Abstract
Scientists such as physiologists, engineers, and nutritionists have often sought to estimate human metabolic strain during daily activities and physical pursuits. The measurement of human metabolism can involve direct calorimetry as well as indirect calorimetry using both closed-circuit respirometry and open-circuit methods that can include diluted flow chambers and laboratory-based gas analysis systems. For field studies, methods involving questionnaires, pedometry, accelerometery, heart rate telemetry, and doubly labelled water exist, yet portable metabolic gas analysis remains the gold standard for most field studies on energy expenditure. This review focuses on research-based portable systems designed to estimate metabolic rate typically under steady-state conditions by critically examining each significant historical innovation. Key developments include Zuntz’s 1906 innovative system, then a significant improvement to this purely mechanical system by the widely adopted Kofranyi–Michaelis device in the 1940s. Later, a series of technical improvements: in electronics lead to Wolf’s Integrating Motor Pneumotachograph in the 1950s; in polarographic O2 cells in 1970–1980’s allowed on-line oxygen uptake measures; in CO2 cells in 1990s allowed on-line respiratory exchange ratio determination; and in advanced sensors/computing power at the turn of the century led to the first truly breath-by-breath portable systems. Very recent significant updates to the popular Cosmed and Cortex systems and the potential commercial release of the NASA-developed ‘PUMA’ system show that technological developments in this niche area are still incrementally advancing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For centuries, scientists have sought ways to accurately estimate human metabolic expenditure during a wide range of work, leisure and sporting activities. A detailed historical review of the measurement of human energy expenditure during field studies already exists (Shephard and Aoyagi 2012). There also already exist several extensive reviews of physiological respiratory equipment that includes some commentary of the historical developments in gas analysis via indirect calorimetry using either closed-circuit or open-circuit methodologies (Consolazio et al. 1963; Douglas 1956; Durnin and Passmore 1967; Edholm and Weiner 1981; Hill 1981; Hodges et al. 2005; Macfarlane 2001; McLean and Tobin 1987; Meyer et al. 2005; Nichols 1994; Overstreet et al. 2017; Patton 1997; Shephard and Aoyagi 2012).
The EJAP is publishing a series of reviews examining some historical insights into the measurement of whole body metabolic rate. This review will focus on portable research-based devices designed to estimate metabolic rates during typically steady-state conditions, and areas that contribute to measurement errors or other reliability and usability issues. It is hoped that a similar review of laboratory-based gas analysis systems will be soon published as an adjourning paper in this series by Professor S. Ward. This current paper will be delimited to portable systems (those designed to be worn by the user), and will not include “mobile systems” that can be easily carried from room-to-room but are not truly portable (e.g., Cosmed FitMate; Korr ReeVue/MetaCheck/CardioCoach devices; Cortex Metalyzer 3B; Aerosport TEEM100). Some limited commentary will be made here on the validity and reliability of these devices, but this paper is not a systematic review of all these validity and reliability studies. Rather, it focusses on key methodological developments over the past 200 odd years of respiratory physiology that have led to the highly complex portable gas analysis systems we have today. Some of the key landmarks in the development of these systems are summarized in Table 1.
Various requirements are needed to accurately measure human metabolic rates in the traditional steady state (Atkinson et al. 2005), which can then used to estimate energy expenditure from portable open-circuit indirect calorimetry data. Traditionally, precise measurements of all inspired gas flows and expired gas flows are needed (flow = volume per unit time), although some systems negate the measurement of inspired flow as it can be accurately estimated using the Haldane Transformation (Luft et al. 1973; Wilmore and Costill 1973). Accurate calibration of the volume or flow sensors beforehand is critical, as is ensuring no leaks or significant gas loss via diffusion. Also needed are quality wide-bore respiratory tubing, a nose-clip plus low-resistance mouthpiece and two-way respiratory valve, or a well-fitting high-quality facemask with a reflected sealing flange that is checked in situ for inspiratory and expiratory leaks. Precise O2 and CO2 analysers are needed (accurately calibrated at the same gas pressures, temperature, and water vapour pressure as the inspired and expired sample gas), plus precise temperature and pressure measures at the sites where volumes and fractional concentrations are measured. For accurate RER and metabolic rate calculations, both \(\dot {V}\)O2 and \(\dot {V}\)CO2 must be known otherwise significant assumptions and errors can be introduced into the metabolic rate calculations. Low-priced metabolic gas analysis systems without a CO2 sensor (only having a O2 sensor and flow sensor) should, therefore, be treated with considered caution if high precision is needed. A summary of some of the potential sources of error, their magnitude and the possible remedies in portable metabolic gas analysis are found in Table 2.
Although steady-state measurements are ideal (as ventilatory RER then matches the cellular Respiratory Quotient), many daily activities are not reflective of periods of true steady-state activity and may involve multiple transitions between different work rates or involve short intermittent activities (although care needs to be taken to exclude any “anaerobic” events that generate lactic acid and result in added CO2 excretion). Portable gas analysis systems are best-suited to steady-state measurements, but can estimate the metabolic demands during daily activities of varying intensity and duration, but this is not recommended and only if the data are temporally averaged over long periods. Modern breath-by-breath systems allow much greater resolution of rapid metabolic gas transients (non-steady-state), but due to the time delays between sudden changes in muscular activity and when \(\dot {V}\)O2 and \(\dot {V}\)CO2 changes are detected (due to varying circulatory lags and fluctuating gas stores), it is difficult to precisely align rapid changes in physical movements with breath-by-breath analysis. Thus, steady-state conditions remain essential for accurate metabolic rate determinations.
Formative steps towards the development of portable gas analysis systems
Early developments that contributed to the future innovations in expired gas analysis were typically limited to laboratory-based systems due to their considerable bulk, with the foundations of direct and indirect calorimetry often ascribed to the work in Paris by the French chemist Antoine Lavoisier. Although oxygen was discovered in 1774 by Priestley, it was Lavoisier who not only named both oxygen and hydrogen (Partington 1962) but also demonstrated in the 1780s using his ice calorimeter that the carbon dioxide produced by an animal was proportional to the heat it produced (Frankenfield 2010). Very little advanced until 1820–1840 when two groups led by Dulong (1841) and Despretz et al. (1824); Dulong independently designed the first respiratory calorimeters for small animals and later Regnault and Reiset (1849) built the earliest, and not so accurate, closed-circuit system for respiratory measurement in animals (McLean and Tobin 1987). In 1892, Haldane made a significant methodological improvement by designing a simple open-circuit gravimetric device for small animals which permitted accurate measurements of both oxygen and carbon dioxide and hence permitted respiratory exchange ratio (RER) analysis (Haldane 1892). The first open-circuit respiratory chamber suited to human use was built in 1862 at Pettenkofer’s Munich lab (Pettenkofer 1862) as he felt a mask or mouthpiece would interfere with breathing and a simple closed system would give off ‘some odorous and possibly toxic volatile substances’ (Douglas 1956). His chamber could not, however, directly measure oxygen uptake, with the true measurement of oxygen uptake later added in 1905 by Atwater and Benedict (1905) using their re-known method described in outstanding detail—see also the review in this series by Kenny et al. (2017).
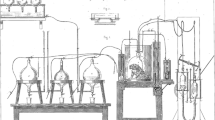
Other innovations contributing to key developments of later gas analysis systems were: first, the work undertaken in 1859 by Smith (1859) of what Douglas (1956) referred to as a “portable open-circuit apparatus”, yet this is better described as a “mobile system” as it could not be carried by the subject (Fig. 1). The subject wore a ‘valved facepiece’ and inspired via a dry gas meter, whilst the expirate passed through a Woulfe bottle containing pumice moistened with strong sulphuric acid to remove water vapour, and then gutta-percha box of potassium hydroxide that removed carbon dioxide, and an identical second Woulfe bottle to dry and remove vapours generated by the potassium hydroxide (Smith 1859). This system could not measure oxygen uptake, but the novel aspect of this system meant the gain in mass of the potassium hydroxide (potash) box was an measure of the carbon dioxide production that was in good agreement with similar measurements taken by Douglas (1956) some 50 years later. Second, the introduction of aliquot sampling of the expirate (Sondén and Tigerstedt 1895). Third, developments of open-circuit systems that permitted “steady-state” collections of expired gas using the Tissot (1904) spirometer, perhaps considered as pioneering work for later “mixing chamber” systems. Fourth, extending Tissot’s measurement to activities outside a laboratory using rubberized Douglas (1911) bags carried on the back—a separate review of the Douglas Bag development has recently been published in this series (Shephard 2017). However, the forefather of modern-day portable gas analysis system is best attributed to Zuntz et al. (1906).
Smith’s early “mobile” respiratory system—modified with permission from The Royal Society (Smith 1859)
Several informative descriptions, reviews and advice on the use of the Tissot, Douglas bag, and the later Kofrayni-Michaelis/Max-Planck systems exist, including sources of potential errors (Consolazio et al. 1963; McLean and Tobin 1987).
1906: Zuntz et al.’s portable respirometer: the first portable gas analysis system
Nathan Zuntz, a dedicated German altitude physiologist, developed this portable open-circuit system from earlier work on a much larger and non-portable system (Geppert and Zuntz 1888) by replacing the wet gas meter with a dry meter (Douglas 1956) and used it on high-altitude studies at the Capanna Margherita research laboratory at Monte Rosa (4559 m) and at Mt Tennerife—see Zuntz’s detailed biography by Gunga (2009). The portable system utilized a face-fitting breathing mask with manually operated valves; a mercury tonometer system for the collection of expired gas samples for later analysis; a steel dry gas meter with bellows connected to a rotating dial for the measurement of expired minute volume (Fig. 2, left); plus an optional hat with an anemometer for measuring wind speed (Fig. 2, right). Although this portable device pre-dated the introduction of the Douglas Bag, Zuntz’s system (Zuntz et al. 1906) has been reported to have been quite accurate, yet heavy and cumbersome (Overstreet et al. 2017), and since this burden outweighed any benefits compared to the Douglas Bag method, it probably contributed to it not being more widely adopted. Zuntz’s innovation did, however, act as a forerunner to the significant development of the Kofrayni-Michaelis/Max-Planck respirometer.
The Zuntz dry gas meter system, also showing it in situ (with anemometer on hat)—modified with permission from the Max-Planck Institute for History of Science archives; http://vlp.mpiwg-berlin.mpg.de
1940: Kofranyi-Michaelis/Max-Planck respirometer
This was a significant development in portable gas analysis systems and estimation of energy expenditure, although curiously Douglas (1956) referred to it only as a “trifling modification” of Zuntz’s system. The Kofranyi-Michaelis device was significant as the first practical estimator of energy expenditure across free-living occupational and recreational activities over extended periods, plus the first to be commercially produced and widely adopted. This pre-electronic device presented a fully mechanical system (Kofranyi and Michaelis 1940) from staff working at the original “Kaiser Wilhem-Institut fur Arbeitsphysiologie” in Dortmund, renamed the Max-Planck Institute in 1949—hence known both as the Kofranyi-Michaelis and/or Max-Planck respirometer.
The original device weighed about 4.3 kg containing a breathing valve with corrugated tubing connected to a twin-bellow dry gas meter (with a thermometer) that could be worn with some comfort on the back using a simple harness (Fig. 3). Perhaps due to earlier suggestions on aliquot sampling by Simonson (Simonson 1928) a pump automatically sampled 0.085% of the expired volume and passed it to small butyl rubber bladders for later chemical analysis (e.g., Haldane apparatus). Later improvements (Müller and Franz 1952), reduced the size of the dry gas meter (20 cm wide, 27 cm high, 11 cm deep), added a new volume counter (rather than the original dial), plus a Perspex viewing lid, and a three-way external sampling valve manually adjustable to (1) off, (2) 0.3% or (3) 0.6% sampling of the expirate; together these reduced the weight to just under 3 kg. As a result, the metabolic cost of wearing the device was estimated and deduced that this added work was insignificant (Consolazio et al. 1963).
Despite being revolutionary, the Kofranyi-Michaelis respirometer had many limitations.
-
1.
Although some of the rubber sampling bladders were treated to reduce carbon dioxide diffusion, this remained an issue and to limit diffusion loss over longer periods it was strongly recommended they be transferred to oiled syringes and analysed within 6 h.
-
2.
Dead space gas within the sampling bladders. In 60 ml bladders the retention and contamination by a small amount of room air (e.g. 3%) prior to measurement would result in a 1% error in oxygen consumption. All bladders needed to be fully evacuated, flushed with expirate (including all tubing), and re-evacuated immediately prior to data collection.
-
3.
Errors in minute ventilation measurement. Considerable variations in errors have been reported, in part due to difference between constant and pulsatile flow calibrations, with ventilator errors varying from 4 to 20%, but with oxygen uptake only being overestimated by 4% (McLean and Tobin 1987). At high gas flows (> 60 l/min—see below), potential existed for the expirate to be quite inaccurately detected. A detailed analysis of all errors contributing to estimation of energy expenditure by the Kofranyi-Michaelis device is presented by Consolazio (p47: maximum negative error of 13.9%, to maximum positive error of 1.5%), and depended on the precision in prior calibrations, of which several methods have been described in detail on their p48-50 (Consolazio et al. 1963). Errors of these nature are also discussed in the review on closed-circuit systems in this series (Archiza et al. 2017).
-
4.
Although the resistance of the system at ventilation rates below 20 l/min was comparable to Douglas bag methods (< 8mmH20), at higher ventilatory rates the resistance increased substantially due to the forces needed to action the bellows and sampling pump (Montoye et al. 1958; Wolff 1956). These resistances are likely due to the Kofranyi-Michaelis system being designed to assess normal working activities with flows of 15–50 l/min (Durnin and Passmore 1967), although Wolff (1956) felt the Kofranyi-Michaelis was not really designed for rates above 30 l/min; the manufacturer considered their device was useable up to 60 l/min (McLean and Tobin 1987). Despite these limitations, the Kofranyi-Michaelis remained a pioneering device in the assessment of energy expenditure across daily, sporting and military activities (Shephard and Aoyagi 2012).
1956: The Wolff Integrating Motor Pneumotachograph
With the development of improved micro-electronics, work at the National Institute for Medical Research (part of the Medical Research Council, in Holly Hill, Hampstead, UK) by Heinz Wolff and his team led to significant improvements over the purely mechanical Kofranyi-Michaelis device, with one reviewer stating “its design was ahead of technology of the time” (McLean and Tobin 1987). Wolff (1956) felt the Kofranyi-Michaelis device could no longer be modified to meet needs of prolonged data collection, or flow rates from 6 to 80 l/min, nor without a significant weight burden to the participant.
Particularly novel in this device was the electronic flowmeter producing an output voltage directly proportional to the instantaneous expired flow, combined with a low flow resistance (< 2.5 cm H2O); the specifics are described in detail elsewhere (Wolff 1958b). Flow was detected via a micro-potentiometer whose signal was integrated over time to provide minute volume using a low friction permanent magnetic electric motor with a linear voltage:motor-speed relationship. Rotation of the motor was measured by a mechanical gear whose count was the time integral of the voltage applied to the motor from the potentiometer and hence directly proportional to the integrated flow rate.
Gas sampling could be undertaken over periods up to 24 h provided collection bags were replaced every 2 h. Aliquot sampling from the flowmeter was done via an adjustable single stroke pump, typically set to take 0.3–0.5 ml from each 1.5 or 4.5 l of expirate and stored in 400 ml of polyvinyl chloride or butyl rubber bag placed in a seamless aluminium canister filled with expired air. A modified Royal Air Force aviator H-type facemask was used, which itself had limitations as it was designed for oxygen delivery and did not have a reflected seal needed to reduce leakage; the bridge of the nose being the main culprit. The H-type mask was made of rubber, lined with chamois leather to improve comfort, but had a substantial deadspace (included nasal chamber and microphone attachment area). To reduce inspiratory resistance, the single RAF mesh-valve on the left cheek was replaced with three spring-loaded mica valves over the nose and each cheek. Modifications of this H-type facemask were also used for the Miser system (below) and in the first successful ascent of Everest (Cotes 1954). The system (Fig. 4 left), including the flowmeter, integrating unit, 90 V battery and sample tin still weighed about 3 kg (comparable to the Kofranyi-Michaelis) and could be worn on the back or chest in a small haversack. Another innovation was the addition of a 250 gm radio-transmitter that permitted transmission of only ventilation data up to 500 yards away (McLean and Tobin 1987), thus showing future trends in this field.
Wolff’s integrating motor pneumotachograph was impressively accurate: when compared to the Douglas Bag over minute volumes ranging 6.4–81.0 l it only varied from − 0.5 to + 0.9% with gas sample differences in expired fractions of O2 and CO2 only varying by − 0.04 to + 0.01% (Wolff 1958a). The integrating motor pneumotachograph was manufactured commercially (J. Langham Thompson Ltd, Bushey Heath, Herts, UK), but it was not widely adopted. This was in part due to it costing four times that of a Kofranyi-Michaelis device (Durnin and Passmore 1967), and despite its clear ingenuity, it was not as rugged as the Kofranyi-Michaelis, requiring skilled maintenance and calibration, with frequent problems with instability of the integrating unit’s transistors; batteries that provided unstable voltages; and damage to connectors (McLean and Tobin 1987).
The Miser 1976
The Miser, introduced in brief (Eley et al. 1976), then later in detail (Eley et al. 1978), was an acronym for Miniature, Indicating (i.e., digital displays), and Sampling Electronic Respirometer from the Physiology Department of Chelsea College in London, as they felt the Kofranyi-Michaelis device and a Dutch portable system (Bleeker and Hoogendoorn 1969) had significant limitations. The Miser was a development of the vacuum bottle sampler (Wright 1961) but swapped electromechanical parts for improved electronic components, yet still was not able to measure expired air on-line and was almost immediately outdated by other systems of the same era (see below).
The Miser had main three parts: a gas meter consisting of a modified H-type facemask with three inspiratory valves and a photo-electronic Wright Respirometer fitted to the expiratory port; a control and display unit with only one moving part (electromagnetic valve) which allowed adjustable sampling of 0.1–0.5 ml of the expirate and taken every 0.4–0.6 l and a vacuum sampler unit (110 ml evacuated aluminium container) with a regulator that kept a constant flowrate into the container until > 93 kPa. The system weighed about 600 gm and the rechargeable battery provided power for 8 h. Tests indicated differences of about 2% in oxygen consumption compared to the Douglas bag method, however, its primary weakness remained leakages around the H-type facemask due to the lack of a reflected seal and the limited accuracy provided by the respirometer (McLean and Tobin 1987).
~ 1970’s : Incorporation of an on-line oxygen electrode
Improvements in the miniaturization of sensors permitted the integration of one or two compact Clark-type polarographic oxygen sensors (Yellow Springs Instruments or Beckman) (Severinghaus 1963) into portable systems allowing the first continuous direct measures of \(\dot {V}\)O2 over extended periods. Modifications of a polarographic O2 electrodes (Clark 1956) introduced a semi-permeable Teflon membrane specific to only oxygen; at a constant polarizing voltage, when O2 diffused through the semi-permeable membrane it is electrochemically reduced at the cathode tip and combined with the KCl solution, simultaneously oxidization at the silver–silver chloride anode occurs resulted in a current that was directly proportional to partial pressure of O2 (PO2) (see also the review in this series by Ward 2017).
New portable systems to use this Clark-type oxygen electrode were: the Aerospace Medical Research Laboratories system (Murray et al. 1968); the Metabolic Rate Monitor (Webb and Troutman 1970); the Oxylog (Humphrey and Wolff 1977); and the Cosmed K2 (Dal Monte et al. 1989), with each providing steady-state \(\dot {V}\)O2 measurements, but as none had on-line CO2 analysis they all required RER assumptions to be made for estimation of metabolic rates. A modification of the Weir equation (Weir 1949) allows estimation of energy expenditure using O2 analysis alone—the Weir “short-cut method” (Consolazio et al. 1963; Durnin and Passmore 1967). Errors in energy expenditure predicted this way vary, with Durnin (p18) claiming only 0.5% error (Durnin and Passmore 1967); yet data from Consolazio (his Tables 5−3 on p. 323, Consolazio et al. 1963) show an average error of 5.7% (Consolazio et al. 1963); this agrees with the typical 6% error seen from indirect calorimetry (Henry 2005) where CO2 production is also not measured. Consolazio also recommended care when using the Weir formula as no check on the normality of respiration is possible without RQ (e.g., hyperventilation).
In the late 1960s, a revolutionary telemetric system was designed at the Wright Patterson Air Force Base in Ohio. This Aerospace Medical Research Laboratories system was a miniaturized, multichannel, pulse-duration modulated and multiplexed, personal radio-telemetry unit (90 m range, total mass of about 840 g) that could simultaneously transmit up to six channels: 3 ECG signals, ambient or body temperature, ventilatory flow (mass flowmeter), plus the difference between inspired and expired oxygen fraction permitting on-line determination and continuous telemetric transmission of \(\dot {V}\)O2 (Murray et al. 1968). The authors claimed excellent results (r = 0.993) compared to spirometric collection and gas chromatograph oxygen analysis up to oxygen consumptions of 3.2 l min.
The Metabolic Rate Monitor (Webb and Troutman 1970) used a very unique facemask design with no valves, no nose-clip, nor breathing resistance due to the motor-blower flow-through arrangement which was apparently well received by users. Limitations of the metabolic rate monitor included that the servo-unit could not be easily carried; it did not measure minute ventilation; and it only produced a time-average \(\dot {V}\)O2 output. But over \(\dot {V}\)O2 ranges from rest to 3.0 l min this device was shown to measure \(\dot {V}\)O2 within 0.1 l min when compared to the Douglas bag method and with good linearity (Webb and Troutman 1970).
The Oxylog (Humphrey and Wolff 1977), later commercially produced by PK Morgan Ltd (Rainham, Kent, UK), was a development by Humphrey and Wolff of the original Integrating Motor Pneumotachograph (see above), as Humphrey helped maintain many of these earlier devices (Shephard and Aoyagi 2012). The system used a facemask with an ambient thermistor [known for leakage issues: (Harrison et al. 1982)] and a Wright respirometer mounted to the inspiratory valve to measure inspired flows. A dynamic sample of mixed expired air was continuously drawn by a small double-piston pump, dried via a tube of anhydrous calcium sulphate and measured by a Beckman polarographic electrode with its own thermistor. Samples of inspired gas were similarly dried and measured by a second oxygen sensor (a unique feature at the time, rather than assuming the fraction of inspired O2 = 0.2093), with electronic circuits reporting the differences between inspired–expired volumes and oxygen tensions, plus digital displays of ventilation and oxygen consumption. The authors reported the system weighed 2.5 kg and was suited to ventilations of 6–80 l min (\(\dot {V}\)O2’s of 0.25–3.0 l min) with its internal rechargeable batteries permitting data collection up to 24 h. The Oxylog was substantially upgraded in 1994 to improve its electronics, data acquisition plus storage capacity, and switched oxygen measurement to small galvanic (electrochemical) fuel cells (Patton 1997). These small galvanic fuel cells generated a very small current proportional to the PO2; when O2 diffuses through the Telfon-covered O2-sensing cathode it undergoes reduction, whilst oxidation of the lead anode simultaneously occurs, with both electrodes separated by a potassium hydroxide electrolyte.
Key studies on the reliability and validity of the Oxylog (Ballal and Macdonald 1982; Harrison et al. 1982; Louhevaara et al. 1985; McNeill et al. 1987) have been summarized by Patton (1997), with the Oxylog comparing well with the Douglas Bag, with discrepancies often less than 3–5%. Its reported limitations included facemask leakage, discomfort of carriage, and the small digital displays (McLean and Tobin 1987). Historically important was the study of Ikegami and colleagues, who modified the Oxylog to incorporate a telemetry system to measure \(\dot {V}\)O2 during an 80-minute tennis game (Ikegami et al. 1988). This was reported as the first continuous measurement of \(\dot {V}\)O2 during an actual sporting event (Patton 1997), although the designers of the Aerospace Medical Research Laboratories system (Murray et al. 1968) may contend their system had this potential 20 years earlier.
Production of the Cosmed K2 (Dal Monte et al. 1989) began a series of significant evolutions towards becoming a leading manufacturer of portable gas analysis systems. The K2 used a specific facemask attached to a photoelectric turbine flowmeter (range 2–300 l min), connected via a capillary tube for measuring the expirate via a polarographic oxygen electrode. This used a novel proportional sampling method where the sampling pump was always in phase with the ventilator signal and whose capacity was also proportional to the ventilation. This patented system acted like a miniature “dynamic mixing chamber” (US-4631966). The total system only weighed ~ 850 g, was capable of also recording heart rate (Polar monitors) and telemetric transmission of all data back to a base-station (~ 100 m range)—(see Fig. 4 right).
Studies on the reliability and validity of the novel K2 have also been summarized by others (Macfarlane 2001; Meyer et al. 2005; Overstreet et al. 2017; Patton 1997), with the K2 being reported as being generally reliable. However, its validity varied—some reported overestimates of resting \(\dot {V}\)O2 up to ~ 20%, but typically during exercise the K2 produced \(\dot {V}\)O2 values that were acceptably close to criterion measures (typically < 6% error).
Readers are reminded that the typical flow sensors in portable systems vary and each has limitations briefly mentioned here (see also the review by Ward 2017). Pneumotachometers require laminar flow for good linearity by sensing a differential pressure drop across a small resistance (Fleisch uses parallel capillaries; Lilly uses 3 mesh screens), but are heavy, and (if not heated) spittle or expired water vapour can accumulate on the screens increasing the flow resistance, and are difficult to clean. Pitot tubes (Porszasz et al. 1994) and variable orifice devices (Osborn 1978) are lightweight, often disposable, less sensitive to blockages and easy to clean, but not as linear in their responses and such as pneumotachometers still need a differential pressure sensor. Turbines have become increasingly popular due to their lightweight (no differential pressure sensor), low deadspace, and relatively insensitive to expirate composition, temperature or humidity. The optical sensor directly measures the vane rotations which should be proportional to the flow rate; although turbines can show impressive reliability (coefficient of variations 0–0.2%) and validity (96–101% accurate) across a full range of sinusoidal flows (Hart and Withers 1996), problems with their “lag before start” and “spin after stop” can cause measurement issues (Ilsley et al. 1993), especially in breath-by-breath systems (Howson et al. 1987; Yeh et al. 1987).
~ 1994–1997 Introduction of a CO2 sensor
The transformative addition of a miniaturized non-dispersive infra-red (NDIR) CO2 sensor supporting the established O2 sensor, permitted the first direct portable measurements of \(\dot {V}\)O2 and \(\dot {V}\)CO2 using the Haldane Transformation and without the need for an assumed RER value; a detailed review of NDIR CO2 sensors exists (Jaffe 2008). Essentially, as CO2 strongly absorbs infra-red radiation, electromagnetic radiation from two nickel–chromium heat sources are sent down two absorption cells (one reference nitrogen cell, one sample cell). The amount of radiation absorbed (relative to the reference cell) is measured by a pressure and temperature-sensitive detector, with changes in its capacitance being proportional to the PCO2 in the sample.
The earliest manufacturers to commercially produce these combined systems included Cosmed with their K4/K4RQ (Hausswirth et al. 1997), Cortex with their X1/MetaMax 1 (Schulz et al. 1997), and Aerosport with their KB1-C (King et al. 1999). These were still not breath-by-breath (B × B) \(\dot {V}\)O2 or \(\dot {V}\)CO2 analysis systems, but still relied on proportional sampling of the expirate typically using a miniature mixing chamber. The benefits of proportional sampling are that only a small “representative” sample of each breath is collected and analysed in a micro-mixing chamber. This avoids large mixing chambers for the entire expirate (not possible for portable systems), and micro-mixing chambers also provide more stable determination of gas fractions than later-developed B × B monitoring (Overstreet et al. 2017)—this can be also visualized by comparing the O2 and CO2 signals from the latest Cosmed K5 “IntelliMET” system that can switch between both modes (see later and Fig. 6).
Released in 1994, the K4/K4RQ replaced the K2’s polarographic electrode with a galvanic fuel cell (Meyer 1990) for O2 measurement (9–22% O2) along with an NDIR CO2 sensor (0–8%). It also retained the DMC (Dynamic Mixing Chamber, ~0.5 cm3: see upper part of Fig. 6) for micro-proportional sampling of the expirate as this lead to greater stability of the expired gas fractions over ventilatory flows from 4 to 250 l min. The system was relatively small (front-mounted unit 170 × 48 × 90 mm; rear-mounted battery 120 × 20 × 80 mm), weighing ~ 800 g, with a unidirectional telemetry range of > 300 m, and an integrated barometer plus ambient temperature sensor. Overviews of the K4/K4RQ performance have been reported (Macfarlane 2001; Meyer et al. 2005; Overstreet et al. 2017), with most studies showing it to be adequately valid across a range of intensities, as well as suitably reliable.
The X1 (Cortex, Leipzig, Germany) comprised a facemask, transmitter and receiver unit of considerable size (4.5 kg). It used Jaeger’s facemask and patented photoelectric TripleV turbine transducer with a capillary tube to sample the expirate proportional to the tidal flow into a micro-mixing chamber. With its standard infra-red CO2 sensor, the evolution in the X1 was the inclusion of a small zirconium oxygen sensor (Benammar 1994) that was very temperature stable (unlike previous polarographic O2 electrodes), and are known to be rapid and accurate (Poole and Maskell 1975). When the zirconium-oxide tube in the oxygen cell is heated > 800 °C it acts as a semi-permeable layer conductive to O2, whilst the inner and outer platinum surfaces act as electrodes. A voltage is generated proportional to the sample PO2 when a sample gas is passed down the central tube and a reference gas (ambient air) passed over the outer surface. The X1 had a telemetry range of ~ 2 km over flat ground, but could buffer data internally for 8.5 h, although normal battery power lasted ~ 1.5 h (Schulz et al. 1997). The X1 showed impressive stability of its O2 and CO2 sensors as well as excellent linearity of the volume transducer up to 288 l/min. When compared to a criterion Oxycon-Gamma system, there was minimal bias in both \(\dot {V}\)O2 and \(\dot {V}\)CO2, with values within normal daily variations of 4–6% (Schulz et al. 1997). The main issue of concern with the X1 was its significant mass (4.5 kg) when compared to its new competitors. The X1 was apparently later referred to as the “MetaMax I” and further developed to the “MetaMax II” that have been shown to be generally valid and reliable (Friedman et al. 1998; Larsson et al. 2004; Medbø et al. 2000, 2012; Meyer et al. 2001, 2005; Schulz et al. 1997).
The Aerosport KB1-C (Ann Arbor, MI) was unique in not only having a pneumotachometer with three flow settings (low 4–50, medium 10–120, and high 25–225 l min) but also adopted gas sampling that took a micro-sample that was directly proportional to the pressure differential across the pneumotachometer’s orifice plate (minute ventilation was similarly determined). The main module contained the galvanic fuel cell (O2 0–25%), NDIR CO2 sensor (0–10%), Polar heart rate sensor and the telemetry unit (~ 300 m range), plus a separate battery pack, all weighing ~ 1.2 kg. Performance of the KB1-C has been summarized before (Macfarlane 2001; Meyer et al. 2005; Overstreet et al. 2017), with it being acceptably reliable during steady-state measures; the medium-flow pneumotachometer was adequately valid at higher work rates but demonstrated considerable errors at Rest and 50 W (where the low-flow pneumotachometer was more acceptable).
~ 1997–2000+: Introduction of breath-by-breath (B × B) capabilities
The advent of improved sensors and advanced computerization permitted the complex algorithms necessary for the first breath-by-breath (B × B) \(\dot {V}\)O2 and \(\dot {V}\)CO2 analysis in portable systems. These systems used low resistance respiratory turbines/tubes and rapid gas sampling near the lips, typically with an integrated Nafion/Permapure “drying” tube (Namieśnik and Wardencki 1999), thus negating the need for proportional sampling micro-mixing chambers. Several informative comparisons of micro-proportional mixing chambers and breath-by-breath methods, including potential sources of errors, have been undertaken (Beijst et al. 2013; Overstreet et al. 2017; Roecker et al. 2005). These B × B systems were highly portable, often with comprehensive sensors (O2, CO2, ventilation, ambient temperature, pressure, humidity, ECG, saturation of arterial oxygen) and typically the option of telemetric transmission of heart rate plus all gas analysis variables over more than 100 m. Common systems included: Cosmed K4b2 (McLaughlin et al. 2001); Cortex MetaMax 3B (also sold as the Sensormedics VMaxST) (Prieur et al. 2003); MedGraphics VO2000 (Crouter et al. 2006); and later the Jaeger Oxycon Mobile (Rosdahl et al. 2010).
The Cosmed K4b2 was released in 1998, a few years after the K4RQ, and was revolutionary as the first commercially available portable B × B system. Although lab-based B × B systems existed for many prior years (Beaver et al. 1973; Roecker et al. 2005), these portable B × B systems allowed not only steady-state metabolic measurements but also additional insights into rapid \(\dot {V}\)O2 kinetics during field studies (Overstreet et al. 2017; Roecker et al. 2005). Yet the inherent noise of B × B systems can also not only impair the study of system linearity of the \(\dot {V}\)O2 kinetic response (Hughson 2009) but also produces greater potential error in \(\dot {V}\)O2 and \(\dot {V}\)CO2 when compared to a mixing chamber system (Beijst et al. 2013), suggesting that mixing chamber systems may have advantages when measuring metabolism in traditional steady-state conditions (Atkinson et al. 2005). Known difficulties exist in the B × B methodology as it requires very precise matching of the ventilatory flow signals with the time delays and dynamic responses of the O2 and CO2 analysers (Hughson et al. 1991; Roecker et al. 2005); these problems are not so critical in micro-proportional sampling systems. Accurate calibration of B × B systems is, therefore, crucial as small errors, and often variable errors (such as varying condensation in the sample line could change the resistance, hence flow and delay time), could influence this alignment process to create significant errors in \(\dot {V}\)O2 (up to 30%), especially at high respiratory frequencies (Boutellier et al. 1987; Hughson et al. 1991; Proctor and Beck 1996). In addition, simple peristaltic pumps used in the sample lines typically do not generate a constant flow and this may exacerbate errors in the correct time delays to the sensors and why more recent B × B systems have tried to incorporate improved constant flow pump technology. The known problems caused by angular momentum of the vane in turbine flow sensors (Yeh et al. 1987) can also provide a greater source of error in the minute ventilation signal in a B × B system than in a mixing chamber system (Atkinson et al. 2005; Beijst et al. 2013).
The Cosmed K4b2 system measured both inspired and expired flow via a bi-directional digital turbine (resistance < 0.7 cm H2O at 14 l/s), and a peristaltic volume pump sampling the expirate at a specific rate that was drawn into the now commonly used gas analysers—galvanic fuel cell (O2) and NDIR (CO2). To align gas flows with fractions, the calibration process determined the two key ‘time delays’ (~ 350 milliseconds from facemask to analysers; ~150 milliseconds for 90% full scale analyser response time), and aligned them using a specific algorithm. The K4b2 is described in detail (Pinnington et al. 2001) and despite its sophistication, it weighed only about 1 kg, and has been used extensively (Cosmed’s website claims > 600 publications in total).
The Cortex MetaMax 3B/VMaxST avoided the front-sensor/rear-battery mounting system used by the Cosmed systems in favour of twin modules (each 120 × 110 × 45 mm) mounted on each side of the chest (one measurement, one battery) and supported by a neck/shoulder harness. It used the well-known Vmask (Hans Rudolph facemask) connected to the Jaeger TripleV turbine, but unlike the MetaMax I and II, the zirconia O2 cell was replaced by the more common galvanic fuel cell, whilst retaining the NDIR CO2 sensor. The system weight about 1.2 kg with a battery life of ~ 2 h, and permitted bi-directional telemetry > 500 m along with ECG data acquisition.
The Jaeger Oxycon Mobile (~ 1 kg) used some similar design features with the MetaMax 3B (twin chest, or back, modules: 126 × 96 × 41 mm each) and its patented TripleV turbine. Whilst also using a galvanic fuel cell for O2 analysis, the Oxycon differed from the Cortex and Cosmed by adopting a thermal conductivity cell for CO2 analysis, with both sensors in the Oxycon being fast responding (claimed 90% response in 80 ms). Two versions were available: Version I in 2002—Jaeger/VIASYS Healthcare; Version II after 2005—Carefusion.
The MedGraphics VO2000 system was very lightweight (~ 800 g) but did not report B × B data, rather only three-breath averages, using MedGraphic’s patented ‘PreVent’ tube (a unique pitot tube of very low resistance and mass that avoided vane-related momentum problems seen in many turbines), and a proportional sampling valve that passed expirate through common galvanic fuel cell and NDIR sensors.
Despite this review not being aimed at providing a detailed summary of the numerous validity and reliability studies for each of these systems, a sample of these reports are cited below to allow readers further consultation and are summarized in Table 3. In their review paper, Meyer and colleagues conclude that modern portable systems in general show acceptable accuracy and sufficient reliability that is typically not inferior to stationary/lab-based metabolic carts (Meyer et al. 2005).
K4b 2 (Darter et al. 2013; Duffield et al. 2004; McLaughlin et al. 2001; Pinnington et al. 2001; Schrack et al. 2010).
MetaMax 3B/VMaxST (Blessinger et al. 2009; Brehm et al. 2004; Laurent et al. 2008; Macfarlane and Wong 2012; Perkins et al. 2004; Prieur et al. 2003; Vogler et al. 2010).
Oxycon Mobile (Attinger et al. 2006; Eriksson et al. 2011; Perret and Mueller 2006; Rosdahl et al. 2010).
VO2000 (Crouter et al. 2006; Wahrlich et al. 2006; Winkle et al. 2011).
Only the VO2000 system was no longer available in 2015, with the review by Overstreet and colleagues reporting that of the remaining available systems all three were found to be acceptably reliable (Overstreet et al. 2017). They also reported that when compared to criterion Douglas Bag methods across a wide range of intensities (Rest to Max), the Cosmed K4b2 and Oxycon Mobile-II were able to provide valid estimates of \(\dot {V}\)O2 (means within ± 0.10 l min), however, the MetaMax 3B tended to overestimate \(\dot {V}\)O2, particularly at higher intensities.
Reports on maintenance issues/problems on any of these more recent portable systems is scarce and is typically anecdotal, although a 2004 Biomechanics web-forum reported a wide range of user comments on Cosmed, Cortex, Medgraphics and Jaeger portable systems. Several users commented on the two more common systems regarding problems, citing some MetaMax 3B issues (e.g., telemetry unit, connectors especially to the volume sensor, rapid O2 cell deterioration), and K4b2 issues (weak soldering, other maintenance issues requiring regular service). As the age or maintenance of these devices was not reported, such anecdotal comments need to be viewed carefully as factory updates are likely to have addressed these issues in later iterations.
~ 2006–2015 NASA PUMA system
The National Aeronautics and Space Administration Glenn Research Center (NASA GRC) in Cleveland OH, in conjunction with Case Western University and the Cleveland Clinic, led by Dan Dietrich, developed a very innovative (patent-pending) system for the International Space Station. In 2006, supported by Cleveland-based Orbital Research, this development became the Portable Unit for Metabolic Analysis (PUMA) that could rapidly monitor \(\dot {V}\)O2 and \(\dot {V}\)CO2 over prolonged periods in flight crew and astronauts without being tethered to a base unit (National Aeronautics and Space Administration 2017).
Inspired and expired flow is measured by a modified commercial ultrasonic sensor and sampled very close to the mouth at 10 Hz (allowing intra-breath measurements), and analysed by very rapidly responding sensors. The unique oxygen sensor is based on the fluorescence quenching of a Ruthenium-based dye sensor developed at the NASA Glenn Research Center. Sinusoidally modulated blue light from a laser diode is used to excite a Ruthenium-based dye which then fluoresces an orange light which is phase-shifted relative to the blue light. The degree of phase shift is proportional to the oxygen fraction and the sensor is reported to have no drift, nor sensitivity to CO2. Carbon dioxide is detected by several infra-red LEDs emitting light at 4.3 μm and a thermoelectrically cooled detector placed ~ 1 cm away. Other commercial sensors detected pressure, temperature and heart rate, with the entire system contained in a unique headgear apparatus (Fig. 5) powered by a commercial camcorder battery, and telemeters data to a laptop via Bluetooth (Dietrich 2013). In April 2016, it was announced that this PUMA system is in the process of being commercialized for the fitness market by AirFlare LLC in Nashville, Tennessee, but as yet no release date has been provided nor have any substantive validity or reliability data been disseminated.
Prototype of the NASA—PUMA metabolic system modified with permission from NASA (National Aeronautics and Space Administration 2017)
~ 2015 + recent updates
In 2015/2016, the two major manufacturers of portable gas analysis systems updated their research-oriented devices.
Cosmed made a significant transformation with their K5 (174 × 64 × 114 mm, 4 h battery, ~900 gm): a unique feature is the option of combining both micro-proportional sampling into a small dynamic mixing chamber, together with B × B technology (via optional ‘IntelliMET’ module—Intelligent Dual Metabolic Sampling Technology: Fig. 6). The option of dual measurement allows users to undertake more conventional steady-state metabolic measurements via the dynamic mixing chamber, or to examine kinetics during transients, permitting greater versatility by allowing users to mitigate criticisms of either sampling method. Additional improvements include: improved dynamic mixing chamber technology to include a constant flow pump instead of the previous peristaltic pump for added reliability; an integrated 10 Hz GPS receiver for navigation/motion; integrated ANT + technology for optional wireless sensors; 3.5″ TFT back-lit LCD touch-screen; weatherproofing (IP54 standard); standard or long-range Bluetooth 2.1; an SD-HC card for additional data storage; new OMNIA PC software (Fig. 7, left). Preliminary data suggest this system is adequately reliable and valid compared against a criterion VacuMed metabolic simulator (Baldari et al. 2015; Bolletta et al. 2016).
Cortex has incrementally updated their MetaMax 3B (Fig. 7, right) to include dynamic flow sampling that ensures a more constant control of sample line flow even when resistances change; 6 h internal battery; modular main electronic board that permits individual components to be replaced (rather than an entire new board); new push/pull cable connectors for greater reliability; long-range Bluetooth 2.1; external GPS; enhanced firmware and new MetaSoft Studio software; new touch-screen Remote Control unit (removing the need for a laptop in the field). No data appears available yet on its updated validity or reliability.
Both the Cosmed K5 and Cortex MetaMax 3B also have a special ventilatory snorkel-type hardware option designed to assist in data acquisition during swimming: the Cortex “MetaSwim” (currently being updated), and the Cosmed “Aquatrainer”.
Over the past decade, there have been developments of several simple yet innovative portable handheld systems designed primarily for consumers that provide a basic measurement of \(\dot {V}\)O2 and an estimate of metabolic rate. These have included the MedGem (FDA approved medical device) and the BodyGem from Microlife (USA), but like some mobile (but not portable) devices (e.g., Cosmed’s FitMate-Pro/Med; Korr’s ReeVue/MetaCheck/CardioCoach) these types of devices have limitations in providing only O2 analysis and require RER assumptions to be made. Despite evidence that suitable predictive equations may provide reasonably valid results for such handheld consumer devices (McDoniel 2007), these handheld devices are unlikely to be accepted in high-quality research where direct measures of \(\dot {V}\)O2 and \(\dot {V}\)CO2 are needed. More recently several handheld consumer-based devices that take both O2 and CO2 measurements have also been devised: “Breezing” (Temple, AZ), with simple validity data reported by the company system’s developers and thus is not sufficiently independent due to potential conflicts of interest (Xian et al. 2015). A new PATH “Breath and Fat Band” sensor that claims to measure flow, O2 and CO2 is also under development via Kickstarter crowd funding. However, all these types of handheld consumer devices are only likely to function at relatively low/resting metabolic rates and unlikely to have a functional role during more intense exercise or in quality research studies.
Conclusions
Over more than 110 years of development in portable gas analysis systems, we have seen many significant advances in the estimation of metabolic rate under steady-state conditions. Beginning in 1906 with Zuntz’s revolutionary, but heavy and purely mechanical device, with limited gas sampling that required chemical analysis afterwards; in 1940 the first commercial portable system, the Kofranyi-Michaelis respirometer, allowed portable collection with aliquot sampling but remained entirely mechanical, yet permitted the first widely accepted instrument for routine field and research studies of metabolic rate; the Wolff Integrating Motor Pneumotachograph (1958) begin a new era of electronic data measurement; whilst the introduction of on-line polarographic O2-cells in the 1970–1980’s allowed the first continuous recording of \(\dot {V}\)O2 data; then in the early 1990s the introduction of small NDIR CO2 cells permitted both on-line \(\dot {V}\)O2, \(\dot {V}\)CO2 and hence RER determination for more accurate metabolic rate estimates, along with proportional sampling and the introduction of dynamic micro-mixing chamber technology (K4RQ) as well as the new galvanic fuel cell for O2 analysis; from 1998 onwards new miniaturization of sensors and computerization permitted the development of the first of several true B × B portable gas analysis systems (allowing both steady-state and kinetic studies). Since then, further incremental developments have been seen, with wireless technologies, GPS, and new miniature sensors allowing a wide range of optional ambient and physiological measurements to be recorded or transmitted. However, the industry has seen a retraction in the number of companies producing these expensive research-grade devices that may reflect a plateau or even a diminution in the research and commercial potential of this area.
The significant cost of product development and the relatively small demand for high-grade portable gas analysis systems means that beyond the existing few commercial manufacturers who can rely on modifications of their existing technologies, typically only government-supported organizations have any potential for substantial new product development in this area. The NASA PUMA system is a good example of such a government-funded project leading to a significant innovation with strong commercial potential due to its unique head-mounted position, lightweight yet powerful sensors and apparent rugged design. Although the future for expansion in demand for these niche portable systems for predominantly steady-state metabolic measurement may seem limited, there still exists sufficient demand for applications requiring portable gas analysis technologies (Meyer et al. 2005) that may allow this field to keep moving incrementally forward.
Abbreviations
- B × B:
-
Breath-by-breath
- CO2 :
-
Carbon dioxide
- CV:
-
Coefficient of variation
- FIO2 :
-
Fraction of inspired oxygen
- FEO2 :
-
Fraction of expired oxygen
- FECO2 :
-
Fraction of expired carbon dioxide
- GESV:
-
Gas exchange system validator
- H2O:
-
Water
- ICC:
-
Intraclass correlation coefficient
- GPS:
-
Global positioning system
- NDIR:
-
Non-dispersive infra-red
- O2 :
-
Oxygen
- PCO2 :
-
Partial pressure of carbon dioxide
- PO2 :
-
Partial pressure of oxygen
- RER:
-
Respiratory exchange ratio
- SEM:
-
Standard error of measurement
- TEM:
-
Technical error of measurement
- \(\dot {V}\)O2 :
-
Oxygen uptake
- \(\dot {V}\)CO2 :
-
Carbon dioxide production
References
Archiza B, Welch JF, Sheel AW (2017) Classical experiments in whole-body metabolism: closed-circuit respirometry. Eur J Appl Physiol 117:1929–1937
Atkinson G, Davison R, Nevill A (2005) Performance characteristics of gas analysis systems: what we know and what we need to know. Int J Sports Med 26:S2-10
Attinger A, Tuller C, Souren T, Tamm M, Schindler C, Brutsche MH (2006) Feasibility of mobile cardiopulmonary exercise testing. Swiss Med Wkly 136:13–18
Atwater WO, Benedict FG (1905) A respiration calorimeter with appliances for the direct determination of oxygen. Carnegie Institute of Washington, Washington
Baldari C, Meucci M, Bolletta F, Gallotta MC, Emerenziani GP, Guidetti L (2015) Accuracy and reliability of COSMED K5 portable metabolic device versus simulating system. Sport Sci Health 11:S58
Ballal M, Macdonald I (1982) An evaluation of the Oxylog as a portable device with which to measure oxygen consumption. Clin Phys Physiol Meas 3:57–65
Beaver WL, Wasserman K, Whipp BJ (1973) On-line computer analysis and breath-by-breath graphical display of exercise function tests. J Appl Physiol 34:128–132
Beijst C, Schep G, van-Breda E, Wijn PFF, van-Pul C (2013) Accuracy and precision of CPET equipment: A comparison of breath-by-breath and mixing chamber systems. J Med Eng Technol 37:35–42
Benammar M (1994) Techniques for measurement of oxygen and air-to-fuel ratio using zirconia sensors. A review. Meas Sci Technol 5:757–767
Bleeker J, Hoogendoorn M (1969) A portable apparatus for continuous measurement of oxygen consumption during work. Acta Physiol Pharmacol Neerl 15:30–36
Blessinger J, Sawyer B, Davis C, Irving BA, Weltman A, Gaesser G (2009) Reliability of the VmaxST portable metabolic measurement system. Int J Sports Med 30:22–26
Bolletta F, Meucci M, Emerenziani GP, Gallotta MC, Guidetti L, Baldari C (2016) Accuracy and reliability of the K5 portable metabolic system in micro-mixing chamber mode using a gas exchange simulator system. Sport Sci Health 12:S43
Boutellier U, Kundig T, Gomez U, Pietsch P, Koller EA (1987) Respiratory phase detection and delay determination for breath-by-breath analysis. J Appl Physiol 62:837–843
Brehm MA, Harlaar J, Groepenhof H (2004) Validation of the portable VmaxST system for oxygen-uptake measurement. Gait Posture 20:67–73
Clark LC (1956) Monitor and control of blood and tissue oxygen tensions. ASAIO Trans 2:41–48
Consolazio CF, Johnson RE, Pecora LJ (1963) Physiological measurements of metabolic functions in man. McGraw-Hill, New York
Cotes J (1954) Ventilatory capacity at altitude and its relation to mask design. Proc R Soc Lond B Biol Sci 143:32–39
Crouter SE, Antczak A, Hudak JR, DellaValle DM, Haas JD (2006) Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol 98:139–151
Dal Monte A, Faina M, Leonardi L, Todaro A, Guidl G, Petrelli G (1989) II consumo massimo di ossígeno in telemetría. Rivista di Cultura Sportiva 15:35–44
Darter BJ, Rodriguez KM, Wilken JM (2013) Test–retest reliability and minimum detectable change using the K4b2: oxygen consumption, gait efficiency, and heart rate for healthy adults during submaximal walking. Res Q Exerc Sport 84:223–231
Despretz CM, Arago F, Gay-Lussac JL (1824) Recherches expérimentales sur les causes de la chaleur animale. Ann Chim Phys 26:337–364
Dietrich DL (2013) NASA’s PUMA provides valuable patient data. Medical Design, http://m.medicaldesign.com/electronics/nasa-s-puma-provides-valuable-patient-data
Douglas CG (1911) A method for determining the total respiratory exchange in man. J Physiol (Lond) 42:17-18P
Douglas CG (1956) The development of experimental methods for determining the energy expenditure of man. Proc Nutr Soc 15:72–77
Duffield R, Dawson B, Pinnington H, Wong P (2004) Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. J Sci Med Sport 7:11–22
Dulong PL (1841) Mémoire sur la chaleur animale. Ann Chim Phys 1:440–455
Durnin JVGA, Passmore R (1967) Energy, work and leisure. Heinemann Educational Books, London
Edholm O, Weiner J (1981) Introduction to the history of human physiology. In: Edholm O, Weiner J (eds) the principles and practice of human physiology. Academic Press, London, pp 1–18
Eley C, Goldsmith R, Layman D, Wright BM (1976) A miniature indicating and sampling electronic respirometer (Miser). J Physiol (Lond) 256 (Suppl):59-60P
Eley C, Goldsmith R, Layman D, Tan GL, Walker E, Wright BM (1978) A respirometer for use in the field for the measurement of oxygen consumption. ‘The Miser’, a miniature, indicating and sampling electronic respirometer. Ergonomics 21:253–264
Eriksson JS, Rosdahl H, Schantz P (2011) Validity of the Oxycon Mobile metabolic system under field measuring conditions. Eur J Appl Physiol 112:345–355
Frankenfield DC (2010) On heat, respiration, and calorimetry. Nutrition 26:939–950
Friedman B, Frese F, Bartsch P (1998) Ergospirometriesysteme vs. Douglas-Bag-Methode: evaluation einer stationaren und einer portablen messeinheit. Dtsch Z Sportmed 49:67–70
Geppert J, Zuntz N (1888) Ueber die regulation der athmung. Pflügers Archiv Eur J Physiol 42:189–245
Gunga HC (2009) Nathan Zuntz: his life and work in the fields of high altitude physiology and aviation medicine. Academic Press, Burlington
Haldane J (1892) A new form of apparatus for measuring the respiratory exchange of animals. J Physiol (Lond) 13:419–430
Harrison MH, Brown GA, Belyavin AJ (1982) The ‘Oxylog’: an evaluation. Ergonomics 25:809–820
Hart JD, Withers RT (1996) The calibration of gas volume measuring devices at continuous and pulsatile flows. Aust J Sci Med Sport 28:61–65
Hausswirth C, Bigard A-X, Le Chevalier J-M (1997) The Cosmed K4 telemetry system as an accurate device for oxygen uptake measurements during exercise. Int J Sports Med 28:449–453
Henry C (2005) Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8:1133–1152
Hill D (1981) Instrumentation for physiological measurements. In: Edholm O, Weiner J (eds) Principles and practice of human physiology. Academic Press, London, pp 529–577
Hodges LD, Brodie DA, Bromley PD (2005) Validity and reliability of selected commercially available metabolic analyzer systems. Scand J Med Sci Sports 15:271–279
Howson MG, Khamnei S, O’Connor DF, Robbins PA (1987) The properties of a turbine device for measuring respiratory volumes in man. J Physiol (Lond) 382:12P
Hughson RL (2009) Oxygen uptake kinetics: historical perspective and future directions. Appl Physiol Nutr Metab 34:840–850
Hughson RL, Northey DR, Xing HC, Dietrich BH, Cochrane JE (1991) Alignment of ventilation and gas fraction for breath-by-breath respiratory gas exchange calculations in exercise. Comput Biomed Res 24:118–128
Humphrey SJE, Wolff HS (1977) The Oxylog. J Physiol (Lond) 267:12P
Ikegami Y, Hiruta S, Ikegami H, Miyamura M (1988) Development of a telemetry system for measuring oxygen uptake during sports activities. Eur J Appl Physiol Occup Physiol 57:622–626
Ilsley AH, Hart JD, Withers RT, Roberts JG (1993) Evaluation of five small turbine-type respirometers used in adult anesthesia. J Clin Monit 9:196–201
Jaffe MB (2008) Infrared measurement of carbon dioxide in the human breath:“breathe-through” devices from Tyndall to the present day. Anesth Analg 107:890–904
Kenny GP, Notley SR, Gagnon D (2017) Direct calorimetry: a brief historical review of its use in the study of human metabolism and thermoregulation. Eur J Appl Physiol:1–21
King GA, McLaughlin JE, Howley ET, Bassett DR, Ainsworth BE (1999) Validation of Aerosport KB1-C portable metabolic system. Int J Sports Med 20:304–308
Kofranyi E, Michaelis H (1940) Ein tragbarer Apparat zur Bestimmung des Grasstoffwechsels. Arbeitsphysiologie (Eur J Appl Physiol Occup Physiol) 11:148–150
Larsson PU, Wadell KME, Jakobsson EJI, Burlin LU, Henriksson-Larsen KB (2004) Validation of the MetaMax II portable metabolic measurement system. Int J Sports Med 25:115–123
Laurent CM, Meyers MC, Robinson CA, Strong LR, Chase C, Goodwin B (2008) Validity of the VmaxST portable metabolic measurement system. J Sports Sci 26:709–716
Louhevaara V, Ilmarinen J, Oja P (1985) Comparison of three field methods for measuring oxygen consumption. Ergonomics 28:463–470
Luft UC, Myhre LG, Loeppky JA (1973) Validity of Haldane calculation for estimating respiratory gas exchange. J Appl Physiol 34:864–865
Macfarlane DJ (2001) Automated metabolic gas analysis systems: a review. Sports Med 31:841–861
Macfarlane DJ, Wong P (2012) Validity, reliability and stability of the portable Cortex Metamax 3B gas analysis system. Eur J Appl Physiol 112:2539–2547
McDoniel SO (2007) A systematic review on use of a handheld indirect calorimeter to assess energy needs in adults and children. Int J Sport Nutr Exerc Metab 17:491–500
McLaughlin JE, King GA, Howley ET, Bassett DR, Ainsworth BE (2001) Validation of the COSMED K4b2 portable metabolic system. Int J Sports Med 22:280–284
McLean J, Tobin G (1987) Animal and human calorimetry. Cambridge University Press, Cambridge
McNeill G, Cox MD, Rivers JP (1987) The Oxylog oxygen consumption meter: a portable device for measurement of energy expenditure. Am J Clin Nutr 45:1415–1419
Medbø JI, Mamen A, Welde B, von Heimburg E, Stokke R (2000) Examination of the Metamax I and II oxygen analysers during exercise studies in the laboratory. Scand J Clin Lab Invest 62:585–598
Medbø JI, Mamen A, Resaland GK (2012) New examination of the performance of the MetaMax I metabolic analyser with the Douglas-bag technique. Scand J Clin Lab Invest 72:158–168
Meyer RM (1990) Oxygen analyzers: Failure rates and life spans of galvanic cells. J Clin Monit Comput 6:196–202
Meyer T, Georg T, Becker C, Kindermann W (2001) Reliability of gas exchange measurements from two different spiroergometry systems. Int J Sports Med 22:593–597
Meyer T, Davison RC, Kindermann W (2005) Ambulatory gas exchange measurements–current status and future options. Int J Sports Med 26(suppl 1):S19-27
Montoye HJ, van Huss WD, Reineke EP, Cockrell J (1958) An investigation of the Müller-Franz calorimeter. Eur J Appl Physiol 17:28–33
Müller E, Franz H (1952) Energieverbrauchsmessungen bei beruflicher Arbeit mit einer verbesserten Respirations-Gasuhr. Eur J Appl Physiol 14:499–504
Murray RH, Marko A, Kissen AT, McGuire DW (1968) A new, miniaturized, multichannel, personal radiotelemetry system. J Appl Physiol 24:588–592
Namieśnik J, Wardencki W (1999) Water vapour removal from gaseous samples used for analytical purposes. A review. Int J Environ Anal Chem 73:269–280
National Aeronautics and Space Administration (2017) Portable unit for metabolic analysis (PUMA). Technology opportunity, TOP3–00231, LEW–17945. NASA Glenn Research Center, Cleveland
Nichols BL (1994) Atwater and USDA nutrition research and service: a prologue of the past century. J Nutr 124(Suppl):1718S-1727S
Osborn JJ (1978) A flowmeter for respiratory monitoring. Crit Care Med 6:349–351
Overstreet BS, Bassett DR Jr, Crouter SE, Rider BC, Parr BB (2017) Portable open-circuit spirometry systems. J Sports Med Phys Fit 57:227–237
Partington J (1962) The discovery of oxygen. J Chem Educ 39:123–125
Patton JF (1997) Measurement of oxygen uptake with portable equipment. In: Carlson-Newberry SJ, Costello RB (eds) Emerging technologies for nutrition research: potential for assessing military performance capability. National Academy Press, Washington, D.C., pp 297–314
Perkins CD, Pivarnik JM, Green MR (2004) Reliability and validity of the Vmax ST portable metabolic analyzer. J Phys Act Health 1:413–422
Perret C, Mueller G (2006) Validation of a new portable ergospirometric device (Oxycon Mobile®) during exercise. Int J Sports Med 27:363–367
Pettenkofer M (1862) Ueber die respiration. Ann der Chem Pharm 2(Suppl):1–52
Pinnington HC, Wong P, Tay J, Green D, Dawson B (2001) The level of accuracy and agreement in measures of FEO2, FECO2 and VE between the Cosmed K4b2 portable, respiratory gas analysis system and a metabolic cart. J Sci Med Sport 4:324–335
Poole G, Maskell R (1975) Validation of continuous determination of respired gases during steady-state exercise. J Appl Physiol 38:736–738
Porszasz J, Barstow TJ, Wasserman K (1994) Evaluation of a symmetrically disposed Pitot tube flowmeter for measuring gas flow during exercise. J Appl Physiol (1985) 77:2659–2665
Prieur F, Castells J, Denis C (2003) A methodology to assess the accuracy of a portable metabolic system (VmaxST). Med Sci Sports Exerc 35:879–885
Proctor DN, Beck KC (1996) Delay time adjustments to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol 81:2495–2499
Regnault VM, Reiset J (1849) Recherches chimiques sur la respiration des animaux des diverses classes. Ann Chim Phys 26:299–519
Roecker K, Prettin S, Sorichter S (2005) Gas exchange measurements with high temporal resolution: the breath-by-breath approach. Int J Sports Med 26:S11-S18
Rosdahl H, Gullstrand L, Salier-Eriksson J, Johansson P, Schantz P (2010) Evaluation of the Oxycon mobile metabolic system against the Douglas bag method. Eur J Appl Physiol 109:159–171
Schrack JA, Simonsick EM, Ferrucci L (2010) Comparison of the Cosmed K4b2 portable metabolic system in measuring steady-state walking energy expenditure. PLoS One 5:e9292
Schulz H, Helle S, Heck H (1997) The validity of the telemetric system Cortex X1 in the ventilatory and gas exchange measurement during exercise. Int J Sports Med 18:1–4
Severinghaus JW (1963) High-temperature operation of oxygen electrode giving fast response for respiratory gas sampling. Clin Chem 9:727–733
Shephard RJ (2017) Open-circuit respirometry: a brief historical review of the use of Douglas bags and chemical analyzers. Eur J Appl Physiol 117:381–387
Shephard RJ, Aoyagi Y (2012) Measurement of human energy expenditure, with particular reference to field studies: an historical perspective. Eur J Appl Physiol 112:2785–2815
Simonson E (1928) Ein neuer Respirationsapparat. Arbeitsphysiologie (Eur J Appl Physiol Occup Physiol) 1:224–257
Smith E (1859) Experimental inquiries into the chemical and other phenomena of respiration, and their modifications by various physical agencies. Philos Trans R Soc Lond 149:681–714
Sondén K, Tigerstedt R (1895) Untersuchungen über die respiration und den Gesammtstoffwechsel des Menschen1, 2. Skandinavisches Archiv Für Physiol 6:1–224
Tissot J (1904) Nouvelle méthode de mesure et d’inscription du débit et des mouvements respiratoires de l’homme et des animaux. J Physiol Pathol Gen 6:688–700
Vogler AJ, Rice AJ, Gore CJ (2010) Validity and reliability of the Cortex MetaMax3B portable metabolic system. J Sports Sci 28:733–742
Wahrlich V, Anjos LA, Going SB, Lohman TG (2006) Validation of the VO2000 calorimeter for measuring resting metabolic rate. Clin Nutr 25:687–692
Webb P, Troutman SJ Jr (1970) An instrument for continuous measurement of oxygen consumption. J Appl Physiol 28:867–871
Weir JBV (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol (Lond) 109:1–9
Wilmore JH, Costill DL (1973) Adequacy of the Haldane transformation in the computation of exercise VO2 in man. J Appl Physiol 35:85–89
Winkle JM, Evans BW, Dilg P, Galparoli S (2011) Comparing a portable and standard metabolic measuring system during rest and exercise. J Exerc Physiol 14:80–88
Wolff HS (1956) Modern techniques for measuring energy expenditure. Proc Nutr Soc 15:77–80
Wolff HS (1958a) The accuracy of the integrating motor pneumotachograph (IMP). J Physiol (Lond) 141:P36-P37 a)
Wolff HS (1958b) The integrating motor pneumotachograph: a new instrument for the measurement of energy expenditure by indirect calorimetry. Q J Exp Physiol Cogn Med Sci 43:270–283 b)
Wright BM (1961) A vacuum bottle sampler for expired air. J Physiol (Lond) 155:2-3P
Xian X, Quach A, Bridgeman D, Tsow F, Forzani E, Tao N (2015) Personalized indirect calorimeter for energy expenditure (EE) measurement. Global J Obesity Diabetes Metab Syndr 2:004–008
Yeh MP, Adams TD, Gardner RM, Yanowitz FG (1987) Turbine flowmeter vs. Fleisch pneumotachometer: a comparative study for exercise testing. J Appl Physiol 63:1289–1295
Zuntz N, Loewy A, Müller F, Caspari W (1906) Höhenklima und Bergwanderungen: in ihrer Wirkung auf den manschen. Deutsches Verlagshaus Bong & Company, Berlin
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Lindinger.
Rights and permissions
About this article
Cite this article
Macfarlane, D.J. Open-circuit respirometry: a historical review of portable gas analysis systems. Eur J Appl Physiol 117, 2369–2386 (2017). https://doi.org/10.1007/s00421-017-3716-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3716-8