Abstract
Sepsis-induced fulminant hepatitis (FH) is a fatal syndrome that has a worse prognosis in clinical practice. Hence, seeking effective agents for sepsis-induced FH treatment is urgently needed. Fibroblast growth factors (FGFs) are vital for tissue homeostasis and damage repair in various organs including the liver. Our study aims to investigate the protective effects and potential mechanisms of FGF9 on lipopolysaccharide (LPS)/D-galactosamine (D-Gal)-induced FH in mice. We found that pre-treatment with FGF9 exhibited remarkable hepaprotective effects on liver damage caused by LPS/D-Gal, as manifested by the concomitant decrease in mortality and serum aminotransferase activities, and the attenuation of hepatocellular apoptosis and hepatic histopathological abnormalities in LPS/D-Gal-intoxicated mice. We further found that FGF9 alleviated the infiltration of neutrophils into the liver, and decreased the serum levels of pro‐inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in LPS/D-Gal-challenged mice. These effects can be explained at least in part by the inhibition of NF-κB signaling pathway. Meanwhile, FGF9 enhanced the antioxidative defense system in mice livers by upregulating the expression of NRF-2-related antioxidative enzymes, including glutamate-cysteine ligase catalytic subunit (GCLC), NAD(P)H: quinone oxidoreductase 1 (NQO-1), and heme oxygenase-1 (HO-1). These data indicate that FGF9 represents a promising therapeutic drug for ameliorating sepsis-induced FH via its anti-apoptotic and anti-inflammatory capacities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fulminant hepatitis (FH) is a severe lethal clinical syndrome induced by pathogen infections, the ingestion of alcohol, hepatotoxic drugs, and other uncommon sources (Fyfe et al. 2018; Stravitz and Lee 2019). Large-scale hepatocyte necrosis accompanied by liver dysfunction is the main characteristic of sepsis-induced FH, which leads to severe coagulation disorder, hydroperitoneum, hepatic encephalopathy, multiple organ failure, and ultimately to death (Bunchorntavakul and Reddy 2017). Unfortunately, available therapeutic approaches to combat sepsis-induced FH are very limited. Therefore, the development of novel drugs for fighting against sepsis-induced FH is urgently needed.
In a recent study, fibroblast growth factor 9 (FGF9) derived from activated hepatic stellate cells is identified, which supports the growth and migration of hepatocellular carcinoma cells in a paracrine manner (Seitz et al. 2020). Besides its role as a potential carcinogenic factor in the tumor microenvironment, FGF9 secreted by activated hepatic stellate cells also provides a mitogenic signal to hepatocytes through a paracrine pathway, and thus exerts hepatoprotective effects during acute hepatic injury (Antoine et al. 2007). However, there remains a paucity of evidence that FGF9 could be potentially applied to sepsis-induced FH treatment.
Lipopolysaccharide (LPS) and D-galactosamine (D-Gal)‑induced acute liver injury in mice is frequently used to resemble the clinical conditions of sepsis-induced FH and to assess therapeutic efficacy aimed at sepsis-induced FH (Galanos et al. 1979). Increasing evidence has shown that the pathogenesis of FH induced by LPS/D-Gal involves various cellular and molecular pathways associated with hepatic inflammatory responses, oxidative stress, and apoptotic hepatocellular death (Li et al. 2018b; Lv et al. 2020). The current study aims to investigate whether FGF9 could protect the liver from sepsis-induced FH in LPS/D-Gal-intoxicated mice, mainly focusing on its ability to prevent hepatocellular apoptosis, oxidative damage, and proinflammatory cytokine production.
Materials and methods
Materials
Recombinant human FGF9 was prepared as our previously described procedure (Wang et al. 2017). Silymarin (a hepatoprotective agent), D-Gal, and LPS (Escherichia coli serotype 0111:B4) were obtained from Sigma Chemical Company (St Louis, MO, USA). We used the following primary antibodies: anti-nuclear factor erythroid 2-related factor 2 (NRF-2) and anti-kelch-like ECH-associated protein 1 (KEAP1) were purchased from Proteintech Company (Chicago, IL, USA); anti-heme oxygenase-1 (HO-1), anti-glutamate-cysteine ligase catalytic subunit (GCLC), and anti-NAD(P)H: quinone oxidoreductase 1 (NQO-1) were obtained from Abcam Company (Cambridge, MA, USA); anti-B-cell lymphoma-2 (BCL-2) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); anti-Poly ADP-ribose polymerase (PARP) was provided from Beyotime Biotechnology (Haimen, Jiangsu, China); anti-caspase-3 was from Cell Signaling Technology Inc. (Beverly, MA, USA); anti-Bcl-2-associated X (BAX) was purchased from ENZO Biochem Inc. (Farmingdale, NY, USA); anti-phospho-(Ser32/Ser36)-nuclear factor kappa B inhibitor α (p-IκBα) was obtained from Signalway Company (Pearland, TX, USA); anti-IκBα and anti-nuclear factor kappa B (NF-κB) were from Bioworld Company (Minneapolis, MN, USA).
Animals
C57BL/6J genetic background mice (male, 20–22 g) were purchased from the Beijing Sibeifu Biotechnology Co., Ltd (Beijing, China). All mice were given water and a standard diet ad libitum and housed under monitored conditions (a 12 h dark/12 h light cycle, 22–25 °C, and 45–55% relative humidity). The animals were acclimated to standard rearing conditions for 1 week before the start of experiments.
Experimental procedure
To examine the potential role of FGF9 in LPS/D-Gal-induced liver damage, mice were classified into six groups of eight mice each: control, FGF9 alone, FH model, FH model + FGF9 low-dose, FH model + FGF9 high-dose, and FH model + silymarin. The sepsis-induced FH model was established in mice by a single intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg) dissolved in normal saline, while an equivalent volume of normal saline (10 mL/kg) instead of LPS/D-Gal was injected intraperitoneally into mice in the control and FGF9 alone groups. Using polyethylene glycol (PEG) 400 as a solubilizer, FGF9 or silymarin was dissolved in normal saline. The volume percentage of PEG400 in normal saline is 30% (v/v), and this was used as solvent. One hour before (pre-treatment) and after (post-treatment) LPS/D-Gal administration, three groups of LPS/D-Gal model mice were treated by intraperitoneal injection with 0.5 or 1.5 mg/kg of FGF9, or 100 mg/kg of silymarin, respectively; the remaining LPS/D-Gal-challenged mice received the same amount of vehicle in parallel. Mice in the FGF9 alone group received 1.5 mg/kg of FGF9 twice but not LPS/D-Gal via intraperitoneal injection. In our pilot experiments, there were no obvious treatment-related liver damage signs observed by histopathological and enzyme activity analyses of liver from mice treated with FGF9 (1.5 mg/kg) alone. The mice were euthanized with inhaled isoflurane 6 h after LPS/D-Gal treatment. The blood by orbital bleed was collected for determining liver enzyme activity. The left lateral lobe of the liver was used to detect pathological lesions and in situ apoptosis. Other parts of liver tissues were used for detection of oxidative stress and apoptosis-related protein.
To evaluate whether FGF9 possesses direct anti-inflammatory and antioxidant capacities at early stage of LPS/D-Gal-induced liver damage, an additional set of mice were divided into four groups of 8 mice each: control, FGF9 alone, FH model, and FH model + FGF9 high dose. We treated these mice following the same protocol outlined above except that the mice were sacrificed 1.5 h after LPS/D-Gal treatment. The parameters of the inflammatory and redox state in liver tissues or blood were detected.
To determine whether FGF9 extends the survival of LPS/D-Gal-intoxicated mice, a separated set of mice were assigned to six groups of 16 mice each: control, FGF9 alone, FH model, FH model + FGF9 low-dose, FH model + FGF9 high-dose, and FH model + silymarin. The only difference compared to the above-mentioned protocol was the dosage of D-Gal and LPS, which was set to 700 mg/kg (D-Gal) and 10 μg/kg (LPS). After mice were intraperitoneally administrated with LPS/D-Gal, mice survival was observed every 2 h up to 24 h. Survival analysis was performed using the Kaplan–Meier method.
To further evaluate whether FGF9 could offer substantial clinical benefits once liver injury has already begun to develop, another set of experiment was conducted following the same protocol as initial experiment except that mice were subjected to an intraperitoneal injection of FGF9 (1.5 mg/kg) at 1, 2 or 4 h after LPS/D-Gal challenge, respectively. Serum total bilirubin (T-BIL) levels and enzyme activities of aminotransferases including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured at 6 h after LPS/ D-Gal exposure.
Assessment of liver damage
The changes in T-BIL concentrations and the activities of AST and ALT in mouse serum were evaluated following the manufacturer’s protocols (Nanjing Jiancheng Institute of Biotechnology, Nanjing, Jiangsu, China). The fixed and embedded liver tissues were cut into 5 μm slices, which were subjected to hematoxylin and eosin (H and E) staining following standard procedures. The stained sections were visualized with a DP70 digital camera fixed on a BX51 microscope (Olympus, Tokyo, Japan).
Cytokine measurement
The concentrations of proinflammatory cytokines tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) in serum samples were quantified using commercial kits from NeoBioscience Technology Co., Ltd. (Shenzhen, Guangdong, China) by referring to the detailed protocol.
Myeloperoxidase (MPO) activity assay
Liver tissue homogenates were prepared by adding hexadecyltrimethylammonium bromide dissolved in phosphate-buffered saline after liver tissues were weighed. A colorimetric detection kit (Nanjing Jiancheng Institute of Biotechnology) was used to measure MPO activity in tissue homogenates, which was normalized by the tissue weight of the same sample.
Analysis of oxidative stress parameters
Malondialdehyde (MDA) and glutathione (GSH) levels, and catalase (CAT) activity in liver tissue homogenates were measured as our previously described using commercially available assay kits (Beyotime; Li et al. 2018a). The activity of glutathione peroxidase (GPX) in liver tissue homogenates was detected with a commercial detection kit (Beyotime) following the instruction manual. Briefly, GPX catalyzes the oxidation of GSH by an organic peroxide reagent (t-Bu-OOH) to produce oxidized glutathione (GSSG), which can be reduced by glutathione reductase using nicotinamide adenine dinucleotide phosphate reduced tetrasodium salt (NADPH) as the reducing substrate. By measuring the consumption of NADPH, the activity of GPX is calculated. The activity of GPX is finally expressed as enzyme activity unit (U) per milligram protein.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
A TUNEL peroxidase apoptosis detection kit (Roche Diagnostics, Indianapolis, IN, USA) was used to detect apoptotic hepatocytes. Briefly, dewaxed and hydrated liver tissue slides were digested by protease K (20 μg/mL, Sigma). The slides were subsequently incubated with 3% (v/v) hydrogen peroxide and labeled with biotinylated dUTP and terminal deoxynucleotidyl transferase dissolved in TUNEL reaction mixture. Thereafter, the slides were probed with horseradish peroxidase-coupled streptavidin and stained with 3, 3′-diaminobenzidine (DAB). Apoptotic cells showed brown staining in the nuclei, which were slightly counterstained with hematoxylin. Finally, the slides were visualized under a microscope (Olympus) and photographed at × 200 magnification.
Caspase activity assay
Colorimetric protease assay kits (Beyotime) were used to measure the activities of caspase-9, -8, and -3 in liver tissues based on the manufacturer’s protocols. Briefly, liver tissue homogenates were prepared in cell lysis buffer and centrifuged at 20,000 × g for 15 min. An aliquot of the supernatant (50 μL) containing 150 μg of protein was mixed with 40 μL of reaction buffer and 10 μL of caspase-9 substrate Ac-LEHD-pNA, caspase-8 substrate Ac-IETD-pNA, or caspase-3 substrate Ac-DEVD-pNA. After 1.5 h incubation at 37 °C, a yellow formazan product p-nitroaniline (pNA) was released from the substrates. The optical density of pNA was detected with a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 405 nm. The caspase protease activities were quantified using a pNA standard curve and presented as fold changes relative to control.
Western blotting analysis
A nuclear and cytoplasmic extraction kit (Beyotime) was used for isolating the nuclear and cytosolic fractions from liver tissue in accordance with the product instruction. β-Actin and lamin B1 were regarded as loading controls for the cytoplasmic and nuclear fractions, respectively. In addition, total protein preparation and Western blotting analysis were performed according to our previously reported method (Li et al. 2018a).
Statistical analysis
All the data shown were from 6 or 8 mice samples and expressed as mean ± standard deviation (SD). Statistical analysis was performed using IBM SPSS software (version 19.0, Chicago, IL, USA). Tukey’s post hoc test was conducted to determine the differences between groups if one-way analysis of variance (ANOVA) revealed a statistically significant difference among multiple groups. Survival curves of mice were plotted by the Kaplan–Meier method and statistical significance was determined by a log-rank test. When the p value was less than 0.05, we judged that the differences were statistically significant between groups.
Results
Effect of FGF9 on the survival rate of LPS/D-Gal-intoxicated mice
For survival analysis, all LPS/D-Gal-challenged mice died within 14 h. In contrast, FGF9 or silymarin extended the survival time and improved survival of LPS/D-Gal-intoxicated mice (Fig. 1). Specifically, after 24 h of LPS/D-Gal treatment, the survival rate (%) of mice was 12.50%, 43.75%, or 18.75% (2, 7, or 3 of 16 mice) when mice were subjected to FGF9 (0.5 and 1.5 mg/kg) or silymarin (100 mg/kg) administration, respectively.
FGF9 reduces mortality in mice after LPS/D-Gal challenge. Mice received an intraperitoneal injection of FGF9 (0.5 or 1.5 mg/kg), silymarin (100 mg/kg), or vehicle (equal volume) 1 h before and after exposure to D-Gal (700 mg/kg) and LPS (10 μg/kg). After LPS/D-Gal challenge, the survival rate of mice was recorded at 2 h intervals for 24 h (n = 16 for each group). *p < 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
Effect of FGF9 on liver damage in LPS/D-Gal-intoxicated mice
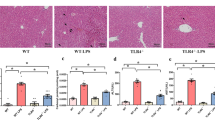
Histopathological changes in the livers of mice after LPS/D-Gal challenge were observed by H and E staining. As shown in Fig. 2A, liver tissues from vehicle or FGF9 (1.5 mg/kg) alone-treated mice exhibited normal-appearing morphology of liver parenchyma with neatly arranged hepatic cords and clear sinusoid architecture. However, LPS/D-Gal-exposed mice showed severe liver damage, including hepatic cord disappearance, inflammatory cell infiltration, narrow sinusoid, hepatocellular necrosis, and hemorrhage. In contrast, these hepatic pathological alterations induced by LPS/D-Gal were significantly attenuated by FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg).
FGF9 alleviates LPS/D-Gal-induced liver damage in mice. A Representative photomicrographs of hematoxylin and eosin-stained mice livers from six groups: control (mice received vehicle twice via intraperitoneal injection with a 2 h interval), FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of saline), FH model (mice received an intraperitoneal injection of vehicle 1 h before and after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg)), LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 0.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), and LPS/D-Gal + silymarin (mice received an intraperitoneal injection of 100 mg/kg of silymarin 1 h before and after intraperitoneal injection of LPS/D-Gal). Arrowheads denote necrotic hepatocytes and arrows denote infiltrated inflammatory cells. Scale bar: 100 μm. B–D At 6 h after LPS/D-Gal challenge, ALT, AST, and T-BIL levels in mouse serum were measured. Values represent mean ± SD for 8 mice. *p< 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
Serum T-BIL, ALT, and AST levels were used to evaluate the extent of liver damage. Compared with those in the vehicle or FGF9 (1.5 mg/kg) alone treatment group, the serum levels of T-BIL, ALT, and AST in the FH model group were markedly elevated (Fig. 2B–D). However, the increase in serum T-BIL, ALT, and AST levels was markedly weakened by pre-treatment with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg).
Effect of FGF9 on hepatic inflammatory responses in LPS/D-Gal-intoxicated mice
Because IL-1β, IL-6, and TNF-α contribute to neutrophil and macrophage infiltration underlying sepsis-induced FH (Ambade et al. 2012), the concentrations of these proinflammatory cytokines in the serum of mice were measured by enzyme-linked immunosorbent assays (ELISA). As shown in Fig. 3A, C , compared with those in the vehicle control mice, serum IL-6 and TNF-α levels in the LPS/D-Gal-challenged mice were remarkably elevated. However, pre-treatment with FGF9 (1.5 mg/kg) led to serum TNF-α and IL-6 levels that were only 62.6% and 34.8% of the levels in LPS/D-Gal-challenged mice. For comparison, FGF9 had no significant impact on the serum level of IL-1β, a critical cytokine in inflammatory processes associated with sepsis-induced FH (Fig. 3B).
FGF9 suppresses hepatic inflammatory responses in mice challenged by LPS/D-Gal. Mice were divided into four groups: control (mice received vehicle twice via intraperitoneal injection with a 2 h interval), FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of saline), FH model [mice received an intraperitoneal injection of vehicle 1 h before and after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg)], and LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal). A–C TNF-α, IL-1β, and IL-6 levels in mouse serum were assayed by ELISA, and D MPO activity in liver homogenates from mice was measured to assess neutrophil infiltration of livers at 1.5 h after LPS/D-Gal challenge. E Representative immunoblot photographs showing expression of NF-κB p65 and lamin B1 in the nuclear fraction, and IκBα, p-IκBα, NF-κB p65, and β-actin in the cytoplasmic fraction from mice liver homogenates. Lamin B1 and β-actin served as nuclear and cytoplasmic fraction controls, respectively. F, G The protein abundance of NF-κB p65 in nuclear extract, and that of IκBα, p-IκBα, and NF-κB p65 in cytoplasmic extract were quantified by densitometry using Image J software, normalized by the value of the corresponding loading reference protein and expressed as fold changes over protein abundance in control mice, which was set as 1.0. Values represent mean ± SD for 8 or 6 mice. *p < 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
Liver neutrophil infiltration, as measured by MPO activity, participates in the amplification of liver damage (Lv et al. 2018). LPS combined with D-Gal treatment resulted in increased MPO activity in mouse livers, suggesting that there was significant infiltration of neutrophils into the liver (Fig. 3D); however, pre-treatment of FGF9 caused a significant decrease in MPO activity (77.2% of the LPS/D-Gal-challenged model group).
Under unstimulated conditions, the inhibitory protein IκB binds to NF-κB in the cytosol, rendering it inactive. Upon inflammatory stimuli, IκB protein is phosphorylated, ubiquitinated, and degraded, allowing NF-κB to translocate into the nucleus where it initiates gene transcription of proinflammatory factors, including the TNF-α and IL-6 genes (Ambade et al. 2012). By Western blotting analysis, we found that LPS combined with D-Gal caused marked phosphorylation and degradation of cytoplasmic IκBα (Fig. 3E, F). As a result, the NF-κB signal was significantly activated, as evidenced by an increase in nuclear NF-κB p65 subunit along with a concomitant decrease in the cytosol (Fig. 3E, G). In contrast, pre-treatment with FGF9 (1.5 mg/kg) notably attenuated LPS/D-Gal-induced IκBα phosphorylation and consequent degradation. After pre-treatment with FGF9, a small amount of NF-κB signal was detected in the nuclear extracts, while a strong signal was observed in the cytoplasmic extracts, suggesting that FGF9 treatment blocked LPS/D-Gal-induced degradation of IκB and retained the NF-κB in the cytoplasm, thereby attenuating the expression of proinflammatory factors.
Effect of FGF9 on hepatocyte apoptosis in LPS/D-Gal-intoxicated mice
To further assess whether FGF9 could protect against liver injury, we applied the TUNEL assay to examine cell apoptosis in mouse livers. Hepatocytes from LPS/D-Gal-intoxicated mice are more vulnerable to apoptosis, as evidenced by the increasing numbers of TUNEL-positive apoptotic hepatocytes (Fig. 4A). However, the hepatocellular apoptosis induced by LPS/D-Gal was markedly relieved in mice pretreated with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg).
FGF9 prevents apoptotic cell death of hepatocytes in mice challenged by LPS/D-Gal. A Representative photomicrographs of TUNEL-stained mouse livers from six groups: control (mice received vehicle twice via intraperitoneal injection with a 2 h interval), FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of saline), FH model [mice received an intraperitoneal injection of vehicle 1 h before and after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg)], LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 0.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), and LPS/D-Gal + silymarin (mice received an intraperitoneal injection of 100 mg/kg of silymarin 1 h before and after intraperitoneal injection of LPS/D-Gal). Arrowheads indicate TUNEL‐labeled positive cells. Scale bar: 100 μm. B The activities of caspase-3, -8, and -9 in liver homogenates from mice were detected using the specific substrates 6 h post-LPS/D-Gal injection. Fold changes in relative caspase activities are presented after normalization with those measured in liver homogenates from control mice. C Representative immunoblot photographs showing expression of BCL-2, BAX, active caspase-3, pro-caspase-3, and cleaved PARP in liver tissue lysates. β-Actin was referred to as an internal control to ensure an equal amount of protein in each lane. D, E The protein abundance of BCL-2, BAX, active caspase-3, pro-caspase-3, and cleaved PARP in liver homogenates from mice were quantified by densitometry using Image J software, normalized by the value of β-actin, and expressed as fold changes over protein abundance in control mice (defined as 1-fold). Values represent mean ± SD for 6 mice. *p < 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
Because the activation of caspase family members suggests initiation of apoptosis, we measured the activities of several key caspases involved in the regulation of apoptotic process. A significant increase in hepatic caspase-3, caspase-9, and caspase-8 activities was found after mice received LPS/D-Gal (Fig. 4B), while the increased activities of these caspases were markedly suppressed when mice were pretreated with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg) prior to LPS/D-Gal challenge. Furthermore, we examined the expression levels of several crucial molecules that regulate hepatocyte apoptosis. As shown in Fig. 4C–E, LPS/D-Gal treatment caused a decrease in the ratio of BCL-2 to Bax protein and up-regulated the expression of cleaved caspase-3 and PARP. Both of these effects were almost completely blocked by the pre-treatment with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg). The above findings suggest that FGF9 possesses strong anti-apoptotic properties in LPS/D-Gal-intoxicated mice.
Effect of FGF9 on hepatic oxidative damage in LPS/D-Gal-intoxicated mice
To evaluate the degree of oxidative damage, MDA, a lipid peroxidation product, whose content in mice livers was measured. As shown in Fig. 5A, compared with vehicle or FGF9 (1.5 mg/kg) alone-treated mice, the MDA content was markedly elevated in liver homogenates from LPS/D-Gal-exposed mice, but the increase was substantially reduced by pre-treatment with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg). Oxidative damage was also evaluated by quantifying the level of non-enzymic antioxidant (GSH) and the activities of antioxidant enzymes (CAT and GPX) in injured mice livers. Figure 5B–D shows a significant decrease in CAT and GPX activities accompanied by a decrease in GSH content in the LPS/D-Gal-intoxicated livers. However, pre-treatment with FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg) activated the antioxidative defense system to reduce LPS/D-Gal-induced oxidative damage.
FGF9 mitigates hepatic oxidative stress in mice challenged by LPS/D-Gal. A–D Mice were divided into six groups: control (mice received vehicle twice via intraperitoneal injection with a 2 h interval), FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of saline), FH model [mice received an intraperitoneal injection of vehicle 1 h before and after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg)], LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 0.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 1 h before and after intraperitoneal injection of LPS/D-Gal), and LPS/D-Gal + silymarin (mice received an intraperitoneal injection of 100 mg/kg of silymarin 1 h before and after intraperitoneal injection of LPS/D-Gal). The contents of MDA and GSH, and activities of CAT and GPX in liver homogenates from mice were measured 6 h after LPS/D-Gal injection. E–H Mice were treated with vehicle or FGF9 (1.5 mg/kg) 1 h before and after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg). After liver tissues were harvested at 1. 5h after LPS/D-Gal administration, the contents of MDA and GSH, and activities of CAT and GPX in liver homogenates from mice were determined. I Representative immunoblot photographs showing expression of NRF-2 and lamin B1 in the nuclear fraction, and KEAP1, NQO-1, GCLC, HO-1, and β-actin in the cytoplasmic fraction of mice liver homogenates. Lamin B1 and β-actin served as nuclear and cytoplasmic fraction controls, respectively. J, K The protein abundance of NRF-2 in nuclear extract, and those of KEAP1, NQO-1, GCLC, and HO-1 in cytoplasmic extract were quantified by densitometry using Image J software, normalized by the value of the corresponding internal reference protein, and expressed as fold changes over protein levels in control mice (defined as onefold). Values represent mean ± SD for 8 or 6 mice. *p < 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
To explore whether FGF9 could directly attenuate LPS/D-Gal-evoked oxidative stress during early stage of liver injury, oxidative stress indicators mentioned above were detected at 1.5 h after LPS/D-Gal exposure. Although LPS/D-Gal challenge still induced a significant increase in MDA content and caused a dramatic reduction in enzyme activities (CAT and GPX) as well as a decrease in GSH content at early stage of injury (Fig. 5E–H), pre-treatment with FGF9 had little effect on LPS/D-Gal-evoked oxidant status.
The analytical data suggested that FGF9 might alleviate the oxidative stress in LPS/D-Gal-induced mice. NRF-2 is a key regulator that protects against various oxidative damage (Ge et al. 2017; Zhao et al. 2018; Hu et al. 2021). Normally, NRF-2 is localized in the cytosol in an inactive state by interaction with its inhibitor KEAP1. In response to oxidative stimuli or antioxidants, the NRF-2 is dissociated from the NRF-2-KEAP1 complex and translocated into the nucleus to activate the transcription of a variety of antioxidative and phase II detoxifying enzyme genes, including HO-1, NQO-1, and GCLC genes (Hu et al. 2021). To investigate the antioxidative mechanism of FGF9, the protein levels of KEAP1 in the cytoplasmic extracts and NRF-2 in the nuclear extracts from mice livers were evaluated. As noted in Fig. 5I, J, the 6 h of LPS/D-Gal treatment elicited a marked increase in KEAP1 protein levels in the cytoplasmic extracts concomitant with a decrease in nuclear NRF-2 protein levels. In contrast, FGF9 (0.5 or 1.5 mg/kg) or silymarin (100 mg/kg) pre-treatment reduced KEAP1 expression in cytosolic extract from the LPS/D-Gal-intoxicated mice livers, but the expression of nuclear NRF-2 protein was up-regulated in response to FGF9 or silymarin treatment. Furthermore, the activation of NRF-2 by FGF9 or silymarin can be also verified by changes in the expression levels of NRF-2 downstream target genes. Immunoblot results suggested that in LPS/D-Gal-induced mice livers, the down-regulation of GCLC, NQO-1, and HO-1 protein expression was completely abrogated by FGF9 or silymarin pre-treatment (Fig. 5I, K ).
Post-treatment with FGF9 relieves liver damage in LPS/D-Gal-intoxicated mice
Since the pre-treatments cannot well simulate the clinic settings, the protective benefit of post-insult administration of FGF9 was also evaluated in murine model of sepsis-induced FH. We found that the mice that received FGF9 treatment at 1 h post-LPS/D-Gal challenge had lower serum levels of ALT, AST and T-BIL than those from model mice (Fig. 6A–C), suggesting that FGF9 may also provide therapeutic benefit in sepsis-induced FH when it is administered in a clinically relevant manner. However, post-treatment with FGF9 at 2 or 4 h following LPS/D-Gal administration had little effect on the induction of serum levels of ALT, AST, and T-BIL in LPS/D-Gal-challenged mice. This can be reasonably interpreted to indicate that hepatitis can be rapidly progressive upon LPS/D-Gal challenge, as evidenced by the peak serum level of a key proinflammatory factor TNF-α was reached at 1.5 h after LPS/D-Gal exposure.
Post-treatment with FGF9 alleviates liver damage in mice challenged by LPS/D-Gal. Mice were divided into five groups: control (mice received vehicle 1 h after intraperitoneal injection of saline), FH model (mice received an intraperitoneal injection of vehicle 1 h after intraperitoneal injection of D-Gal (500 mg/kg) and LPS (5 μg/kg), and LPS/D-Gal + FGF9 (mice received an intraperitoneal injection of 1.5 mg/kg of FGF9 at 1, 2, or 4 h after intraperitoneal injection of LPS/D-Gal). A–C ALT, AST, and T-BIL levels in mouse serum were measured at 6 h post-LPS/D-Gal injection. Values represent mean ± SD for 8 mice. *p < 0.05 in comparison with vehicle-treated control group; #p < 0.05 in comparison with FH model group
Discussion
Sepsis-induced FH is a rapidly deteriorating clinical syndrome that severely endangers human health (Fyfe et al. 2018). Currently, no effective therapeutic agents for sepsis-induced FH are available. In an ex vivo model of carbon tetrachloride-induced liver injury, FGF9 is found to elevate the proliferation rate of hepatocytes and exert a hepatoprotective effect in a paracrine fashion (Antoine et al. 2007). Despite this, the in vivo role of FGF9 in sepsis-induced FH remains elusive. In the current study, we established an experimental sepsis-induced FH mice model by combined administration of LPS and D-Gal to mice, and treated the LPS/D-Gal-intoxicated mice with FGF9 to examine its hepatoprotective effects and underlying molecular mechanisms in sepsis-induced FH. We found that FGF9 effectively reduced the mortality of sepsis-induced FH mice. In addition, the lethal hepatotoxicity inflicted by LPS/D-Gal, as evidenced by overt histological lesions accompanied by elevated serum transaminase activities and T-BIL level, was relieved by FGF9 pre-treatment. Therefore, these observations suggest that FGF9 may serve as a potential drug candidate for the prevention of sepsis-induced FH.
In early stage of liver injury, LPS/D-Gal recruits macrophages to the liver. Infiltrated macrophages secrete TNF-α, which initiates hepatocyte injury by interacting with TNF-receptor 1 (TNF-R1) and adaptor protein TNF-R1-associated death domain (TRADD) on the membrane of hepatocytes (Jing et al. 2019). The signaling complex recruits and activates caspase-8, which transduces two different caspase-dependent apoptotic signaling (Bajt et al. 2001). On the one hand, caspase-8 directly stimulates the activation of the downstream effector caspase-3; on the other hand, caspase-8 truncates Bid, which enters the mitochondria where it activates BAK and/or BAX, leading to release of mitochondrial cytochrome C and activation of caspase-9 and -3. In this study, we found that FGF9 markedly attenuated the increase in serum TNF-α level in mice inflicted by LPS/D-Gal. Consequently, FGF9 inhibited TNF-α-induced apoptotic pathways and blocked apoptosis, as indicated by an increased BCL-2/BAX ratio and lower activities of caspase -8, -9, and -3 in mice livers. This is probably one of the reasons why FGF9 could ameliorate the lethal impairment of mice induced by LPS/D-Gal.
In middle stage of LPS/D-Gal-induced liver injury, infiltrated macrophages produce a myriad of proinflammatory factors, including TNF-α and IL-6, which recruit and activate neutrophils (Ambade et al. 2012). Once recruited, neutrophils release toxic substances such as proteases and reactive oxygen species (ROS). Furthermore, ROS in injured mice livers transduce signaling cascades linked to inflammation, thus promoting the secretion of plenty of proinflammatory cytokines (Miyauchi et al. 2019). The synergistic interplay between hepatic ROS and proinflammatory cytokines accelerates the process of liver injury. Here, we provide evidence that pre-treatment with FGF9 inhibited LPS/D-Gal-induced neutrophil infiltration and activation in mice livers, as determined by measuring the activity of MPO—an enzyme abundantly expressed in activated neutrophils. Meanwhile, FGF9 also significantly reduced LPS/D-Gal-induced MDA generation, promoted GSH synthesis, and enhanced antioxidant capacity by restoring GPX and CAT activities. However, FGF9 did not exert a direct antioxidant effect in early stage of LPS/D-Gal-induced liver injury. Indeed, FGF9 has been shown to completely prevent LPS/D-Gal-induced TNF-α production, which is a critical event in the development of sepsis-induced FH. Therefore, FGF9 might regulate redox homeostasis primarily by suppression of TNF-α production rather than direct effects on antioxidant defense system, because inflammation and oxidative stress largely relies on the deleterious stimuli of TNF-α.
The production of proinflammatory chemokines and cytokines including TNF-α is tightly regulated by the NF-κB signaling pathway (Ambade et al. 2012). Our experimental results found that FGF9 markedly decreased the phosphorylation and degradation of IκB induced by LPS combined with D-Gal, which gave rise to a marked decrease in nuclear localization and transcriptional activity of NF-κB. Our findings support this view that the suppressive effect of FGF9 on LPS/D-Gal-initiated NF-κB activation partly accounts for reduced TNF-α generation and results in restraint of oxidative damage during inflammatory responses. Previous studies have also reported that liver damage is further exacerbated when NF-κB enters the nucleus and initiates the transcription of genes coding proinflammatory enzyme cyclooxygenase-2 (COX-2) (Wang et al. 2018; Gong et al. 2017; Xia et al. 2014). In another study, however, prostaglandin E2 (PGE2) generated by COX-2 in hepatocytes acts to counter LPS/D-Gal-induced proinflammatory cytokine production and hepatocyte apoptosis (Mayoral et al. 2008). In line with the latter observation, prophylactic administration of FGF20, a member of the FGF9 subfamily, is known to play a protective role in dextran sulfate sodium-induced colitis in mice and indomethacin-induced small intestinal ulceration/inflammation in rats through inducing COX-2-derived PGE2 production (Jeffers et al. 2002). Thus, additional studies will be needed to investigate whether FGF9 ameliorates LPS/D-Gal-induced FH via increased COX-2 protein levels, independent of the transcriptional activity of NF-κB.
In conclusion, our present study suggests that FGF9 ameliorates LPS/D-Gal-induced FH through attenuating aggressive inflammation and hepatocyte apoptosis, thus maintaining redox homeostasis in mouse livers. The potential mechanism underlying the anti-inflammatory activity of FGF9 might be partially attributed to suppression of the NF-κB signaling pathway, and the potential antioxidative activity of FGF9 might be associated with the activation of NRF-2 (Fig. 7). Thereby, FGF9 may be a potential therapeutic agent for sepsis-induced FH, although additional experiments are warranted to confirm the tolerability and safety of FGF9.
References
Ambade A, Catalano D, Lim A, Mandrekar P (2012) Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology 55(5):1585–1595
Antoine M, Wirz W, Tag CG, Gressner AM, Marvituna M, Wycislo M, Hellerbrand C, Kiefer P (2007) Expression and function of fibroblast growth factor (FGF) 9 in hepatic stellate cells and its role in toxic liver injury. Biochem Bioph Res Co 361(2):335–341
Bajt ML, Vonderfecht SL, Jaeschke H (2001) Differential protection with inhibitors of caspase-8 and caspase-3 in murine models of tumor necrosis factor and Fas receptor-mediated hepatocellular apoptosis. Toxicol Appl Pharma 175(3):243–252
Bunchorntavakul C, Reddy KR (2017) Acute liver failure. Clin Liver Dis 21(4):769–792
Fyfe B, Zaldana F, Liu C (2018) The pathology of acute liver failure. Clin Liver Dis 22(2):257–268
Galanos C, Freudenberg MA, Reutter W (1979) Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA 76(11):5939–5943
Ge M, Yao WF, Yuan DD, Zhou SL, Chen X, Zhang YH, Li HB, Xia ZY, Hei ZQ (2017) Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia-reperfusion injury. Cell Death Dis 8(6):e2841
Gong XB, Yang Y, Huang LG, Zhang QY, Wan RZ, Zhang P, Zhang BS (2017) Antioxidation, anti-inflammation and anti-apoptosis by paeonol in LPS/D-GalN-induced acute liver failure in mice. Int Immunopharmacol 46:124–132
Hu J, Zhu ZJ, Ying HL, Yao J, Ma HB, Li L, Zhao YF (2021) Oleoylethanolamide protects against acute liver injury by regulating Nrf-2/HO-1 and NLRP3 pathways in mice. Front Pharmacol 11:2338
Jeffers M, McDonald WF, Chillakuru RA, Yang MJ, Nakase H, Deegler LL, Sylander ED, Rittman B, Bendele A, Sartor RB, Lichenstein HS (2002) A novel human fibroblast growth factor treats experimental intestinal inflammation. Gastroenterology 123(41):1151–1162
Jing ZT, Liu W, Xue CR, Wu SX, Chen WN, Lin XJ, Lin X (2019) AKT activator SC79 protects hepatocytes from TNF-α-mediated apoptosis and alleviates D-Gal/LPS-induced liver injury. Am J Physiol-Gastr L 316(3):G387-396
Li A, Zhang JY, Xiao X, Wang SS, Wan JB, Chai YS, Li P, Wang YT (2018a) Hepatorenal protective effects of medicinal herbs in An-Gong-Niu-Huang Wan (AGNH) against cinnabar-and realgar-induced oxidative stress and inflammatory damage in mice. Food Chem Toxicol 119:445–456
Li M, Wang S, Li X, Jiang LL, Wang XJ, Kou RR, Wang Q, Xu L, Zhao N, Xie KQ (2018b) Diallyl sulfide protects against lipopolysaccharide/D-galactosamine-induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. Food Chem Toxicol 120:500–509
Lv HM, Fan XY, Wang LD, Feng HH, Ci XX (2018) Daphnetin alleviates lipopolysaccharide/D-galactosamine-induced acute liver failure via the inhibition of NLRP3, MAPK and NF-κB, and the induction of autophagy. Int J Biol Macromol 119:240–248
Lv HM, An BY, Yu QL, Cao Y, Liu Y, Li SZ (2020) The hepatoprotective effect of myricetin against lipopolysaccharide and D-galactosamine-induced fulminant hepatitis. Int J Biol Macromol 155:1092–1104
Mayoral R, Mollá B, Flores JM, Boscá L, Casado M, Martín-Sanz P (2008) Constitutive expression of cyclo-oxygenase 2 transgene in hepatocytes protects against liver injury. Biochem J 416(3):337–346
Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, Ueda T, Okajima H, Terajima H, Uemoto S (2019) Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci USA 116(27):13533–13542
Seitz T, Freese K, Dietrich P, Thasler WE, Bosserhoff A, Hellerbrand C (2020) Fibroblast growth factor 9 is expressed by activated hepatic stellate cells and promotes progression of hepatocellular carcinoma. Sci Rep 10(1):1–9
Wang S, Lin HP, Zhao TT, Huang SS, Fernig DG, Xu N, Wu FF, Zhou M, Jiang C, Tian HS (2017) Expression and purification of an FGF9 fusion protein in E. coli, and the effects of the FGF9 subfamily on human hepatocellular carcinoma cell proliferation and migration. Appl Microbiol Biot 101(21):7823–7835
Wang W, Wu LL, Li Q, Zhang Z, Xu LB, Lin CX, Gao L, Zhao KL, Liang F, Zhang Q, Zhou M, Jiang WZ (2018) Madecassoside prevents acute liver failure in LPS/D-GalN-induced mice by inhibiting p38/NF-κB and activating Nrf2/HO-1 signaling. Biomed Pharmacother 103:1137–1145
Xia XM, Su CY, Fu JL, Zhang P, Jiang XJ, Xu DM, Hu LH, Song EQ, Song Y (2014) Role of α-lipoic acid in LPS/ D-GalN induced fulminant hepatic failure in mice: studies on oxidative stress, inflammation and apoptosis. Int Immunopharmacol 22(2):293–302
Zhao N, Guo FF, Xie KQ, Zeng T (2018) Targeting Nrf-2 is a promising intervention approach for the prevention of ethanol-induced liver disease. Cell Mol Life Sci 75(17):3143–3157
Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LY19H160029), the National Natural Science Foundation of China (82174058), the Guangdong Basic and Applied Basic Research Foundation (2021A1515012229), the Chongqing Natural Science Foundation (cstc2019jcyj-msxmX0045), and the TCM project of Chongqing Municipal Health and Family Planning Commission (ZY201702122).
Author information
Authors and Affiliations
Contributions
Ao Li and Xue-Mei Li carried out the experiments and should be considered co-first authors. Ao Li and Xiao Xiao drafted the manuscript. Chu-Ge Song conducted data analysis. Hai-Shan Tian prepared the recombinant FGF9 protein. Ao Li, Wei-Min Yao, and Hai-Shan Tian conceived and designed this project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Ethical approval
All procedures involving animals were approved by the Animal Care and Use Committee of Chongqing University of Technology, and were conducted in accordance with the NIH guidelines for the care and use of laboratory animals (8th Edition, 2011).
Availability of data and materials
All experimental data and analysis are included in this article.
Additional information
Handling editor: M. S. Palma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, A., Li, XM., Song, CG. et al. Fibroblast growth factor 9 attenuates sepsis-induced fulminant hepatitis in mice. Amino Acids 54, 1069–1081 (2022). https://doi.org/10.1007/s00726-022-03143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03143-7