Abstract
Homocysteine is an amino acid produced in the liver that, when present in high concentrations, is thought to contribute to plaque formation and, consequently, increased risk of cardiovascular disease. However, daily physical activity and training programs may contribute to controlling atherosclerosis. Given that physical exercise induces changes in protein and amino acid metabolism, it is important to understand whether homocysteine levels are also affected by exercise and to determine possible underlying mechanisms. Moreover, regarding the possible characteristics of different training programs (intensity, duration, repetition, volume), it becomes prudent to determine which types of exercise reduce homocysteine levels. To these ends, a systematic review was conducted to examine the effects of daily physical activity and different training programs on homocysteine levels. EndNote® was used to locate articles on the PubMed database from 2002 to 2013 with the keyword combinations “physical activity and homocysteine”, “training and homocysteine”, and/or “exercise and homocysteine”. After 34 studies were identified, correlative and comparative studies of homocysteine levels revealed lower levels in patients engaged in greater quantities of daily physical activity. Regarding the acute effects of exercise, all studies reported increased homocysteine levels. Concerning intervention studies with training programs, aerobic training programs used different methods and analyses that complicate making any conclusion, though resistance training programs induced decreased homocysteine levels. In conclusion, this review suggests that greater daily physical activity is associated with lower homocysteine levels and that exercise programs could positively affect homocysteine control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is associated with a high rate of mortality (Romaldini et al. 2004; Ito et al. 2013) and characterized by fatty plaque accumulation in blood vessels (Gottlieb et al. 2005). The main risk factors for the development of atherosclerosis are related to diabetes, obesity, smoking, high blood pressure, sedentary lifestyle, and poor diet habits, all of which potentially increase total cholesterol, triglyceride levels, very low-density lipoprotein (VLDL) levels, low-density lipoprotein (LDL) levels, and decreased high-density lipoprotein (HDL) levels (Gottlieb et al. 2005). The control of these variables has been elucidated in the literature.
Homocysteine, another risk factor for the development of atherosclerosis, is an amino acid produced in the liver after methionine metabolism (Brustolin et al. 2010). Its synthesis occurs in the transsulfuration of methionine overload, but can also occur in demethylation related to fasting conditions (Neves et al. 2004; Brustolin et al. 2010). The increase in plasma levels of homocysteine is related to physiological, genetic, and nutritional factors and can cause atheromatous plaques to form (Neves et al. 2004). Some mechanisms proposed to explain how homocysteine harms vascular endothelial cells are related to increased production of several pro-inflammatory cytokines, vasodilatation impairment, and oxidative stress increases (König et al. 2003).
Physical activity and training programs contribute to controlling cardiovascular risk factors and reducing the risk of atherosclerosis development (Prado and Dantas 2002). However, the understanding and review of studies related to the effects of physical activity and training programs on homocysteine levels are rarely discussed in the literature, despite being important knowledge for the diagnosis and control of atherosclerosis. To reverse this trend, this study conducted a systematic review to examine the effects of physical activity and training programs on homocysteine levels.
Given physical exercise induces changes in protein and amino acid metabolism, it is important to understand whether homocysteine levels are also affected by exercise and to determine its possible underlying mechanisms. Furthermore, regarding different exercise programs’ possible characteristics (e.g., intensity, duration, repetition, volume), it becomes prudent to discern which types of exercise reduce homocysteine levels.
Methodology
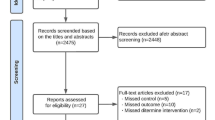
A systematic review was conducted of electronic searches using EndNote® on the PubMed Central database. The descriptors used for searches were “physical activity and homocysteine”, “training and homocysteine”, and “exercise and homocysteine”. As shown in Fig. 1, 34 original articles were identified in 31 peer-reviewed scientific journals. Priority was given to studies published from 2002 to 2013 with samples of participants who engaged in physical activity at least weekly, were performed with humans aged more than 18 years, and with aerobic training and resistance training lasting for more than 6 weeks. Review studies were excluded, as well as meta-analyses, theses, dissertations, and monographs (Table 1). The date of the last search of the literature was in April 2013. Articles were grouped as either cross-sectional or longitudinal studies. For cross-sectional studies, three analyses were conducted based on correlative, comparative, and acute effects of exercise, while for longitudinal studies comparative analysis was performed.
Results
This study examined the effects of physical activity and training programs on PH levels. The characteristics of articles (i.e., author, year, title, and journal) of cross-sectional studies, their correlation of daily physical activity with PH, and their respective results are presented in Table 2. All of the presented studies (n = 4) revealed an inverse association between daily physical activity and homocysteine levels (Chrysohoou et al. 2004; Hellgren et al. 2005; Unt et al. 2008; Loprinzi and Cardinal 2012).
The importance of daily physical activity level in PH control was also addressed in five cross-sectional, comparative studies, which are shown in Table 3, along with another three studies comparing PH levels in athletes to sedentary participants. Regarding daily physical activity levels, most of the studies (Dankner et al. 2007; Czajkowska et al. 2008; Nascimento et al. 2011) addressing the topic refer that subjects with higher daily physical activity had lower PH. In one study (Murakami et al. 2011), this relation was verified only in genotype (TT), while only one other study revealed no differences in pH given changes in daily physical activity levels (Czajkowska et al. 2011).
For those comparing PH in athletes to sedentary subjects, two studies found lower values in athletes (Rousseau et al. 2005; Joubert and Manore 2008), while another reported no significant difference (di Santolo et al. 2009). At the same time, Rousseau et al. (2005) described reduced pH in athletes with greater energy expenditure.
All studies of the acute effects of exercise on PH (Table 4) consistently reported increased PH during different types of exercises (Herrmann et al. 2003a, b; Gelecek et al. 2007; Bizheh and Jaafari 2011; Inglesias-Gutiérrez et al. 2012).
When searching for studies of training effects on PH, four studies were found that used aerobic training programs and another two that used resistance training programs (Table 5). Results regarding aerobic training programs’ effect on PH had conflicting results; two reported no changes (Randeva et al. 2002; Boreham et al. 2005), while another two reported increased PH levels (Guzel et al. 2012; Molina-López et al. 2013). Regarding resistance training, another two studies described decreased PH (Vincent et al. 2003, 2006).
Discussion
This systematic review primarily sought to understand whether homocysteine levels are affected by exercise and training, which could thus contribute to preventing blood vessel damage. Since regular exercise influences total daily physical activity and possibly induces chronic physiological adaptations that could differ, at least in magnitude, from those induced by training-oriented programs, this review focused on studies addressing the association of daily physical activity with PH.
In studies of daily physical activity and PH, two kinds of analysis were identified: correlation and comparison. Results from both kinds consistently report an inverse association between levels of physical activity and PH concentration (Chrysohoou et al. 2004; Hellgren et al. 2005; Dankner et al. 2007; Czajkowska et al. 2008; Unt et al. 2008; Nascimento et al. 2011; Loprinzi and Cardinal 2012).
In these studies, participants with the longest moderate-to-vigorous activity periods also had the lowest levels of PH concentration (Rousseau et al. 2005), which suggests active lifestyles may help to control PH levels. However, given that subjects with active lifestyles tend to be more conscious of other beneficial factors of health, such as reduced alcohol consumption, balanced diet, and not smoking (Paffenbarger et al. 1993), it is difficult to establish any causal relation between daily physical activity and PH.
Understanding physiological adaptations to exercise usually relies on two kinds of studies: the analysis of acute changes given exercise and the assessment of chronic adaptations to exercise and/or training.
Considering acute changes in PH during exercise, all articles found an increase in PH (Herrmann et al. 2003a, b; Gelecek et al. 2007; Bizheh and Jaafari 2011; Inglesias-Gutiérrez et al. 2012). During prolonged exercise, skeletal muscles increase protein and amino acid catabolism (Rennie and Tipton 2000), a cortisol-dependent regulation that results in simultaneous increased liver amino acid uptake to induce glucose synthesis (Powers and Howley 2007). Concerning resistance and strength exercises, mechanical contraction may also induce skeletal muscle protein catabolism, which favors the pool of methionine in the blood stream. Moreover, the fate of amino acids released likely differs between cells; skeletal muscles oxidize more glutamate and branched-chain amino acids, while the liver uses the remainder to produce ketone bodies and glucose (Wu 2009). Liver methionine metabolism pathways in the methionine cycle and transsulfuration sequence include the conversion of methionine to S-adenosylmethionine that can, in diverse transmethylation reactions, yield a methylated product plus S-adenosylhomocysteine, the cleavage of which yields homocysteine and adenosine (Brustolin et al. 2010). Since increased homocysteine depends on methionine availability (König et al. 2003), if an acute bout of physical exercise increases methionine availability, then increased PH during exercise may be partly explained by increased methionine availability and transsulfuration activity. It is important to note that such PH increase is transient and may return to basal values in <24 h (Inglesias-Gutiérrez et al. 2012). As such, it can be concluded only that acute exercise induces a temporary increase in PH.
Regarding the effects of different exercise programs on PH, two types of exercise were found: aerobic and resistance. For aerobic training, decreased PH is expected, since aerobic training increases protein turnover (Rennie and Tipton 2000) and antioxidant capacity (Venditti and di Meo 1997). In fact, aerobic programs stimulate liver glutathione synthesis (Leeuwenburgh et al. 1997), which favors a decline in homocysteine. On this point, however, studies have reported conflicting results. While one study reported decreased pH (Randeva et al. 2002), another reported no changes (Boreham et al. 2005) and two others reported an increase (Guzel et al. 2012; Molina-López et al. 2013). Several aspects related to the type of exercise’s duration and intensity, as well as supplementation, must be considered. The exercise program used by Randeva et al. (2002) involved fast walking for 20–60 min for 5 days per week, which revealed significant changes in both PH and maximum oxygen uptake. These results align with our expectations, since aerobic training favors protein turnover and increases the use of amino acids for the Krebs cycle (via succinyl-CoA synthase) or glucose synthesis and favors antioxidant synthesis. However, this study was performed with a small sample size of overweight women, which complicates result generalizability.
Boreham et al. (2005) also reported no changes in PH after 8 weeks of exercise that consisted of bouts of climbing 199 stairs (i.e., 90 stairs/min) at an increase of one bout every 2 weeks. A brief analysis of this exercise program revealed that subjects performed exercise about 2 min per day at the beginning, which at the end of the program increased to nearly 9 min. This very brief period of training thus induced physiological changes that could or could not favor a metabolic pathway.
Meanwhile, the use of callisthenic exercises in a period of 12 weeks, for 50 min, 3 times a week, induced increased PH (Guzel et al. 2012). Interestingly, this study’s exercise program induced changes in body composition—namely, decreases in body fat and total body weight—as well as decreased systolic and diastolic blood pressure. However, since PH and nitric oxide (NO) increased, these results contradict the proposal that hyperhomocysteine somehow impairs NO bioavailability (Stühlinger et al. 2001). Moreover, though PH increased with this exercise program, those values remained in the normal range. Since the sample studied consisted of 42 healthy middle-aged women whose PH levels were usually lower than in men (Cappuccio et al. 2002), these results are difficult to explain. Nevertheless, a change in lipid profile is possible to explain these changes in PH with this exercise program.
It has been suggested that hypomethylation associated with increased homocysteine could be responsible for lipid accumulation in tissues (Obeid and Herrmann 2009). In fact, decreased S-adenosylmethionine or increased S-adenosylhomocysteine decreases the synthesis of phosphatidylcholine, which is a major phospholipid required for VLDL protein assembly and homeostasis (Olthof et al. 2005). As such, a reduction in VLDL would be expected. Decreased plasma VLDL is frequently observed after aerobic exercise training (Halverstadt et al. 2007) and could help to explain changes in PH, according to Guzel et al. (2012). In Guzel et al. (2012), the probability of this hypothesis increased once lipid parameters declined significantly after training.
More recently, Molina-López et al. (2013) studied the effect of 16 weeks of aerobic training in handball players. This study alone used folic acid supplementation during the first 8 weeks of training and was also the only study in which volume and intensity of exercise were the concerns. Though it would be expected that variations in PH clearly related to aerobic training, PH increased despite folic acid supplementation, while plasma folic acid concentration declined. In fact, PH concentration markedly increased in cases of folate deficiency, as well as in participants with low-to-normal concentrations of plasma folate (König et al. 2003). It thus remains unclear why plasma folic acid declined with aerobic training. A possible explanation could be related to increased oxidative stress during this type of training, which could partly explain folic acid depletion, given folic acid’s importance as a liver antioxidant (Jacob and Burri 1996). Regarding lipid metabolism, Molina-López et al. (2013) did not measure VLDL, and though plasma LDL increased, plasma LDL remained in the normal range.
To assess aerobic training’s effects on PH, the studies do not allow any conclusion and, in turn, clearly demonstrate the need for further research. In fact, it seems that exercise duration is related more often to decreased homocysteine and, by contrast, that intensity favors increased homocysteine. As such, research involving an accurate control of exercise type, especially related to duration, intensity, volume, and frequency, should be conducted. At the same time, it is also important to study related changes in folic acid concentration, antioxidant status, and VLDL concentration.
Only two studies, both conducted by the same researchers and neither of which used any supplementation, were found that address the effects of resistance training on PH (Vincent et al. 2003, 2006). Different exercise intensities were used in the studies, and even then, PH decreased after the exercise program. These results can be explained by increased skeletal muscle turnover induced by resistance training, which may consequently decrease methionine availability. Regarding the exercise programs proposed by Vincent et al. (2003, 2006), it is possible that some participants performed exercise at 50 % of the maximum repetition. In this case, resistance training would also depend on an oxidative pathway to produce energy, which could, in turn, depend to some extent on increased amino acid use. These events would not favor methionine availability for homocysteine synthesis. Despite plausible explanations for decreased PH following resistance training, more studies are needed to make reliable conclusions. By extension, variables related to changes in body composition—namely, body fat and lean body mass—and markers of protein turnover with resistance training should also be assessed.
Altogether, this systematic review identified the necessity of additional studies with different exercise programs, with combined exercise programs (i.e., aerobic and resistance training), with other samples, and with different control variables in order to determine possible physiological and biochemical mechanisms involved in homocysteine control.
Conclusion
This systematic review underscored that an active lifestyle (i.e., of high daily physical activity) can help to control homocysteine levels and thus reduce risk factor for the development of atherosclerosis. While acute exercise usually induces increased PH, no consensus exists regarding the effect of aerobic training on PH, though duration seems to reduce it and intensity could increase it.
Furthermore, though resistance training programs can reduce homocysteine levels, further studies are needed. Data suggest that participation in resistance exercise can lower cardiovascular risk factors, yet while duration and intensity seem to influence homocysteine levels after participation in an aerobic exercise program, no influence was found for resistance training. It would therefore be interesting to study changes in homocysteine induced by combined exercise programs (i.e., aerobic and resistance) either during the same training session or in separate sessions.
References
Bizheh N, Jaafari M (2011) The effect of a single bout circuit resistance exercise on homocysteine, hs-CRP and fibrinogen in sedentary middle aged men. Iran J Basic Med Sci 14:568–573
Boreham CAG, Kennedy RA, Murphy MH, Tully M, Wallace WFM, Young I (2005) Training effects of short bouts of stair climbing on cardiorespiratory fitness, blood lipids, and homocysteine in sedentary young women. Br J Sports Med 39:590–593. doi:10.1136/bjsm.2002.001131
Brustolin S, Giugliani R, Félix TM (2010) Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res 43:1–7
Cappuccio FP, Bell R, Perry IJ, Gilg J, Ueland PM, Refsum H, Sagnella GA, Jeffery S, Cook DG (2002) Homocysteine levels in men and women of different ethnic and cultural background living in England. Atherosclerosis 164:95–102
Chrysohoou C, Panagiotakos DB, Pitsavos C, Zeimbekis A, Zampelas A et al (2004) The associations between smoking, physical activity, dietary habits and plasma homocysteine levels in cardiovascular disease-free people: the ‘ATTICA’ study. Vasc Med 9:117–123
Czajkowska A, Lutoslawska G, Mazurek K, Ambroszkiewics J, Zmijewski P (2008) The relationship between physical activity and plasma homocysteine level in young men. Pediatr Endocrinol Diabetes Metab 14:177–180
Czajkowska A, Lutoslawska G, Mazurek K, Ambroszkiewicz J, Zmijewski P (2011) Plasma homocysteine levels, physical activity and macronutrient intake in young healthy men. Pediatr Endocrinol Diabetes Metab 17:30–34
Da Cunha MJ, da Cunha AA, Ferreira AG, Machado FR, Schmitz F et al (2012) Physical exercise reverses glutamate uptake and oxidative stress effects of chronic homocysteine administration in the rat. Int J Dev Neurosci 30:69–74. doi:10.1016/j.ijdevneu.2012.01.001
Dankner R, Chetrit A, Dror GK, Sela BA (2007) Physical activity is inversely associated with total homocysteine levels, independent of C677T MTHFR genotype and plasma B vitamins. Age 29:219–227. doi:10.1007/s11357-007-9041-0
De Jong N, Chin A, Paw MJ, de Groot LC, Rutten RA, Swinkels DW et al (2001) Nutrient-dense foods and exercise in frail elderly: effects on B vitamins, homocysteine, methylmalonic acid, and neuropsychological functioning. Am J Clin Nutr 73:338–346
Di Santolo M, Banfi G, Stel G, Cauci S (2009) Association of recreational physical activity with homocysteine, folate and lipid markers in young women. Eur J Appl Physiol 105:111–118. doi:10.1007/s00421-008-0880-x
Gaume V, Figard H, Mougin F, Guilland JC, Alberto JM et al (2005) Effect of a swim training on homocysteine and cysteine levels in rats. Amino Acids 28:337–342
Gelecek N, Teoman N, Ozdirenc M, Pinar L, Akan P et al (2007) Influences of acute and chronic aerobic exercise on the plasma homocysteine level. Ann Nutr Metab 51:53–58
Gottlieb MGV, Bonardi G, Moriguchi EH (2005) Fisiopatologia e aspectos inflamatórios da aterosclerose: artigo de Revisão. Scientia Medica 15:203–207
Guzel NA, Pinar L, Colakoglu F, Karacan S, Ozer C (2012) Long-term callisthenic exercise-related changes in blood lipids, homocysteine, nitric oxide levels and body composition in middle-aged healthy sedentary women. Chin J Physiol 55:202–209. doi:10.4077/CJP.2012.AMM122
Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM (2007) Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metab, Clin Exp 56:444–450
Hammouda O, Chtourou H, Chaouachi A, Chahed H, Ferchichi S et al (2012) Effect of short-term maximal exercise on biochemical markers of muscle damage, total antioxidant status, and homocysteine levels in football players. Asian J Sports Med 3:239–246
Hayward R, Ruangthai R, Karnilaw P, Chicco A, Strange R et al (2003) Attenuation of homocysteine-induced endothelial dysfunction by exercise training. Pathophysiology 9:207–214
Hellgren M, Melander A, Ostgren CJ, Rastam L, Lindblad U (2005) Inverse association between plasma homocysteine, sulphonylurea exposure and physical activity: a community-based sample of type 2 diabetes patients in the Skaraborg hypertension and diabetes project. Diabetes Obes Metab 7:421–429
Herrmann M, Schorr H, Obeid R, Scharhag J, Urhausen A et al (2003a) Homocysteine increases during endurance exercise. Clin Chem Lab Med 41:1518–1524
Herrmann M, Wilkinson J, Schorr H, Obeid R, Georg T et al (2003b) Comparison of the influence of volume-oriented training and high-intensity interval training on serum homocysteine and its cofactors in young, healthy swimmers. Clin Chem Lab Med 41:1525–1531
Inglesias-Gutiérrez E, Egan B, Díaz-Martínez ÁE, Peñalvo JL, González-Medina A et al (2012) Transient increase in homocysteine but not hyperhomocysteinemia during acute exercise at different intensities in sedentary individuals. PLoS ONE 7:1–8. doi:10.1371/journal.pone.0051185
Ito H, Antoku S, Furusho M, Shinozaki M, Abe M et al (2013) The prevalence of the risk factors for atherosclerosis among type 2 diabetic patients is greater in the progressive stages of chronic kidney disease. Nephron Extra 3:66–72. doi:10.1159/000353592
Jacob RA, Burri BJ (1996) Oxidative damage and defense. Am J Clin Nutr 63:985S–990S
Joubert LM, Manore MM (2006) Exercise, nutrition, and homocysteine. Int J Sport Nutr Exerc Metab 16:341–361
Joubert LM, Manore MM (2008) The role of physical activity level and B-vitamin status on blood homocysteine levels. Med Sci Sports Exerc 40:1923–1931. doi:10.1249/MSS.0b013e31817f36f9
König D, Bissé E, Deibert P, Müller HM, Wieland H et al (2003) Influence of training volume and acute physical exercise on the homocysteine levels in endurance-trained men: interactions with plasma folate and vitamin B12. Ann Nutr Metab 47:114–118
Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL (1997) Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J of Physiol 272:R363–R369
Loprinzi PD, Cardinal BJ (2012) Interrelationships among physical activity, depression, homocysteine, and metabolic syndrome with special considerations by sex. Prev Med 54:388–392. doi:10.1016/j.ypmed.2012.03.016
Mishra PK, Awe O, Metreveli N, Qipshidze N, Joshua IG et al (2011) Exercise mitigates homocysteine -β2-adrenergic receptor interactions to ameliorate contractile dysfunction in diabetes. Int J Physiol Pathophysiol Pharmacol 3:97–106
Molina-López J, Molina JM, Chirosa LJ, Florea DI, Sáez L et al (2013) Effect of folic acid supplementation on homocysteine concentration and association with training in handball players. J Int Soc Sports Nutr 10:1–8. doi:10.1186/1550-2783-10-10
Murakami H, Iemitsu M, Sanada K, Gando Y, Ohmori Y et al (2011) Associations among objectively measured physical activity, fasting plasma homocysteine concentration, and MTHFR C677T genotype. Eur J Appl Physiol 111:2997–3005. doi:10.1007/s00421-011-1926-z
Nascimento CM, Stella F, Garlipp CR, Santos RF, Gobbi S et al (2011) Serum homocysteine and physical exercise in patients with Parkinson’s disease. Psychogeriatrics 11:105–112. doi:10.1111/j.1479-8301.2011.00356.x
Neves LB, Macedo DM, Lopes AC (2004) Homocisteína. J Bras Patol Med Lab 40:311–320
Obeid R, Herrmann W (2009) Homocysteine and lipids: s-adenosyl methionine as a key intermediate. FEBS Lett 583:1215–1225
Okura T, Rankinen T, Gagnon J, Lussier-Cacan S, Davignon J et al (2006) Effect of regular exercise on homocysteine concentrations: the HERITAGE Family Study. Eur J Appl Physiol 98:394–401
Olthof MR, Vliet TV, Verhoef P, Zock PL, Katan MB (2005) Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med 2(5):e135
Paffenbarger RS, Hyde RT, Alvin MA, Wing AL et al (1993) The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl Jf Med 328:538–545
Powers SK, Howley ET (2007) Exercise physiology: theory and application to fitness and performance. Human kinetics, Champaign IL
Prado ES, Dantas EHM (2002) Efeitos dos exercícios físicos aeróbio e de força nas lipoproteínas HDL, LDL e lipoproteína(a). Arq Bras Cardiol 79:429–433
Randeva HS, Lewandowski KC, Drzewoski J, Brooke-Wavell K, O’Callaghan C et al (2002) Exercise decreases plasma total homocysteine in overweight young women with polycystic ovary syndrome. J Clin Endocrinol Metab 87:4496–4501
Rennie MJ, Tipton KD (2000) Protein and amino acid metabolism during and after exercise and the effects of nutrition. Ann Rev of Nutrit 20:457–483
Romaldini CC, Issler H, Cardoso AL, Diament J, Forti N (2004) Fatores de risco para aterosclerose em crianças e adolescentes com história familiar de doença arterial coronariana premature. J Pediatr 80:135–140
Rousseau AS, Robin S, Roussel AM, Ducros V, Margaritis L (2005) Plasma homocysteine is related to folate intake but not training status. Nutr Metab Cardiovasc Dis 15:125–133
Ruiz JR, Hurtig-Wennlöf A, Ortega FB, Patterson E, Nilsson TK et al (2007) Homocysteine levels in children and adolescents are associated with the methylenetetrahydrofolate reductase 677C>T genotype, but not with physical activity, fitness or fatness: the European Youth Heart Study. Br J Nutr 97:255–262
Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP (2001) Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104:2569–2575
Unt E, Zilmer K, Mägi A, Kullisaar T, Kairane C et al (2008) Homocysteine status in former top-level male athletes: possible effect of physical activity and physical fitness. Scand J Med Sci Sports 18:360–366
Venditti P, Di Meo SD (1997) Effect of training on antioxidant capacity, tissue damage, and endurance of adult male rats. Int J Sports Med 18:497–502
Vincent KR, Braith RW, Bottiglieri T, Vincent HK, Lowenthal DT (2003) Homocysteine and lipoprotein levels following resistance training in older adults. Prev Cardiol 6:197–203
Vincent HK, Bourguignon C, Vincent KR (2006) Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 14:1921–1930
Wright M, Francis K, Cornwell P (1998) Effect of acute exercise on plasma homocysteine. J Sports Med Phys Fitness 38:262–265
Wu G (2009) Amino acids: metabolism function and nutrition. Amino Acids 37:1–17
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, A.S., da Mota, M.P.G. Effects of physical activity and training programs on plasma homocysteine levels: a systematic review. Amino Acids 46, 1795–1804 (2014). https://doi.org/10.1007/s00726-014-1741-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1741-z