Abstract
The effects of β-alanine (BA) and sodium bicarbonate (SB) on energy metabolism during work-matched high-intensity exercise and cycling time-trial performance were examined in 71 male cyclists. They were randomised to receive BA + placebo (BA, n = 18), placebo + SB (SB, n = 17), BA + SB (BASB, n = 19), or placebo + placebo (PLA, n = 18). BA was supplemented for 28 days (6.4 g day−1) and SB (0.3 g kg−1) ingested 60 min before exercise on the post-supplementation trial. Dextrose and calcium carbonate were placebos for BA and SB, respectively. Before (PRE) and after (POST) supplementation, participants performed a high-intensity intermittent cycling test (HICT-110%) consisting of four 60-s bouts at 110% of their maximal power output (60-s rest between bouts). The estimated contribution of the energy systems was calculated for each bout in 39 of the participants (BA: n = 9; SB: n = 10; BASB: n = 10, PLA: n = 10). Ten minutes after HICT-110%, cycling performance was determined in a 30-kJ time-trial test in all participants. Both groups receiving SB increased estimated glycolytic contribution in the overall HICT-110%, which approached significance (SB: + 23%, p = 0.068 vs. PRE; BASB: + 18%, p = 0.059 vs. PRE). No effects of supplementation were observed for the estimated oxidative and ATP-PCr systems. Time to complete 30 kJ was not significantly changed by any of the treatments, although a trend toward significance was shown in the BASB group (p = 0.06). We conclude that SB, but not BA, increases the estimated glycolytic contribution to high-intensity intermittent exercise when total work done is controlled and that BA and SB, either alone or in combination, do not improve short-duration cycling time-trial performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
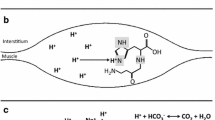
Repeated bouts of high-intensity exercise lead to a pronounced accumulation of hydrogen cations (H+), a by-product of anaerobic metabolism that has been shown to play causative roles in peripheral fatigue (Debold et al. 2016; Fitts 2016). Muscle acidosis has been shown to impair energy transfer via anaerobic pathways, since the accumulation of H+ inhibits phosphorylcreatine resynthesis (Sahlin et al. 1975) and the activity of key glycolytic enzymes (e.g., glycogen phosphorylase and phosphofructokinase) (Sutton et al. 1981). Intramuscular acidosis, therefore, appears to limit the ability of the muscle cells to cope with the high-energy demands during high-intense exercise, thereby impairing performance (Bishop and Claudius 2005; Gladden 2004; Hermansen and Osnes 1972). In this context, strategies have been used to attenuate acidosis and improve performance (Oliveira et al. 2017; Sakamoto et al. 2018; Saunders et al. 2017b), with β-alanine (BA) and sodium bicarbonate (SB) supplementation being two of the most effective strategies capable of increasing H+ buffering capacity and exercise performance (Carr et al. 2011; Saunders et al. 2017b).
BA supplementation is effective to increase intracellular carnosine content (Harris et al. 2006; Saunders et al. 2017a), a dipeptide whose pKa of 6.83 (Abe 2000) falls within the mid-point of the pH transit range in skeletal muscle (pH ~ 7.0 at rest and ~ 6.5 at fatigue) (Cady et al. 1989). Carnosine may contribute with up to ~ 20% of the total physiochemical buffering capacity in type II muscle fibres (Harris et al. 2006). Whilst BA supplementation increases the intracellular muscle buffering capacity, SB supplementation, in turn, increases the extracellular buffering capacity via increased blood bicarbonate (Jones et al. 2016; Oliveira et al. 2017). Increased blood bicarbonate enhances H+ efflux from working muscles to the blood where they are neutralised (Bishop et al. 2004; Siegler et al. 2016). Interestingly, both BA and SB seem to be particularly effective during exercises of very similar characteristics, namely high-intensity exercise lasting from ~ 1 to ~ 5 min with high reliance on glycolysis (Carr et al. 2011; Saunders et al. 2017b; Tobias et al. 2013). Likewise, BA and SB co-supplementation has been shown to result in additive performance improvements in comparison to SB or BA alone in tasks of these same characteristics (Hobson et al. 2013; Painelli et al. 2013; Tobias et al. 2013), which further support their roles on intramuscular pH regulation.
The peripheral mechanisms underlying the ergogenic properties of BA and SB, being either initiated by increased H+ removal or increased intracellular buffering capacity, are probably similar and both involve retardation of the deleterious effects of intramuscular acidification on Ca++ homeostasis, contractile properties, and energy homeostasis. With regards to energy homeostasis, attenuated muscle acidosis is suggested to improve phosphorylcreatine resynthesis and increase the activity of glycolytic enzymes such as glycogen phosphorylase and phosphofructokinase (Siegler et al. 2016). Therefore, some of the most important ergogenic mechanisms of both BA and SB may involve increased activation of the glycolytic and the ATP-PCr systems. Increased post-exercise blood lactate has been shown following both BA (Tobias et al. 2013) and SB (Artioli et al. 2007) supplementation, providing support to the increased glycolytic activation hypothesis. Increased post-exercise muscle lactate has also been shown following SB supplementation (Bishop et al. 2004). However, the previous investigations have not controlled intensity and duration of the exercise, rendering them unclear as to whether increased lactate was caused by a direct effect on the regulation of the energetic systems or by an indirect effect secondary to the increased total mechanical work done during exercise. Moreover, a quantitative estimation of the increase in glycolytic activation has never been reported.
Although BA and SB have been studied for years, most investigations have focused on their ergogenic properties, with their underpinning mechanisms overlooked. Most of the theoretical mechanisms remain poorly examined and their impact on the energy systems is yet to be demonstrated. In this study, we used a non-invasive method (di Prampero and Ferretti 1999) to estimate the contribution of the three energy systems (aerobic, ATP-PCr, and glycolysis) to examine how BA, SB, and their combination alter the regulation of the energy system in skeletal muscle during work-matched high-intensity intermittent exercise. A secondary aim of this investigation was to examine the effects of these supplements, alone or in combination, on a work-based cycling time-trial designed to simulate 1-km cycling performance.
Methods
Participants
To be included in the study, participants were required to meet the following criteria: non-vegetarian men between 18 and 45 years of age, actively training cycling at least three times per week, and a minimum of 6 h week−1, with at least 3 years of experience in cycling training. Exclusion criteria were: diagnosed chronic diseases, use of BA or creatine 6 months prior to the study, smoking, and continued use of medications. Volunteers were fully informed of any risks and discomforts associated with the study before giving their signed consent for participation. The study was approved by the institution’s ethics committee.
Eighty trained cyclists were initially enrolled. However, nine participants dropped out after randomisation: five after the familiarisation sessions and four after the first main trial. All drop-outs were due to reasons unrelated to the study: injury during training or competition (n = 3); moved away (n = 1); change in lifetime/work routine and the impossibility of attending subsequent sessions (n = 2); personal reasons not disclosed (n = 3). Seventy-one cyclists (Table 1) completed all the sessions and were included in the analyses [mean ± standard deviation (SD): 5 ± 4 years of experience in cycling, 9 ± 3 h of training/week, consisting of 235 ± 92 km/week].
Experimental design
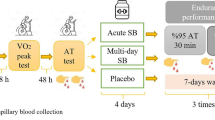
In this double-blind, randomised, parallel-group, placebo-controlled trial, participants attended the laboratory on five separate occasions (Fig. 1). In the first visit, maximal aerobic power output (Wmax) and peak oxygen consumption (VO2peak) were determined. The next two visits (48 h apart) were used to familiarise the participants with the main trial procedures (i.e., a high-intensity intermittent cycling protocol followed by a 30-kJ time-trial performance test, as detailed below). On the two remaining visits, participants completed the main experimental trials before (PRE) and after (POST) the 28-day supplementation period.
Participants were randomly allocated to one of the following groups: BA (β-alanine + placebo; n = 20, 3 drop-outs), SB (placebo + sodium bicarbonate; n = 20, 3 drop-outs), BASB (β-alanine + sodium bicarbonate; n = 20, 1 drop-out), or PLA (placebo + placebo; n = 20, 2 drop-outs). Maltodextrin and calcium carbonate were the placebos for BA and SB. Randomisation was performed in blocks of 4 with groups being matched for time to complete a 30-kJ test. Due to a persistent malfunction of the gas analyses’ equipment, we were unable to collect expired air data of 32 participants; therefore, energy system contribution was estimated in 39 volunteers (BA: n = 9, SB: n = 10, BASB: n = 10, PLA: n = 10). The current study was designed and undertaken in full by the researchers, with no financial, intellectual, or other input from any company.
Preliminary testing and main trials
In the first visit, participant’s height, body mass (BM), and 24-h dietary intake were recorded. Volunteers were asked to replicate the same food and portion sizes ingested in the 24 h prior to the next visits; compliance with this request was verbally confirmed. Thereafter, participants underwent a graded exercise test to determine Wmax and VO2peak.
For the main trials, participants arrived at the same time of day 2–4 h after their last meal. Upon arrival, they rested for 10 min before blood collection for the determination of lactate, pH, and bicarbonate at rest. Another blood sample was collected at rest during each main trial to assess the effects of BA on blood markers of renal, hepatic, and muscle health. In the last trial only (post-supplementation), participants ingested either SB or placebo, followed by 60-min rest and an additional blood sample was collected for the determination of the same variables immediately prior to exercise. Resting oxygen consumption was measured for 5 min; volunteers then performed a 5-min cycling warm up at 100 W, followed by a high-intensity intermittent cycling test (HICT-110%) consisting of four 60-s bouts at 110% of their Wmax interspersed by 60-s recovery periods. Post-exercise blood samples were collected from the finger tip for lactate determination immediately after each bout. A venous blood sample was also collected immediately after the last bout for the determination of pH and bicarbonate. Ten minutes after the HICT-110%, participants undertook a 30-kJ time trial; finger-prick and venous blood samples were collected immediately pre- and post 30-kJ time trial.
Participants were instructed to maintain their usual diet and levels of training for the duration of the study as well as to abstain from alcohol, caffeine, and strenuous exercise 24 h prior to all the experimental trials. Participants chose the time of day of their preference to perform the tests, with this being individually replicated in all subsequent trials. All tests were performed in a controlled environment with a temperature of 25 ± 0.6 °C and 63 ± 4.8% relative humidity.
Supplementation protocol
All supplements were prepared by an individual not involved in data collection. Each participant received an opaque white-sealed bottle containing either 800-mg tablets of sustained-release BA (CarnoSyn™, Natural Alternatives International, USA) or maltodextrin (Natural Alternatives International, USA). They were instructed to ingest two capsules, four times a day (totalising 6.4 g day−1), along with meals, for 28 days. To verify compliance, the participants were asked to complete a supplementation log, which showed a high degree of compliance in all the groups (BA: 97.1 ± 3.7%; SB: 97.1 ± 4.3%; BA + SB: 96.9 ± 3.3% and PLA: 97.9 ± 6.3%).
In addition to the chronic supplementation with BA or maltodextrin, the participants ingested 0.3 g kg−1 BM of SB or calcium carbonate 60 min before the tests in the post-supplementation trial. These were given in 1-g gelatin capsules, which were ingested in no more than 5 min under supervision to ensure full compliance. Gastrointestinal discomfort was reported prior to and 60 min after the capsules ingestion using a 10-point scale where a score of ‘0’ corresponded to the absence of symptoms and a score ‘10’ corresponded to extreme discomfort (Price et al. 2003). Supplements and their respective placebos were identical in appearance and were obtained from a certified manufacturer. To verify the efficacy of the blinding procedure, participants were asked to identify their group allocation (i.e., BA or maltodextrin and SB or calcium carbonate).
Graded exercise test
The test was conducted on an electromagnetically braked cycle ergometer (Lode Excalibur, Lode, Groningen, The Netherlands) from a starting power output of 100 W, with intensity being increased by 6 W every 15 s. Participants were told to maintain a pedal frequency between 90 and 100 rev min−1, and the test was terminated when cadence could not be maintained > 60 rev min−1 for 5 s. Wmax was calculated as the power of the last completed stage plus the fraction of time spent in the final non-completed stage multiplied by 6 W. VO2peak was determined as the highest 15-s average recording of oxygen consumption (VO2) measured by a calibrated portable gas analyser (K4b2, Cosmed®, Rome, Italy). Validity and reliability of this analyser have been previously determined (for review, see Macfarlane 2017) with a typical error of ~ 4% (Duffield et al. 2004). Participants were allowed to freely adjust bike settings on their first visit; these were recorded and replicated on the next visits.
High-intensity intermittent cycling test
The HICT-110% was conducted in an electromagnetically braked cycle ergometer (Lode Excalibur, Lode, Groningen, The Netherlands) and consisted of 4 × 1-min cycling bouts at 110% of the Wmax attained in the graded exercise test. Cadence was constant (90–100 rev min−1) and the bouts were interspersed by 1-min passive recovery intervals, during which volunteers remained seated on the ergometer. Following the completion of the last bout, participants rested for 10 min. Oxygen consumption was measured at rest, throughout the HICT-110%, and up to 5 min after the completion of the test using a calibrated portable gas analyser (K4b2, Cosmed®, Rome, Italy).
30-kJ cycling time-trial test
Ten minutes after the HICT-110%, participants completed a 30-kJ time-trial on the same ergometer to assess tolerance to high-intensity exercise. The fixed amount of mechanical work was selected to last ~ 60–90 s, thereby being an approximation of a 1-km cycling race. Participants received verbal encouragement throughout the test, but no performance-related information was provided. The only feedback provided was the mechanical work completed when they reached 50, 75, 90, and 100% of the 30 kJ. The intra-class correlation coefficient and the coefficient of variation (CV) data for the 30-kJ TT were 0.995 and 4.8%. The CV data were calculated between the second familiarisation session and the pre-supplementation session (trials without supplement intervention) for 71 individuals.
Blood collection and analyses
Finger-prick blood samples (20 μL) were collected and stored in 2% NaF (20 µl). Samples were centrifuged (2000g for 5 min at 4 °C) to separate plasma, which was stored at − 80 °C until analysis. Plasma lactate was determined spectrophotometrically using an enzymatic assay (Katal, Interteck, Sao Paulo, Brazil). Venous blood samples (1 ml) from the antecubital vein were collected using heparin-coated syringes and directly injected into the blood gas analyser (Rapid Point 350, Siemens, Germany) to determine pH and PCO2. Blood bicarbonate concentration was calculated according to the Henderson–Hasselbalch equation. Day-to-day coefficient of variation was determined in 45 individuals for blood pH (0.5%; 95% CI 0.4–0.6%), blood bicarbonate (17.4%; 95% CI 13.8–20.3%), and blood lactate (14%; 95% CI 11.3–16.2). To examine the effects of BA supplementation on blood markers of clinical health, resting venous blood samples were collected PRE- and POST-supplementation for the analysis of aspartate transaminase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase, albumin, globulin, creatinine, creatinine kinase, and uric acid. Estimated glomerular filtration rate (GFR) was determined using blood creatinine values by the Cockcroft–Gault formula: GFR = [(140 − age) × BM]/72 × creatinine. All analyses were performed by the Clinics Hospital Central Laboratory within 4 h from collection. Since blood samples were drawn before SB ingestion, the participants were grouped as BA (i.e., those in the BA and BA + SB groups) or PLA (i.e., those in the SB + PLA groups). These analyses were carried out in 58 participants due to scheduling problems with the Central Hospital Laboratory. The reference values provided by the Central Laboratory were used to classify whether each blood variable was within the normal range.
Estimation of the energy system contribution
The contribution of energy systems was estimated during each bout according to the assumptions of di Prampero and Ferretti (1999). To estimate the contribution of the oxidative systems, resting oxygen consumption was subtracted from the area under the oxygen consumption curve obtained during each HICT-110% bout. Oxygen consumption at rest was obtained by multiplying the average of the last 30 s of baseline oxygen consumption by the bout duration time (60 s), whilst areas under the curve were calculated using the trapezoidal method. Units for calculation were converted accordingly. The contribution of the ATP-PCr system was calculated by subtracting resting oxygen consumption from the oxygen consumed during the 1-min recovery between bouts, as previously used in intermittent exercise protocols where the interval between bouts is too short to allow the formation of either a mono- or a bi-exponential curve of the excess post-exercise oxygen consumption (Lopes-Silva et al. 2015). The contribution of the glycolytic system was estimated, assuming that 1 mM of lactate corresponds to 3 ml of oxygen consumption per kilogram of BM and that 1 l of oxygen corresponds to 20.92 kJ (di Prampero and Ferretti 1999). Therefore, delta lactate (i.e., difference between the concentrations before and after each bout) was multiplied by 3 and by the participant’s BM.
Food intake assessment
During the supplementation period, participants were required to complete a 3-day food diary on 2 non-consecutive weekdays and 1 weekend day. Habitual dietary consumption of BA was calculated based on data from Abe (2000) and Jones et al. (2011).
Statistical analyses
Mixed-model analyses were performed for blood variables (pH, bicarbonate, and lactate) with group (BA, SB, BASB, and PLA), time (PRE and POST), and moment (time-points within main trials) being fixed factors. For energy system contribution (oxidative, ATP-PCr, and glycolytic), mixed models were performed with group, time, and bouts being fixed factors. For 30-kJ TT performance, mixed-models were performed with group and time being fixed factors. For blood markers of health, group (BA and PLA) and time (PRE and POST) were fixed factors. To examine the overall effect of supplementation on energy system contribution throughout the entire trials, total energy contribution (1st + 2nd + 3rd + 4th bouts) was calculated and analysed using mixed models with group and time being fixed factors. In all the cases, participants were random factors. Four different covariance matrices were tested to determine the model that best fitted each data set, according to the Schwarz’s Bayesian criterion. Single degree of freedom contrast analysis was used for specific hypothesis-driven comparisons. Fisher’s exact test was used to evaluate the efficacy of the double-blind design. Analyses were performed using the SAS software v.9.3. The significance level adopted was 5%. Differences between or within groups were considered to approach significance when p < 0.1 (i.e., type I error rate of 10%).
The effect of supplementation on the energy system contribution and on 30-kJ time-trial performance was also analysed a posteriori using the effect sizes and confidence intervals for within-group comparisons, as previously described (Nakagawa and Cuthill 2007). Positive and negative confidence intervals (not crossing zero) were deemed significant. To account for outliers, all the analyses were conducted without these individuals; since their exclusion did not change any outcome, the entire data set was used in the final analyses.
Results
Effects of BA and SB supplementation on energy system contribution
The absolute estimated glycolytic contribution throughout the four HICT-110% bouts was not significantly affected by supplementation (group-by-time-by-bout interaction: F = 1.23, p = 0.23). However, a significant decrease was shown throughout bouts, irrespective of supplementation (main effect of bout: F = 15.62, p < 0.0001) (Table 2).
The absolute estimated ATP-PCr contribution throughout the four HICT-110% bouts was not significantly affected by supplementation (group-by-time-by-bout interaction: F = 0.39, p = 0.99). However, a significant increase was shown throughout bouts, irrespective of supplementation (main effect of bout: F = 9.42, p < 0.0001) (Table 2).
The absolute estimated oxidative contribution throughout the four HICT-110% bouts was not significantly affected by supplementation (group-by-time-by-bout interaction: F = 0.39, p = 0.99). However, a significant increase was shown throughout bouts, irrespective of supplementation (main effect of bout: F = 133.54, p < 0.0001) (Table 2).
When analysing the absolute estimated contribution of the energy systems to the total energy cost of the four bouts combined (1st + 2nd + 3rd + 4th bouts), no significant group-by-time interactions were shown for the glycolytic (F = 1.0, p = 0.398), ATP-PCr (F = 0.57, p = 0.638), or oxidative systems (F = 1.82, p = 0.152) (Fig. 2). However, a significant main effect of time was shown for the glycolytic system, indicating a higher glycolytic contribution POST supplementation, irrespective of group (F = 4.65; p = 0.034); contrast analysis revealed that the groups receiving SB displayed a higher glycolytic contribution in comparison to PRE-supplementation that approached significance (SB: + 10.1 kJ or + 23%, t = − 1.85; p = 0.068; BASB: + 8.3 kJ or + 18%, t = − 1.92, p = 0.059), while no effects were shown for the other groups (BA: − 0.7 kJ or + 1.2%, t = 0.13; p = 0.90; PLA: + 3.5 kJ or + 10.3%, t = − 0.65, p = 0.52). Effect size analysis has also shown that both groups receiving SB displayed increased glycolytic contribution; no effects were observed for the ATP-PCr and oxidative systems (Fig. 3).
Effects of β-alanine (BA) and sodium bicarbonate (SB) supplementation on the absolute contribution of the energy systems [glycolytic (a); ATP-PCr (b); oxidative (c)] to the overall energy cost of the HICT-110% (all four bouts summed). Left panels display mean ± SD for PRE and POST; right panels display mean (white horizontal bars) and individual (black circles) post–pre-absolute changes. p values displayed on a refer to contrast analysis. PLA placebo. BA: n = 9; SB: n = 10; BASB: n = 10; PLA: n = 10
Effect sizes (black circles) and 95% confidence intervals (black lines) calculated for the within-group differences of the energy transferred in each system. Significance was considered where confidence interval did not cross zero. BA β-alanine, SB sodium bicarbonate. BA: n = 9; SB: n = 10; BASB: n = 10; PLA: n = 10
30-kJ time-trial performance
No significant group-by-time interaction was shown for time to complete the 30-kJ performance test (F = 0.8, p = 0.496). However, contrast analysis revealed a reduction in TT performance in the BASB group that approached significance (t = 1.88; p = 0.064; Fig. 4a). Individual analysis showed that 6 out of 17 participants in both BA and SB groups, 7 out of 19 in BASB group, and 3 out of 18 participants in PLA group improved performance above the CV of the test (4.8%) (Fig. 4c). Effect size analysis revealed a trend for small performance improvements from PRE- to POST-supplementation in all groups, except in the PLA group; however, these changes did not reach statistical significance (Fig. 4d).
Effects of β-alanine (BA) and sodium bicarbonate (SB) on the time to complete 30-kJ time-trial performance. p value on a refers to single degree of freedom contrast analysis (all other comparisons are p > 0.1); percentages on b refers to the chance of a positive effect according to magnitude-based inference analysis. The grey bar on c represents the 95% confidence interval of the coefficient of variation of the test. Positive and negative confidence intervals crossing zero on d were deemed nonsignificant BA: n = 17; SB: n = 17; BASB: n = 19; PLA: n = 18
Blood variables
Blood pH was significantly reduced after HICT-110% in comparison with resting condition, irrespective of group or supplementation (Fig. 5a) (t = 28.45, p < 0.0001). Blood pH then significantly increased following the 10-min interval before the 30 kJ TT (t = − 5.88, p < 0.0001) and dropped again following the 30 kJ TT to reach the lowest values in comparison with all other time-points (all p < 0.0001, Fig. 5). Contrast analysis showed that pH was significantly higher in both groups that ingested SB (i.e., SB and BASB) in comparison with the PRE-supplementation trial (within-group effect: all p < 0.005, Fig. 5a).
Effects of β-alanine (BA) and sodium bicarbonate (SB) on blood pH (a), blood bicarbonate (b), and plasma lactate (c) PRE- (black square) and POST-supplementation (white square). For clarity, values obtained at rest from all the groups were averaged and displayed in a dashed line (all other time-points are significantly different from rest, p < 0.01). BA: n = 17; SB: n = 17; BASB: n = 19; PLA: n = 18. #p < 0.01 (effect of time). *p < 0.05 (within-group effect; single degree of freedom contrast analysis). †p = 0.074 (within-group effect; single degree of freedom contrast analysis)
Blood bicarbonate was significantly reduced after HICT-110% in comparison with resting condition, irrespective of group or supplementation (Fig. 5b) (t = 23.07, p < 0.0001). Blood bicarbonate then decreased further following the 10-min interval before the 30-kJ TT (t = 6.05, p < 0.0001) and tended to stabilise after the 30-kJ TT (t = 2.54, p = 0.06). Contrast analysis showed that blood bicarbonate was significantly higher in both groups that ingested SB (i.e., SB and BASB) in comparison with the PRE-supplementation trial (within-group effect: all p < 0.001).
Plasma lactate was significantly increased after HICT-110% in comparison with resting condition, irrespective of group or supplementation (Fig. 5c) (t = 30.2, p < 0.0001). Plasma lactate then decreased slightly but significantly following the 10-min interval before the 30-kJ TT (t = 3.77, p = 0.0004) and increased again after the 30-kJ TT (t = 14.48, p < 0.0001). Multiple comparison analysis showed higher post-HICT-110% plasma lactate in the SB group following supplementation in comparison with PRE, which approached significance (t = − 1.82, p = 0.074) and significantly higher after the 30-kJ TT (t = − 2.67, p = 0.01). Plasma lactate was significantly higher in the BASB group POST supplementation in comparison with PRE after 30-KJ TT (t = − 2.61, p = 0.011).
Blood markers of health
No significant main effect of time or group-by-time interaction was shown for any of the measured health variables, except for uric acid (group-by-time interaction: F = 4.21, p = 0.045); the group-by-time interaction for albumin approached significance (group-by-time interaction: F = 3.34, p = 0.073). Contrast analyses revealed that the PLA group displayed higher uric acid values POST- vs. PRE-supplementation that approached significance (t = − 1.76, p = 0.084), with no changes in the BA group (t = 1.15, p = 0.255). Contrast analyses also revealed that the BA group displayed lower albumin levels POST- vs. PRE-supplementation that approached significance (t = 1.82, p = 0.075), with no changes in the PLA group (t = − 0.77, p = 0.445) (Supplementary Table 1).
Food intake
No significant differences were found between-groups for any of the variables assessed (Table 3).
Side effects and blinding efficacy
Three volunteers reported paresthesia with BA (BASB group) and nine participants (SB: 5; BASB: 4) reported gastrointestinal discomfort after ingesting sodium bicarbonate. The most common symptoms were belching, nausea, gastric fullness, and intestinal discomfort. Only one volunteer in SB group scored 8 on the 10-point gastric discomfort scale, and no other participant reported a score above 4. Although the blinding may have been compromised for these individuals, we decided to include their dada on analysis as there was a little difference in the mean overall values if they were excluded.
In BA group, a total of seven volunteers correctly guessed the supplement which they were taking during the 28 days as well as five individuals in SB, six in BASB, and five in PLA group (Fischer’s exact test, p = 0.85). Corresponding data for correctly guessing ingestion of acute SB or PLA in each group were of 5, 6, 4, and 5 individuals in BA, SB, BASB, and PLA, respectively (Fisher’s exact test, p = 0.82).
Discussion
This is the first study to examine the effect of BA supplementation, alone or combined with SB ingestion, on the estimated contribution of energy systems during high-intensity intermittent exercise. We used a non-invasive quantitative method that allows the estimation of the metabolic energy transferred by all three energy systems (ATC-PCr, glycolytic, and oxidative). We hypothesised that both supplements would have a similar impact on the energy systems, namely, the ability to increase the glycolytic contribution. In partial agreement with our hypothesis, we showed that only SB was capable of increasing the glycolytic contribution, with no effect being observed with BA supplementation.
The magnitude of increase in glycolytic contribution was substantial (~ 20% in SB and BASB groups, although small in absolute values, since the total mechanical work was constrained and, therefore, similar in all trials). However, the mixed-model analysis did not allow drawing firm conclusions as to whether SB increased the glycolytic contribution in our study (i.e., no significant group-by-time interaction, with contrast analysis indicating only effects that approached significance). This suggests that this analysis in particular is somewhat underpowered, which is attributable to the impossibility to analyse expired air in nearly half of the total sample. To circumvent this issue and help us to draw firmer conclusions, we used an effect size-based approach, which more clearly indicated that SB, but not BA, increased the estimated contribution of the glycolytic system (see Fig. 3).
Although it is difficult to reconcile why such an effect was observed with SB, but not with BA supplementation, it is possible that the exercise protocol used to estimate the energy systems may have played a role. In our study, we opted to use two separated exercise protocols: one being only for studying energy systems and the other only for assessing performance. This was necessary, since the control of the total mechanical work was indispensable for the proper examination of our research question, which obviously excludes the possibility of assessing performance in the same protocol. Given that total mechanical work was fixed, only the effects truly brought about by supplementation, and not those brought about by increased work done, were present. Interestingly, the previous studies have shown increased post-exercise blood lactate responses following BA supplementation (Sale et al. 2011; Tobias et al. 2013). Since lactate is a product of glycolysis and, hence, a marker of glycolytic activation, these studies suggested that BA can increase glycolytic activation during exercise. However, it must be noted that none of these previous studies controlled total mechanical work done; therefore, it is not possible to differentiate the direct effect of BA on glycolytic activation from its indirect effect on glycolysis secondary to the increased total work done. Based on the results of the present study, it might be possible that BA does not exert any direct effect on glycolysis and that any increased glycolytic activation would be dependent on increased mechanical work, therefore, not being detectable when intensity and duration of exercise are fixed. In support of this, Bellinger and Minahan (2016a) observed that BA supplementation alone increased anaerobic capacity (1.1 ± 0.7 kJ) when volunteers cycled until exhaustion, but not when the test time was matched with that achieved at the pre-supplementation trial.
Recently, Lopes-Silva et al. (2018) showed that SB ingestion resulted in greater estimated glycolytic energy contribution (31%) when compared with the placebo condition in the first round of a simulated taekwondo combat, that is, when total mechanical work done was not controlled. In the present study, SB appeared to increase glycolytic activation independent of any increase in total work done. Although it is difficult to explain why SB and BA had different effects on the glycolytic system, one possibility could relate to the total amount of H+ that can be neutralised by the working muscles by the typical increases in muscle carnosine and in blood bicarbonate. Assuming the typical 6 mmol l−1 increase in blood bicarbonate following SB ingestion and 5 l of total blood volume, then SB ingestion would allow the neutralisation of ~ 30 mmoles of H+ above baseline (based on the 1:1 stoichiometry of HCO3− and H+ reaction). This is approximately two times more than the ~ 15 mmoles of H+ that a typical 80% increase in muscle carnosine (from 20 to 35 mmol kg−1 dry muscle after BA supplementation) can neutralise in both legs (assuming that legs correspond to 30% of BM, that 40% of leg mass is skeletal muscle, and that 70% of wet muscle is water—therefore, 3 kg of dry muscle times ~ 5 mmoles of H+ per kg of dry muscle that can be neutralised by a 15 mmol kg−1 dry muscle increase in muscle carnosine). Therefore, the effects of SB on the glycolytic activation could be more easily detectable than those of BA due a possibly greater ability of SB to neutralise H+.
Alternatively, the increased glycolytic contribution shown with SB could be accounted for by the indirect nature of the method used to estimate glycolytic contribution, which might be more sensitive to changes under conditions where the rates of lactate efflux to the blood, or lactate removal from circulation into other tissues, are altered. Since SB ingestion increases lactate efflux from the working muscles to the blood, thereby increasing blood lactate levels during exercise (Bishop et al. 2004; Siegler et al. 2016), it is possible that the increased glycolytic contribution with SB shown in our study could be an overestimation caused by increased lactate efflux rates, and not by a true increase in glycolytic activation. In fact, it has been difficult to differentiate whether increased lactate efflux during alkalosis is a consequence of increased flux rates per se or increased lactate production due to enhanced glycolytic activation (Spriet et al. 1986). However, the previous research has shown an increase in muscle lactate production as a direct result of higher glycogenolysis and glycolysis rates with SB during exercise at a controlled constant load (Hollidge-Horvat et al. 2000), thus providing support for the concept that the increased glycolytic contribution estimated in our study reflects, at least in part, an actual increase in glycolytic activation. Moreover, SB ingestion has been shown to increase muscle lactate content (Sutton et al. 1981), suggesting that lactate production, and thereby glycolytic flux, is increased with alkalosis. In line with our findings, Brisola et al. (2015) reported increased anaerobic (lactic + alactic) contribution to high-intensity exercise using the maximal accumulated oxygen deficit method, which relies on post-exercise oxygen consumption kinetics rather than blood lactate values (Brisola et al. 2015). The similarity between our results and those reported by Brisola et al. (2015) further reinforces our hypothesis that increased glycolytic activation accounts, at least partially, for the increased estimated glycolytic that we showed after SB ingestion. Nevertheless, in view of the limitation of our method and based on the previous literature, it seems reasonable to speculate that our results are likely explained by a combination of a true effect of SB on glycolytic activation as well as an artifactual increase in glycolytic contribution driven by increased lactate efflux.
In our study, SB ingestion increased blood bicarbonate concentrations by ~ 5–6 mmol l−1, which appears to be within the range that appears necessary to promote performance improvements (Carr et al. 2011; Heibel et al. 2018; Siegler et al. 2016). Blood pH was also increased only in the groups ingesting SB. Elevation in blood bicarbonate and pH is associated with the increased rates of lactate− and H+ efflux from the working cells, followed by a consequential reduction in H+ concentration inside muscle fibres (Costill et al. 1984). The attenuation in intramuscular acidosis would reduce the inhibitory effect of H+ on glycolytic enzymes, thereby increasing energy transfer via glycolysis (Siegler et al. 2016). This has been considered a major mechanism underpinning the ergogenic effects of SB, although other mechanisms have also been proposed (Siegler et al. 2016). This mechanism can also explain the increased glycolytic contribution estimated in the current, and other, studies (Brisola et al. 2015). In line with the previous research (Jones et al. 2016; Sale et al. 2011; Saunders et al. 2014a), blood bicarbonate was significantly reduced in response to acute exercise, which is an expected response and reflects the fact that the H+ produced within muscle cells are released to the circulation where they are neutralised by bicarbonate anions. Although it seems clear that BA did not elicit any changes in plasma lactate responses to exercise in our study, the effects of SB were less consistent. While a significant increase was observed only in the SB group following HICT-110%, a significant increase was shown in both SB and BASB groups following the time-trial. This could be a consequence of constrained pacing and mechanical work in the HICT-110% protocol in contrast with the time-trial where pacing is not constrained. Similar results were reported by Danaher et al. (2014), where plasma lactate increased in the SB- and BASB-supplemented conditions, but reached significance only in the BASB condition. Although we cannot provide any physiological explanation to these discrepancies, it seems likely that the elevated number of levels in the statistical analyses along with substantial interindividual variation and relatively small samples sizes contributed to inconsistent statistical outcomes. Nonetheless, it appears to exist a clear tendency for SB to increase lactate responses to high-intensity exercise, an effect that seems to be far less evident with BA.
In the present study, time to complete the 30-kJ TT was not significantly improved with BA or SB supplementation, although an improvement that approached significance was shown in the BASB group. The previous research suggests that the ergogenic effects of these buffering agents are more pronounced during exercise capacity tests (Carr et al. 2011; Saunders et al. 2017b), which require the individuals to exert themselves to the point of volitional exhaustion, as opposed to performance tests with a fixed point of cessation (Bellinger et al. 2012). Exercise capacity tests are thought to result in maximal H+ production (Saunders et al. 2017b), thereby being more susceptible to increased buffering capacity. In our study, we opted for a cycling time-trial performance test instead of an exercise capacity test, because it relates more closely to performance in real-world events (Currell and Jeukendrup 2008). However, this type of test has consistently been shown to be less sensitive to detect the ergogenic effects of supplements than capacity tests, since their effects are typically small (Bellinger and Minahan 2016b; Saunders et al. 2017b). This may explain, at least in part, why an effect that approached significance was only shown in the group supplemented with both BA and SB, as the combination of both supplements typically results in a larger effect size (Saunders et al. 2017b). Nonetheless, the effect sizes reported herein (Fig. 4d) are similar to those shown in a previous meta-analysis showing the efficacy of BA on exercise (i.e., ~ 0.1 to ~ 0.2 for exercise performance tests) (Saunders et al. 2017b), which suggests that the lack of statistical significance could be due to insufficient statistical power to detect small effects.
Our results also indicate that BA is safe and free of adverse health effects, at least under the supplementation protocol used in this study, and considering the blood markers analysed. Only 3 out of 36 participants reported to have felt symptoms of paraesthesia, which were mild in all the cases, although we confirmed that the blinding efficacy remained intact for the study as a whole and none of these three individuals behaved as outliers in the performance tests. Although we were unable to examine the effect of SB on blood health markers, 9 out of the 36 participants in the groups ingesting SB reported acute symptoms of gastrointestinal discomfort, one of which reported severe discomfort. This is in line with the previous studies (De Araujo Dias et al. 2015; Saunders et al. 2014b), showing that SB may elicit moderate-to-severe gastrointestinal distress, reinforcing that practitioners must be cautious when recommending SB to athletes in actual competitive settings.
One limitation of this study is the lack of direct assessment of muscle carnosine. Although we could not confirm increased muscle carnosine, it is well established that a similar 4-week BA supplementation protocol (6.4 g day−1) is effective to elevate intramuscular carnosine in 40–80% (Harris et al. 2006; Hill et al. 2007). A recent study showed that the largest part of such increase occurs in the first 4 weeks of supplementation (Saunders et al. 2017a), and that further increases in muscle carnosine are only modest if supplementation is extended to 10 or even 24 weeks (Saunders et al. 2017a; Hill et al. 2007). The lack of muscle analyses also limits the confirmation of the effects of the supplements on energy metabolism. Another limitation is that we were unable to undertake the estimation of the energy systems in all participants, which have reduced the statistical power of this specific analysis. Since the method used to estimate the energy systems is indirect and reliant on blood lactate values to estimate the energy release from glycolysis, we cannot entirely rule out that the treatments have affected lactate transport across sarcolemma, which could, in turn, influence the estimation of glycolytic contribution. In fact, it is possible that this has led us to overestimate the effects of SB on the glycolytic contribution to exercise to some extent, although it seems unlikely that this could explain the increased glycolytic contribution in its entirety. There is robust evidence from studies using more direct measurements of glycolytic activation (Costill et al. 1984; Hollidge-Horvat et al. 2000), indicating that SB increases not only lactate efflux from the working cells, but also glycolytic flux within the working cells.
In conclusion, our results show that SB, but not BA, increases the estimated glycolytic contribution to high-intensity intermittent exercise when total work done is controlled. In addition, both BA and SB alone and in combination did not promote increases in short-duration cycling time-trial performance.
References
Abe H (2000) Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochemistry (Mosc) 65:757–765
Artioli GG, Gualano B, Coelho DF, Benatti FB, Gailey AW, Lancha AH Jr (2007) Does sodium-bicarbonate ingestion improve simulated judo performance? Int J Sport Nutr Exerc Metab 17:206–217
Bellinger PM, Minahan CL (2016a) Metabolic consequences of β-alanine supplementation during exhaustive supramaximal cycling and 4000-m time-trial performance. Appl Physiol Nutr Metab 41:864–871
Bellinger PM, Minahan CL (2016b) The effect of β-alanine supplementation on cycling time trials of different length. Eur J Sport Sci 16:829–836
Bellinger PM, Howe ST, Shing CM, Fell JW (2012) Effect of combined β-alanine and sodium bicarbonate supplementation on cycling performance. Med Sci Sports Exerc 44:1545–1551
Bishop D, Claudius B (2005) Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med Sci Sports Exerc 37:759–767
Bishop D, Edge J, Davis C, Goodman C (2004) Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc 36:807–813
Brisola GM, Miyagi WE, da Silva HS, Zagatto AM (2015) Sodium bicarbonate supplementation improved MAOD but is not correlated with 200- and 400-m running performances: a double-blind, crossover, and placebo-controlled study. Appl Physiol Nutr Metab 40:931–937
Cady EB, Jones DA, Lynn J, Newham DJ (1989) Changes in force and intracellular metabolites during fatigue of human skeletal muscle. J Physiol 418:311–325
Carr AJ, Hopkins WG, Gore CJ (2011) Effects of acute alkalosis and acidosis on performance: a meta-analysis. Sports Med 41:801–814
Costill DL, Verstappen F, Kuipers H, Janssen E, Fink W (1984) Acid-base balance during repeated bouts of exercise: influence of HCO3. Int J Sports Med 5:228–231
Currell K, Jeukendrup AE (2008) Validity, reliability and sensitivity of measures of sporting performance. Sports Med 38:297–316
Danaher J, Gerber T, Wellard RM, Stathis CG (2014) The effect of β-alanine and NaHCO3 co-ingestion on buffering capacity and exercise performance with high-intensity exercise in healthy males. Eur J Appl Physiol 114:1715–1724
De Araujo Dias G, Silva VE, Painelli VS, Sale C, Artioli GG, Gualano B, Saunders B (2015) (In)Consistencies in responses to sodium bicarbonate supplementation. PLoS One 10:e0143086. https://doi.org/10.1371/journal.pone.0143086
Debold EP, Fitts RH, Sundberg CW, Nosek TM (2016) Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc 11:2270–2280
Di Prampero PE, Ferretti G (1999) The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respir Physiol 118:103–115
Duffield R, Dawson B, Pinnington HC, Wong P (2004) Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. J Sci Med Sport 7:11–22
Fitts RH (2016) The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc 11:2335–2338
Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA (2006) The absorption of orally supplied β-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30:279–289
Heibel AB, Perim PHL, Oliveira LF, McNaughton LR, Saunders B (2018) Time to optimise supplementation: modifying factors influencing individual responses to extracellular buffering agents. Front Nutr 5:35. https://doi.org/10.3389/fnut.2018.00035
Hermansen L, Osnes JB (1972) Blood and muscle pH after maximal exercise in man. J Appl Physiol 32:304–308
Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007) Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32:225–233
Hobson RM, Harris RC, Martin D, Smith P, Macklin B, Gualano B, Sale C (2013) Effect of β-alanine, with and without sodium bicarbonate, on 2000-m rowing performance. Int J Sport Nutr Exerc Metab 23:480–487
Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJ (2000) Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol Endocrinol Metab 278:E316–E329
Jones G, Smith M, Harris R (2011) Imidazole dipeptide content of dietary sources commonly consumed within the British diet. Proc Nutr Soc 70:E363
Jones RL, Stellingwerff T, Artioli GG, Saunders B, Cooper S, Sale C (2016) Dose–esponse of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int J Sport Nutr Exerc Metab 26:445–453
Lopes-Silva JP, Silva Santos JF, Branco BH, Abad CC, Oliveira LF, Loturco I, Franchini E (2015) Caffeine ingestion increases estimated glycolytic metabolism during taekwondo combat simulation but does not improve performance or parasympathetic reactivation. PLoS One 10:e0142078. https://doi.org/10.1371/journal.pone.0142078
Lopes-Silva JP, Da Silva Santos JF, Artioli GG, Loturco I, Abbiss C, Franchini E (2018) Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur J Sport Sci 20:1–10. https://doi.org/10.1080/17461391.2018.1424942
Macfarlane DJ (2017) Open-circuit respirometry: a historical review of portable gas analysis systems. Eur J Appl Physiol 117:2369–2386
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc 82:591–605
Oliveira LF, de Salles Painelli V, Nemezio K, Gonçalves LS, Yamaguchi G, Saunders B, Gualano B, Artioli GG (2017) Chronic lactate supplementation does not improve blood buffering capacity and repeated high-intensity exercise. Scand J Med Sci Sports 27:1231–1239
Painelli VS, Roschel H, Jesus FD, Sale C, Harris RC, Solis MY, Benatti FB, Gualano B, Lancha AH Jr, Artioli GG (2013) The ergogenic effect of β-alanine combined with sodium bicarbonate on high-intensity swimming performance. Appl Physiol Nutr Metab 38:525–532
Price M, Moss P, Rance S (2003) Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc 35:1303–1308
Sahlin K, Harris RC, Hultman E (1975) Creatine kinase equilibrium and lactate content compared with muscle pH in tissue samples obtained after isometric exercise. Biochem J 152:173–180
Sakamoto A, Naito H, Chow CM (2018) Effects of hyperventilation on repeated pedaling sprint performance: short vs. long intervention duration. J Strength Cond Res 32:170–180
Sale C, Saunders B, Hudson S, Wise JA, Harris RC, Sunderland CD (2011) Effect of β-alanine plus sodium bicarbonate on high-intensity cycling capacity. Med Sci Sports Exerc 43:1972–1978
Saunders B, Sale C, Harris RC, Sunderland C (2014a) Effect of sodium bicarbonate and β-alanine on repeated sprints during intermittent exercise performed in hypoxia. Int J Sport Nutr Exerc Metab 24:196–205
Saunders B, Sale C, Harris RC, Sunderland CD (2014b) Sodium bicarbonate and high-intensity cycling capacity: variability in responses. Int J Sports Physiol Perform 9:627–632
Saunders B, De Salles Painelli V, De Oliveira LF, Da Eira Silva V, Da Silva RP, Riani L, Franchi M, Gonçalves LS, Harris RC, Roschel H, Artioli GG, Sale C, Gualano B (2017a) Twenty-four weeks of β-alanine supplementation on carnosine content, related genes, and exercise. Med Sci Sports Exerc 49:896–906
Saunders B, Elliott-Sale K, Artioli GG, Swinton PA, Dolan E, Roschel H, Sale C, Gualano B (2017b) β-Alanine supplementation to improve exercise capacity and performance: a systematic review and meta-analysis. Br J Sports Med 51:658–669
Siegler JC, Marshall PW, Bishop D, Shaw G, Green S (2016) Mechanistic insights into the efficacy of sodium bicarbonate supplementation to improve athletic performance. Sports Med Open 2:41. https://doi.org/10.1186/s40798-016-0065-9
Spriet LL, Lindinger MI, Heigenhauser GJ, Jones NL (1986) Effects of alkalosis on skeletal muscle metabolism and performance during exercise. Am J Physiol 251:R833–R839
Sutton JR, Jones NL, Toews CJ (1981) Effect of pH on muscle glycolysis during exercise. Clin Sci (Lond) 61:331–338
Tobias G, Benatti FB, de Salles Painelli V, Roschel H, Gualano B, Sale C, Harris RC, Lancha AH Jr, Artioli GG (2013) Additive effects of β-alanine and sodium bicarbonate on upper-body intermittent performance. Amino Acids 45:309–317
Acknowledgements
We wish to thank the Laboratório de Determinantes Energéticos de Desempenho Esportivo (LADESP) for the access to their facilities. We also would like to thank Hamilton Roschel and Manuel Lixandrão for the advice with statistical analysis, Eimear Dolan for the helpful insights for the discussion, and all the volunteers for their participation. Rafael Pires da Silva, Vitor S. Painelli, Bruno Gualano, Bryan Saunders, and Guilherme Artioli have been financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grants number: 2012/13026-5, 2013/04806-0, 2013/14746-4, 2016/50438-0, and 2014/11948-8). Luana F. de Oliveira has been financially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Bruno Gualano has been financially supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant number 150513/2015-1). Bryan Saunders has previously received financial support from Natural Alternatives International (NAI) to undertake a study unrelated to the current one. NAI have not had any input (financial, intellectual, or otherwise) into this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling Editor: W. Derave.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, R.P., de Oliveira, L.F., Saunders, B. et al. Effects of β-alanine and sodium bicarbonate supplementation on the estimated energy system contribution during high-intensity intermittent exercise. Amino Acids 51, 83–96 (2019). https://doi.org/10.1007/s00726-018-2643-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2643-2