cysteine and tyrosine: early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. Biomed Res Int 2015:418681. https://doi.org/10.1155/2015/418681
Muoio DM, Newgard CB (2008) Mechanisms of disease:Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9:193–205. https://doi.org/10.1038/nrm2327
Muroyama K, Murosaki S, Yamamoto Y et al (2003) Effects of intake of a mixture of thiamin, arginine, caffeine, and citric acid on adiposity in healthy subjects with high percent body fat. Biosci Biotechnol Biochem 67:2325–2333. https://doi.org/10.1271/bbb.67.2325
OuYang Y, Jin Y, Zhao X et al (2020) Revealing metabolic pathways relevant to prediabetes based on metabolomics profiling analysis. Biochem Biophys Res Commun 533:188–194. https://doi.org/10.1016/j.bbrc.2020.09.016
Pan X-F, Wang L, Pan A (2021) Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol 9:373–392. https://doi.org/10.1016/S2213-8587(21)00045-0
Rom O, Villacorta L, Zhang J et al (2018) Emerging therapeutic potential of glycine in cardiometabolic diseases: dual benefits in lipid and glucose metabolism. Curr Opin Lipidol 29:428–432. https://doi.org/10.1097/MOL.0000000000000543
Short KR, Chadwick JQ, Teague AM et al (2019) Effect of obesity and exercise training on plasma amino acids and amino metabolites in American Indian adolescents. J Clin Endocrinol Metab 104:3249–3261. https://doi.org/2019091011595970200
Smith JD, Fu E, Kobayashi MA (2020) Prevention and management of childhood obesity and its psychological and health comorbidities. Annu Rev Clin Psychol 16:351–378. https://doi.org/10.1146/annurev-clinpsy-100219-060201
Sohn M-J, Chae W, Ko J-S et al (2022) Metabolomic signatures for the effects of weight loss interventions on severe obesity in children and adolescents. Metabolites 12:27. https://doi.org/10.3390/metabo12010027
Sookoian S, Pirola CJ (2012) Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterology: WJG 18:3775–3781. https://doi.org/10.3748/wjg.v18.i29.3775
Wadden TA, Tronieri JS, Butryn ML (2020) Lifestyle modification approaches for the treatment of obesity in adults. Am Psychol 75:235–251. https://doi.org/10.1037/amp0000517
Xie G, Wang L, Chen T et al (2021) A metabolite array technology for precision medicine. Anal Chem 93:5709–5717. https://doi.org/10.1021/acs.analchem.0c04686
Zhang G-F, Jensen MV, Gray SM et al (2021) Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab 33:804–817e5. https://doi.org/10.1016/j.cmet.2020.11.020
Zhou X, Zhang H, He L et al (2018) Long-term l-serine administration reduces food intake and improves oxidative stress and sirt1/NFκB signaling in the hypothalamus of aging mice. Front Endocrinol 9:476. https://doi.org/10.3389/fendo.2018.00476
Zou K, Turner K, Zheng D et al (2020) Impaired glucose partitioning in primary myotubes from severely obese women with type 2 diabetes. Am J Physiol Cell Physiol 319:C1011–C1019. https://doi.org/10.1152/ajpcell.00157.2020
Acknowledgements
This work was supported by the National Office of Philosophy and Social Science of China (No. 23ATY007), (Ministry of Education in China) Project of Humanities and Social Sciences (No. 22YJC890014), General project of Guangdong philosophy and Social Science Foundation (GD21CTY01). We gratefully acknowledge the valuable contributions of THE BIGGEST LOSER in data collection.
Author information
Authors and Affiliations
Contributions
LZ designed research and performed research and revised the manuscript, XL wrote the manuscript, performed research, analyzed data and wrote and revised the manuscript. JL, NZ and QJ performed research and revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by E. Closs.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Zhu, L., Liu, J. et al. Effect of weight loss interventions on metabolomic signatures in obese children with insulin resistance. Amino Acids 56, 54 (2024). https://doi.org/10.1007/s00726-024-03409-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00726-024-03409-2