Abstract
Affibody molecules constitute a novel class of molecular display selected affinity proteins based on non-immunoglobulin scaffold. Preclinical investigations and pilot clinical data have demonstrated that Affibody molecules provide high contrast imaging of tumor-associated molecular targets shortly after injection. The use of cysteine-containing peptide-based chelators at the C-terminus of recombinant Affibody molecules enabled site-specific labeling with the radionuclide 99mTc. Earlier studies have demonstrated that position, composition and the order of amino acids in peptide-based chelators influence labeling stability, cellular processing and biodistribution of Affibody molecules. To investigate the influence of the amino acid order, a series of anti-HER2 Affibody molecules, containing GSGC, GEGC and GKGC chelators have been prepared and characterized. The affinity to HER2, cellular processing of 99mTc-labeled Affibody molecules and their biodistribution were investigated. These properties were compared with that of the previously studied 99mTc-labeled Affibody molecules containing GGSC, GGEC and GGKC chelators. All variants displayed picomolar affinities to HER2. The substitution of a single amino acid in the chelator had an appreciable influence on the cellular processing of 99mTc. The biodistribution of all 99mTc-labeled Affibody molecules was in general comparable, with the main difference in uptake and retention of radioactivity in excretory organs. The hepatic accumulation of radioactivity was higher for the lysine-containing chelators and the renal retention of 99mTc was significantly affected by the amino acid composition of chelators. The order of amino acids influenced renal uptake of some conjugates at 1 h after injection, but the difference decreased at later time points. Such information can be helpful for the development of other scaffold protein-based imaging and therapeutic radiolabeled conjugates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant cells are marked by distinctive phenotypic and genotypic alterations. These pathotypic molecular features can be utilized for molecular recognition and selective treatment of cancer cells. Molecular radionuclide imaging can visualize aberrantly expressed gene products in vivo (Britz-Cunningham and Adelstein 2003) which can be utilized for stratification of patients who could benefit from a particular targeting therapy and for monitoring of response to such therapy (Mankoff 2009; Tolmachev et al. 2010a). Molecular radionuclide imaging employs the specific binding of radiolabeled targeting antibodies and peptides to certain molecular cancer-associated targets in order to selectively deliver radionuclides to the abnormal cells (Tolmachev et al. 2010a).
The human epidermal growth factor (HER) receptor tyrosine kinase family (designated in non-human species as erbB) is often overexpressed in many malignancies, for example breast, ovary and colorectal carcinomas (Hynes and MacDonald 2009). Signaling of the HER family receptors triggers proliferation and decreases apoptosis, i.e. they act as proto-oncogenes. Overexpression of HER is considered as a part of malignant phenotype (Citri and Yarden 2006). The transmembrane protein HER2 (erbB2) is the most well-known member of this receptor family. HER2 is a major molecular target for both immunotherapy and tyrosine kinase inhibition due to its significant role in proliferation of several malignancies, especially breast cancer (Chang 2010; Cameron and Stein 2008).
Several classes of targeting molecules (antibodies, antibody fragments, short peptides and scaffold proteins) have been evaluated for radionuclide in vivo imaging of HER2 expression (Tolmachev 2008). Analysis suggests that small targeting proteins have advantages in sensitivity and specificity over full-length intact antibodies or their fragments (Tolmachev 2008). Affibody molecules are a new class of scaffold proteins derived from the randomization of 13 surface amino acids of the B domain of S. aureus protein A, commonly selected using phage display technique (Löfblom et al. 2010; Nygren 2008). These robust, small (7 kDa) proteins with affinities in the low nano- to picomolar range, are characterized by high rate of extravasation and rapid clearance of non-bound tracer from the circulation, as well as other nonspecific compartments, when compared to antibodies and their fragments (Ahlgren and Tolmachev 2010). The ZHER2:342 Affibody molecule with picomolar affinity to the HER2 receptor was developed earlier (Orlova et al. 2006). The ZHER2:342 and its derivatives were labeled successfully with a variety of radionuclides, including 99mTc, 111In, 68Ga, 124I, demonstrating a high potential for radionuclide imaging of HER2 in pre-clinical studies (Ahlgren and Tolmachev 2010) and in a pilot clinical study (Baum et al. 2010).
Generator-produced 99mTc (T 1/2 = 6 h, E γ = 140.5 keV) is an attractive radionuclide for imaging using single photon emission computed tomography (SPECT). Availability, favorable photon energy and a well-developed chemistry all add to the advantages of this radionuclide as an optimal imaging label candidate. Earlier, several approaches to site-specifically label derivatives of the anti-HER2 ZHER2:342 Affibody molecule has been investigated with an emphasis on the use of peptide-based chelators (Engfeldt et al. 2007a, b; Tran et al. 2007a, b, 2008, 2009; Ekblad et al. 2008, 2009; Ahlgren et al. 2009). Studies using synthetic derivatives of ZHER2:342 with mercaptoacetyl-based chelators in murine models have shown that increasing the hydrophilicity of the peptide-based chelators by the use of polar (serine) or charged (glutamate, lysine) amino acids suppresses hepatobiliary excretion, but increases renal retention of radioactivity (Engfeldt et al. 2007a, b; Tran et al. 2007b, 2008). Additionally, the use of amino acids with electron-donating side-chains improved the stability of the chelators in circulation. However, an excessive use of the positively charged lysine was associated with an elevated hepatic uptake, but low hepatobiliary excretion (Tran et al. 2008). Moreover, it was shown that the order of the amino acids in the peptide-based chelators influences the renal retention of radioactivity by affecting the rate of the urinary excretion of low-molecular-weight radiocatabolites (Ekblad et al. 2008). Furthermore, it has been demonstrated that the positioning of the mercaptoacetyl-based chelators, either at the N-terminus or in helix 3 of the Affibody molecule, plays a role for the stability of the chelate, pathway that predominates (Ekblad et al. 2009). The best construct, 99mTc-maESE-ZHER2:342, provided an uptake of 0.33 ± 0.06 and 33 ± 5 %IA/g (per cent of injected activity per gram) in liver and kidney, respectively, at 4 h after injection (Ekblad et al. 2008). This conjugate also demonstrated specific imaging of HER2-expressing xenografts in murine models.
Affibody molecules with mercaptoacetyl-containing chelators can only be produced by peptide synthesis. This limits the flexibility in the production route of an imaging tracer. Instead, the use of cysteine-containing peptide-based chelators permits direct recombinant production of targeting agents suitable for site-specific labeling. The use of cysteine-based CGG- and CGGG-chelators at the N-terminus allowed labeling of Affibody molecules with 99mTc and enabled visualization of HER2-expressing xenografts in mice (Tran et al. 2007a). However, these conjugates suffered from limited in vivo stability and an appreciable degree of hepatobiliary excretion. Positioning of the cysteine at the C-terminus (forming a -VDC chelator) enabled 99mTc-labeling of Affibody molecules with improved stability in vivo (Ahlgren et al. 2009), most likely due to a more favorable geometry of the chelate. Biodistribution studies of several derivatives of this construct showed that the placement of Ala1, Glu2 on the N-terminus of Affibody molecules, site-specifically labeled at the C-terminus, was associated with low hepatic uptake and low levels of hepatobiliary excretion (Ahlgren et al. 2008, 2009, 2010a; Tran et al. 2009). Furthermore, it was shown that the renal retention of 99mTc could be reduced by the use of the GSECG chelating system, a C-terminal “mirror” homolog of the maESE-chelator at the N-terminus (Tran et al. 2009).
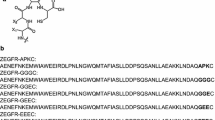
Recently we have studied a series of five derivatives of the ZHER2:342 Affibody molecule [Ala1,Glu2,Gly56,Ser57,Glu58,Cys59]ZHER2:342 (designated as ZHER2:V1), [Ala1,Glu2,Gly56,Gly57,Gly58, Cys59]ZHER2:342 (designated as ZHER2:V2), [Ala1,Glu2,Gly56,Gly57,Ser58,Cys59]ZHER2:342 (designated as ZHER2:V3), [Ala1,Glu2,Gly56,Gly57,Glu58,Cys59]ZHER2:342 (designated as ZHER2:V4), and [Ala1,Glu2 Gly56, Gly57,Lys58,Cys59]ZHER2:342 (designated as ZHER2:V5) labeled site-specifically at the C-terminus with 99mTc (Fig. 1) (Wållberg et al. 2011a). Single amino acid modifications in the C-terminal chelating GGXC moiety were translated into an appreciable difference in the uptake and retention of these conjugates and their radiocatabolites in excretory organs. Summarizing the previous data, both the position and the composition of the N3S chelating moiety influences the labeling stability, biodistribution and targeting properties of the Affibody molecules.
Overview of the peptide-based chelating system used for chelation of 99mTc. a General structure of the N3S chelators formed by a C-terminal cysteine and three adjacent amino acids. X1, X2 and X3 denoting the side chains of the amino acids involved in the coordination of the radionuclide. b The studied HER2-binding Affibody molecules including the C-terminal chelating sequence aligned to the parent molecule ZHER2:342. The variable amino acids are marked in bold. Positions of α-helices 1 through 3 are indicated by boxes. Constructs ZHER2:V6, ZHER2:V7, and ZHER2:V8 have been investigated in the current study and data concerning ZHER2:V3, ZHER2:V4, and ZHER2:V5 were taken from (Wållberg et al. 2011a)
The goal of this study was to investigate the effect, if any, on the in vitro and in vivo properties resulting from changing the position of one single amino acid in the GGXC system. A glycine residue was placed between the substituted amino acid X and the C-terminal cysteine. Three Affibody variants [Ala1,Glu2,Gly56,Ser57,Gly58,Cys59]ZHER2:342 (designated as ZHER2:V6), [Ala1,Glu2,Gly56,Glu57,Gly58,Cys59]ZHER2:342 (designated as ZHER2:V7), and [Ala1,Glu2 Gly56, Lys57,Gly58,Cys59]ZHER2:342 (designated as ZHER2:V8) (Fig. 1b) were labeled with 99mTc, and the influence of the chelators on the cellular processing and biodistribution was studied. The properties of ZHER2:V6, ZHER2:V7, and ZHER2:V8 were compared with the properties of the previously studied ZHER2:V3, ZHER2:V4, and ZHER2:V5 (Fig. 1b) (Wållberg et al. 2011a).
Materials and methods
Materials
α-d-Gluconic acid sodium salt and ethylenediaminetetraacetic acid (EDTA) were from Sigma-Aldrich, tin(II)-chloride dihydrate (SnCl2·2H2O) and pyridine were from Fluka Chemika. Phosphate buffered saline, pH 7.4 (PBS) was produced in house. 99mTc was obtained as pertechnetate from an Ultra-TechneKow generator (Covidien) eluted with sterile 0.9% sodium chloride (Covidien). An automated gamma counter (Perkin-Elmer) was used to measure the radioactivity. Yield, radiocolloid content and radiochemical purity of the labeled Affibody constructs were analyzed using instant thin layer chromatography (ITLC) strips (150-771 DARK GREEN, Tec-Control Chromatography strips from Biodex Medical Systems). NuPAGE® 10% Bis–Tris gels (Invitrogen) were used for analysis of conjugates and for in vitro stability studies. The Cyclone Storage Phosphor System and the OptiQuant image analysis software (Perkin-Elmer) were used to measure the radioactivity distribution on chromatography strips or PAGE gels. Ovarian carcinoma SKOV-3 cells (ATCC, purchased via LGC Promochem) were used for binding specificity and cellular processing studies. The cells were counted using an electronic cell counter (Beckman Coulter). Unpaired two-tailed t test was used to determine significant difference (p < 0.05) between groups of samples.
Production and characterization of Affibody molecules
A detailed description of the preparation, purification and characterization of the investigated Affibody molecules is provided elsewhere (Wållberg et al. 2011b). The purified proteins were divided into aliquots of 100 μg, freeze-dried and stored at −20°C.
Labeling and stability test
The C-terminally engineered Affibody molecules ZHER2:V6, ZHER2:V7 and ZHER2:V8 were site-specifically labeled with technetium-99m using lyophilized kits. Each kit contained 5 mg of sodium α-d-glucoheptonate dihydrate, 100 μg of edetate disodium (Na2EDTA) and 75 μg of tin(II) chloride dihydrate (SnCl2·2H2O) as has been optimized earlier by (Ahlgren et al. 2010b). Labeling was performed by adding the contents of the freeze-dried kit, dissolved in 100 μL degassed PBS, to 100 μg of Affibody molecule. To the reaction mixture, 100 μL (600 MBq) of the generator eluted 99mTc-pertechnetate was added and the vial was filled with argon gas to protect the mixture from oxidation. The reaction vial was thoroughly vortexed and incubated at 90°C for 1 h. Labeling yield and reduced hydrolyzed technetium colloids (RHT) was thereafter determined by ITLC after cooling for 15 min. PBS was used as mobile phase for analysis of the labeling efficiency and radiochemical purity. In this system, unbound radioactivity migrates with the eluent front and radiolabeled Affibody molecule remains at the application point. A mixture of pyridine:acetic acid:water (5:3:1.5) was used as eluent for ITLC to estimate the presence of RHT. Purification was performed by size-exclusion chromatography using disposable NAP-5 columns (GE Healthcare) pre-equilibrated and eluted with PBS.
For serum stability studies, a serum sample (240 μL) was mixed with freshly labeled Affibody molecules (10 μL) to obtain an Affibody concentration similar to the concentration in blood at the moment of injection. The samples were incubated for 1 h at 37°C and analyzed using radio-SDS-PAGE in MES buffer (200 V constant). Another sample of conjugate, which was kept at room temperature in PBS was used as a control. A sample of 99mTc-pertechnetate was used as a reference standard for low-molecular-weight compounds. Both control and pertechnetate samples were run in parallel with the tested sample on the same gel.
Binding specificity test
For cell studies, the HER2-expressing ovarian carcinoma cell line SKOV-3 was used. For each conjugate, a group of six petri dishes containing a cell monolayer (1 × 106 cells/dish) was used. In each group, cells in three dishes were pre-saturated with 500-fold excess unlabeled Affibody molecules 5 min before addition of the labeled Affibody conjugates. The cells were incubated with 0.2 nM labeled conjugate in a humidified incubator (5% CO2, 37°C) for 1 h. The medium was collected and the cells were washed with cold serum-free medium before 0.5 mL trypsin–EDTA solution/dish was added and incubated for 10 min. Detached cells were diluted with 0.5 mL complete medium, re-suspended and transferred to fraction tubes. The radioactivity of cells and media was measured using an automated gamma counter and the per cent of cell-bound radioactivity was calculated.
Cellular processing and retention
Cellular retention and processing were studied using the methods validated for Affibody molecules earlier (Wållberg and Orlova 2008). Cells (1.2 × 106 cells/dish) were incubated with 0.2 nM solution of labeled conjugates at 4°C. After 1-h incubation, the medium with the labeled compound was removed and cells were washed three times with ice-cold serum-free medium. 1 mL of complete media was added to each dish and cells were further incubated at 37°C in an atmosphere containing 5% CO2. At designated time points (0 h, 1 h, 2 h, 4 h, 8 h and 24 h), a group of three dishes was removed from the incubator, the media was collected and cells were washed three times with ice-cold serum-free medium. Thereafter, cells were treated with 0.5 mL 0.2 M glycine buffer, pH 2, containing 4 M urea, for 5 min on ice. The acidic solution was collected and cells were additionally washed with 0.5 mL glycine buffer. The acidic fractions were pooled. The cells were then incubated with 0.5 mL 1 M NaOH at 37°C for 10 min. The cell debris was collected, and the dishes were additionally washed with 0.5 mL of NaOH solution. The alkaline solutions were pooled. The radioactivity in the acidic solution was considered as membrane bound, and in the alkaline fractions as internalized.
Animal studies
The animal experiments were planned and performed in accordance with national legislation on laboratory animals protection. The animal study plans have been approved by the local Ethics Committee for Animal Research in Uppsala.
Biodistribution studies were performed in female immunocompetent Naval Medical Research Institute (NMRI) mice. All mice were acclimatized for 1 week at the Rudbeck Laboratory animal facility before any experimental procedures. For each conjugate, 12 mice, randomized into groups of four, were used. Animals were injected intravenously with 1 μg (40 kBq) conjugate per animal in 100 μL PBS. One group of mice was killed at predetermined time points (1, 4 and 24 h after injection) by an intraperitoneal injection of anesthesia, Ketalar-Rompun solution (20 μL/g body weight; Ketalar: 10 mg/mL, Pfizer; Rompun[xylazine]: 1 mg/mL) with subsequent exsanguination by heart puncture using a 1 mL syringe prewashed with diluted heparin (5,000 IU/mL). Blood, lung, liver, spleen, stomach, kidney, salivary glands, muscles, intestines, and the remaining carcass were collected. Organs and tissue samples were weighed, and their radioactivity was measured. The tissue uptake values were calculated as percent of injected activity per gram tissue (%IA/g) except for the intestines and the carcass, which was calculated as %IA per whole sample.
Results
Characterization of Affibody molecules
Data concerning the ZHER2:V6, ZHER2:V7 and ZHER2:V8 Affibody molecules (taken from Wållberg et al. 2011b) are presented in Table 1. Data for ZHER2:V3, ZHER2:V4 and ZHER2:V5 are provided for comparison. All Affibody variants are characterized by high melting points (over 63°C) and high affinity to HER2: 90, 269 and 283 pM for ZHER2:V6, ZHER2:V7, and ZHER2:V8, respectively.
Labeling and in vitro stability
Data concerning the labeling and stability of ZHER2:V6, ZHER2:V7, ZHER2:V8 are presented in Table 2. All three variants were efficiently labeled with 99mTc, with labeling yields exceeding 95% in the case of ZHER2:V6 and ZHER2:V8, and 90% for ZHER2:V7. The radiochemical purity of the labeled products after purification using NAP-5 columns was above 99% for all the three conjugates.
The results of the stability test are presented in Fig. 2. The serum stability test using SDS-PAGE revealed exceptionally high-serum stability of all three conjugates after 1 h incubation in serum. The only observed radioactivity peak corresponded to the expected molecular weight of the Affibody molecule. The SDS-PAGE analysis showed no indications of aggregation or binding to serum proteins or release of radionuclide, since no radioactivity bands of higher or lower molecular weight could be observed.
SDS–PAGE analysis of the stability of 99mTc-labeled Affibody molecules in murine serum. 1 control 99mTc-Affibody molecule (kept in PBS at room temperature for 1 h); 2 99mTc-Affibody molecule incubated in murine serum at 37°C for 1 h; 3 99mTcO4 −, used as a marker for low-molecular-weight compounds. The signal was measured as digital light units (DLU) and is proportional to radioactivity at a given point of a lane in the SDS-PAGE gel
Binding specificity and cellular retention
All conjugates showed specific binding to the HER2 receptor as the uptake by SKOV-3 cells was blocked by pre-saturation with 500-fold molar excess of unlabeled protein. The cell-associated radioactivity decreased from above 30%, to less than 2% for all conjugates upon blocking (Fig. 3).
Specificity of the binding of technetium-99m-labeled Affibody molecules to HER2-expressing cells in vitro. The specificity test was performed using SKOV-3 ovarian cancer cell line. For the pre-saturation of HER2, a 500-fold molar excess of non-radioactive Affibody molecule was added. Data are presented as mean values from three cell dishes with standard deviations. Pre-saturation of HER2 caused a significant decrease of the binding, demonstrating specific binding to HER2
The composition of the peptide-based chelators had an apparent influence on both the overall retention and the cellular processing of the 99mTc-labeled conjugates after binding to HER2-expressing SKOV-3 cells (Fig. 4). There was no significant difference in the overall retention between 99mTc-ZHER2:V7 and 99mTc-ZHER2:V8, both containing charged amino acids in the chelating moiety, while the cellular retention of the serine-containing 99mTc-ZHER2:V6 conjugate was significantly weaker. Moreover, there was an appreciable difference in both the level and the pattern of internalized radioactivity. The lysine-containing 99mTc-ZHER2:V8 demonstrated a tendency of continuous growth of the fraction of internalized radioactivity, and was associated with the highest level of intracellular radioactivity. For the serine-containing 99mTc-ZHER2:V6, the membrane-bound activity decreased appreciably during the experiment, while the internalized fraction reached a plateau after 4 h. Such retention profile could not be explained by dissociation of the membrane-bound conjugate since 99mTc-ZHER2:V3 with a lower affinity to HER2 showed better retention of membrane-bound activity. More likely, a steady state was reached in the cellular internalization and externalization of the 99mTc-ZHER2:V6-associated radioactivity after 4 h. The level of internalized radioactivity was the lowest for 99mTc-ZHER2:V6. The glutamyl containing ZHER2:V7 showed an intermediate level of internalized radioactivity. The analysis of the cellular retention and internalization of 99mTc-labeled ZHER2:V6, ZHER2:V7 and ZHER2:V8 showed an appreciable difference from the levels of cellular retention previously obtained for their counterparts with GGXC chelators (ZHER2:V3, ZHER2:V4 and ZHER2:V5). The GXGC-containing conjugates were less efficiently retained by HER2 expressing cells. Conversely, the difference in the internalization and intracellular retention of radioactivity for the serine- and glutamate-containing chelators was small between GXGC and GGXC chelators. This difference was most pronounced for the serine-containing conjugates.
Influence of the amino acid composition in the cysteine-containing chelators on the cellular processing of 99mTc-labeled Affibody molecules by SKOV-3 cells. Total cell-bound radioactivity is marked red, internalized radioactivity is marked blue. a Solid lines 99mTc-ZHER2:V6 (-GSGC), dashed lines 99mTc-ZHER2:V3 (-GGSC); b solid lines 99mTc-ZHER2:V7 (-GEGC), dashed lines 99mTc-ZHER2:V4 (-GGEC); c solid lines 99mTc-ZHER2:V8 (-GKGC), dashed lines 99mTc-ZHER2:V5 (-GGKC). Data for 99mTc-ZHER2:V3, 99mTc-ZHER2:V4, and 99mTc-ZHER2:V5 are taken from Wållberg et al. (2011a) for comparison
Animal studies
The biodistribution data for 99mTc-labled ZHER2:V6, ZHER2:V7 and ZHER2:V8 in NMRI mice are presented in Tables 3, 4 and 5, respectively. For comparison, data for the GGXC-containing counterparts (Wållberg et al. 2011a) are provided in the same Tables. All comparative findings reported are statistically significant unless otherwise stated.
All three studied 99mTc-labeled Affibody molecules, ZHER2:V6, ZHER2:V7 and ZHER2:V8, showed a rapid blood clearance. No significant differences in radioactivity were observed in organs that are in direct equilibrium with blood pool (spleen, salivary glands, lungs) except from a somewhat elevated lung uptake of 99mTc-ZHER2:V4 (3.04 ± 1.04 %IA/g 1 h p.i.) compared to the other variants. There was also a clear resemblance in uptake in non-excretory organs of non-tumor bearing NMRI mice between the GXGC-containing Affibody molecules and their previously studied GGXC-containing counterparts 99mTc-ZHER2:V3, 99mTc-ZHER2:V4 and 99mTc-ZHER2:V5 (Wållberg et al. 2011a). However, the uptake in excretory organs (liver and kidneys) differed considerably. The distribution and uptake pattern in excretory organs showed a remarkable difference at early time points after injection. The seryl- and glutamyl-containing GGXC variants (ZHER2:V3 and ZHER2:V4) showed significantly higher kidney uptake in comparison with their GXGC counterparts (ZHER2:V6 and ZHER2:V7) at 1 h p.i. The renal uptake values at that time point were 45 ± 11 and 94 ± 9 %IA/g for seryl-containing 99mTc-ZHER2:V6 and 99mTc-ZHER2:V3, respectively (p < 0.0005). For glytamyl-containing 99mTc-ZHER2:V7 and 99mTc-ZHER2:V4, the renal uptake values were 97 ± 10 and 132 ± 13 %IA/g (p < 0.01), respectively. The difference in renal uptake at 1 h p.i. was not significant for lysyl-containing variants 99mTc-ZHER2:V8 (122 ± 12 %IA/g) and 99mTc-ZHER2:V5 (139 ± 10 %IA/g). However, the retention of radioactivity at 4 h p.i. was significantly (p < 0.01) higher for GGKC-containing 99mTc-ZHER2:V5 (121 ± 9 %IA/g) than for GKGC-containing 99mTc-ZHER2:V8 (81 ± 11 %IA/g).
The renal uptake of conjugates with a glutamyl- (ZHER2:V4 and ZHER2:V7) or lysyl- (ZHER2:V5 and ZHER2:V8) in the chelating sequence were higher than for the conjugates with a non-charged, less hydrophilic seryl-containing chelator (ZHER2:V3 and ZHER2:V6) at 1 h p.i. The lysine-containing variants showed a four to sixfold higher uptake in the kidney compared to the glutamyl containing counterparts at later time points. Regardless of their rate of uptake in the kidneys, the seryl- and glutamyl-containing variants showed an extensive clearance from the body and a marked decrease in renal retention of radioactivity already at 4 h p.i. However, this was not the case with the lysine-containing chelators, 99mTc-ZHER2:V5 and ZHER2:V8, which were apparently retained in the kidneys. Interestingly, the renal retention of the GGKC variant, ZHER2:V5, was higher than its GKGC counterpart, ZHER2:V8, throughout the study.
There was no significant difference in radioactivity in the liver between the variants 99mTc-ZHER2:V6 and ZHER2:V7 or their counterparts, 99mTc-ZHER2:V3 and ZHER2:V4. However, the hepatic uptake of the lysine-containing variants 99mTc-ZHER2:V5 and 99mTc-ZHER2:V8 were markedly (two- to threefold) higher than the hepatic uptake of the other variants.
Discussion
Targeting proteins based on non-immunoglobulin affinity ligands, such as Affibody molecules, are currently under active evaluation as probes for molecular imaging (Miao et al. 2010). The small size of these tracers (4–15 kDa) enables an imaging contrast unattainable by monoclonal antibodies. Still, many aspects of targeting using scaffold proteins are not yet well studied, which necessitates extensive research. One such aspect is how the labeling chemistry influences the biodistribution of the targeting proteins, in particular their uptake and retention in healthy tissues, since this can appreciably influence the imaging contrast. This issue is closely connected with the cellular processing of conjugates bound to cell surface proteins.
Previous studies have demonstrated that the amino acid composition of peptide-based N3S chelators at the N-terminus strongly influences the excretion pathways of 99mTc-labeled Affibody molecules and the retention of radioactivity in excretory organs (Ahlgren and Tolmachev 2010). Moreover, the renal retention of 99mTc was significantly dependant on the order of the amino acids in such chelators (Ekblad et al. 2008). This is a very interesting effect since experiments with strongly residualizing labels suggest that the renal re-absorption of ZHER2:342 from primary urine is nearly complete (Tolmachev et al. 2006; Orlova et al. 2007; Ahlgren et al. 2008). The difference in renal radioactivity between different chelators for 99mTc must be due to different degrees of intracellular retention of radiocatabolites by the renal proximal tubular cells. Recently, we have reported that the amino acid composition of cysteine-containing peptide-based GGXC chelators (where X = G, S, E or K) had an appreciable influence on the uptake and retention of 99mTc-labeled Affibody molecules in the liver and kidneys (Wållberg et al. 2011a). However, the effect of reordering the amino acids in this N3S chelating system at the C-terminus has not been studied earlier. This study was designed to investigate the influence of such reordering on the stability, labeling efficiency and, more interestingly, the biodistribution properties of 99mTc-labeled variants of ZHER2:342 Affibody molecules. The variants ZHER2:V6, ZHER2:V7 and ZHER2:V8 with a GXGC chelator at the C-terminus were studied for the first time. For comparison, the previously studied 99mTc-labeled ZHER2:V3, ZHER2:V4 and ZHER2:V5, variants with homologous GGXC chelators were included in the study (Wållberg et al. 2011a). These conjugates differ from ZHER2:V6, ZHER2:V7 and ZHER2:V8 only by the order of the chelating amino acids. All the experimental protocols, cell lines and animal strains were the same in both studies, and the experiments were performed by the same researchers, which provided good comparability of the data.
Surface plasmon resonance (Biacore) experiments (Wållberg et al. 2011b) showed that the ZHER2:V6, ZHER2:V7 and ZHER2:V8 Affibody molecules bind with high affinity to HER2, although a certain influence of the C-terminal sequence could be seen. 99mTc-labeling using a two-vial freeze-dried kit method was successful with labeling yield over 90% for all studied proteins, indicating no influence from the amino acid position on the labeling properties of the N3S system. After purification using disposable size-exclusion columns, the radiochemical purity was over 99% for the all three conjugates. All conjugates showed high stability when incubated with murine serum for 1 h and no signs of radionuclide release, no transchelation to blood proteins or aggregation could be observed. The biodistribution data were in good agreement with the data from the in vitro stability test. Rapid clearance of radioactivity from blood indicated that no transchelation of 99mTc form the labeled conjugates to blood proteins occurred (Tables 3, 4, 5). The radioactivity uptake in the stomach and salivary glands were low, thus indicating no release of free pertechnetate into blood circulation. All three labeled conjugates showed preserved binding specificity to HER2 expressing SKOV-3 cells, implying that the labeling procedure had no negative influence on the binding properties of these Affibody molecules.
The cellular processing data for 99mTc-ZHER2:V6, ZHER2:V7 and ZHER2:V8 by SKOV-3 carcinoma cells were significantly different from data obtained for their counterparts with a GGXC chelator. The GXGC variants were characterized by an overall lower cellular retention. The GXGC variant ZHER2:V6 showed an almost twofold higher affinity to HER2 than ZHER2:V3; however, the cellular retention of 99mTc-ZHER2:V6 was appreciably lower. Internalization data (Fig. 4a), showed better accumulation of intracellular radioactivity for 99mTc-ZHER2:V3 in comparison with 99mTc-ZHER2:V6. This can be explained by the formation of “leaky” cellular radiocatabolites with low residualizing capacity after internalization of 99mTc-ZHER2:V6 and intracellular degradation. The low renal retention of radioactivity in vivo at 1 h p.i. (Table 3) also indicates poor residualizing properties of the 99mTc-GSGC chelate. The difference between 99mTc-ZHER2:V5 and 99mTc-ZHER2:V8 was mainly in the membrane-bound fraction of radioactivity. It is likely that the observed better membrane retention of ZHER2:V5 was due to the twofold higher affinity of this compound to HER2. The difference in retention of 99mTc-ZHER2:V4 and 99mTc-ZHER2:V7 is more difficult to explain, since the affinities to HER2 are almost identical, and the difference in intracellular radioactivity was very small. Still, in vivo data on renal retention suggest a somewhat lower intracellular retention of 99mTc-ZHER2:V4. One has to be cautious, however, in comparison of in vitro data for cancer cells and in vivo data for excretory organs. The common aspect here is the retention of radiocatabolites. This depends mainly on the physico-chemical properties of 99mTc-GXYC chelate, such as stability, charge distribution and lipids solubility. There is, however, a major difference in the internalization rate. In cancer cell lines, the internalization rate depends mainly on the nature of the cellular membrane target and the nature of the binding probe. The results of this study, as well as others (Wållberg and Orlova 2008; Wållberg et al. 2011a; Ahlgren et al. 2008, 2009, 2010a; Tolmachev et al. 2010b), suggest that the internalization of radiolabeled ZHER2:342 derivatives by HER2-expressing cells is relatively slow (less than 30% after 24 h). In vivo, the internalization in kidneys and liver occurs by scavenger receptors with a rather high internalization rate.
The in vivo data showed that the uptake of the 99mTc-labeled GXGC-containing Affibody molecules was very similar in a majority of organs, except from excretory ones. It was also similar to the biodistribution of the GGXC-containing counterparts (Tables 3, 4, 5). The observed difference in renal activity between GGXC variants containing amino acids with charged side chains and their GXGC counterparts is most likely a matter of residualizing properties, since the re-absorption of ZHER2:342 derivatives is close to 100%. The major influence was at 1 h p.i., but at 4 h this difference was appreciably decreased and remained low up to 24 h. Overall, positively charged lysine-containing variants displayed the strongest residualizing effect. Interestingly, the use of lysine-containing chelators also caused an elevated uptake in the liver. This effect was more pronounced for the GGKC chelator than for GKGC (Table 5). Most likely, this is not due to the residualizing properties of lysine-containing chelators, since earlier data concerning ZHER2:342 derivatives labeled with strongly residualizing 111In, 57Co and 68Ga labels showed several fold lower hepatic activities (Tolmachev et al. 2006, 2010c; Orlova et al. 2007; Ahlgren et al. 2008, 2010a; Wållberg et al. 2010). On the other hand, the lysine-rich maKKK-chelator positioned at the N-terminus caused high hepatic uptake of 99mTc-labeled ZHER2:342 (Tran et al. 2008). It is possible that positively charged 99mTc chelates can be preferentially taken up by one of the scavenger receptors on hepatocytes. This may be an indication that positively charged chelators should be avoided for the labeling of Affibody molecules.
Conclusion
The use of cysteine-containing peptide-based chelators is an efficient way of labeling Affibody molecules with technetium with high stability. However, both the composition of such chelators and the order of the amino acids are essential for both the cellular processing by cancer cells and the retention of the radionuclide in excretory organs. This requires a careful optimization of such chelators. These findings may be crucial for future development of therapeutic conjugates labeled with 186Re or 188Re, where small differences in the uptake would be crucial for nephrotoxicity and/or radiation dose to the liver and other normal organs. This information can be helpful for the development of other scaffold protein-based radiolabeled conjugates.
References
Ahlgren S, Tolmachev V (2010) Radionuclide molecular imaging using Affibody molecules. Curr Pharm Biotechnol 11:581–589
Ahlgren S, Orlova A, Rosik D, Sandström M, Sjöberg A, Baastrup B, Widmark O, Fant G, Feldwisch J, Tolmachev V (2008) Evaluation of maleimide derivative of DOTA for site-specific labeling of recombinant Affibody molecules. Bioconjug Chem 19:235–243
Ahlgren S, Wållberg H, Tran TA, Widström C, Hjertman M, Abrahmsén L, Berndorff D, Dinkelborg LM, Cyr JE, Feldwisch J, Orlova A, Tolmachev V (2009) Targeting of HER2-expressing tumors using a site-specifically 99mTc-labeled recombinant Affibody molecule ZHER2:2395 with C-terminal engineered cysteine. J Nucl Med 50:781–789
Ahlgren S, Orlova A, Wållberg H, Hansson M, Sandström M, Lewsley R, Wennborg A, Abrahmsén L, Tolmachev V, Feldwisch J (2010a) Targeting of HER2-expressing tumors using 111In-ABY-025, a second generation Affibody molecule with a fundamentally re-engineered scaffold. J Nucl Med 51:1131–1138
Ahlgren S, Andersson K, Tolmachev V (2010b) Kit formulation for 99mTc-labeling of recombinant anti-HER2 Affibody molecules with a C-terminally engineered cysteine. Nucl Med Biol 37:539–546
Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, Tolmachev V, Feldwisch J (2010) Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled Affibody. J Nucl Med 51:892–897
Britz-Cunningham SH, Adelstein SJ (2003) Molecular targeting with radionuclides: state of the science. J Nucl Med 44:1945–1961
Cameron DA, Stein S (2008) Drug insight: intracellular inhibitors of HER2—clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol 5:512–520
Chang HR (2010) Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer 116:2856–2867
Citri A, Yarden Y (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516
Ekblad T, Tran T, Orlova A et al (2008) Development and preclinical characterisation of 99mTc-labelled Affibody molecules with reduced renal uptake. Eur J Nucl Med Mol Imaging 35:2245–2255
Ekblad T, Orlova A, Feldwisch J, Wennborg A, Eriksson Karlström A, Tolmachev V (2009) Positioning of 99mTc-chelators influences radiolabeling, stability and biodistribution of Affibody molecules. Bioorg Med Chem Lett 19:3912–3914
Engfeldt T, Orlova A, Tran T et al (2007a) Imaging of HER2-expressing tumours using a synthetic Affibody molecule containing the 99mTc-chelating mercaptoacetyl-glycyl-glycyl-glycyl (MAG3) sequence. Eur J Nucl Med Mol Imaging 34:722–733
Engfeldt T, Tran T, Orlova A, Widström Ch, Eriksson Karlström A, Tolmachev V (2007b) 99mTc-chelator engineering to improve tumour targeting properties of a HER2-specific Affibody molecule. Eur J Nucl Med Mol Imaging 34:1843–1853
Hynes NE, MacDonald G (2009) ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 21:177–184
Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY (2010) Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett 584:2670–2680
Mankoff DA (2009) Molecular imaging to select cancer therapy and evaluate treatment response. Q J Nucl Med Mol Imaging 53:181–192
Miao Z, Levi J, Cheng Z (2010) Protein scaffold-based molecular probes for cancer molecular imaging. Amino Acids. doi:10.1007/s00726-010-0503-9
Nygren PA (2008) Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J 275:2668–2676
Orlova A, Tolmachev V, Pehrson R, Lindborg M, Tran T, Sandström M, Nilsson FY, Wennborg A, Abrahmsén L, Feldwisch J (2007) Synthetic affibody molecules: a novel class of affinity ligands for molecular imaging of HER2-expressing malignant tumors. Cancer Res 67:2178–2186
Orlova A, Magnusson M, Eriksson T, Nilsson M, Larsson B, Höiden-Guthenberg I, Widström C, Carlsson J, Tolmachev V, Ståhl S, Nilsson F (2006) Tumor imaging using a picomolar affinity HER2 binding Affibody molecule. Cancer Res 66:4339–4348
Tolmachev V (2008) Imaging of HER-2 overexpression in tumors for guiding therapy. Curr Pharm Des 14:2999–3011
Tolmachev V, Nilsson FY, Widström C, Andersson K, Rosik D, Gedda L, Wennborg A, Orlova A (2006) 111In-benzyl-DTPA-ZHER2:342, an affibody-based conjugate for in vivo imaging of HER2 expression in malignant tumors. J Nucl Med 47:846–853
Tolmachev V, Stone-Elander S, Orlova A (2010a) Current approaches to the use of radiolabeled tyrosine kinase-targeting drugs for patient stratification and treatment response monitoring: prospects and pitfalls. Lancet Oncol 11:992–1000
Tolmachev V, Hofström C, Malmberg J et al (2010b) HEHEHE-tagged affibody molecule may be purified by IMAC, is conveniently labeled with [99(m)Tc(CO)3](+), and shows improved biodistribution with reduced hepatic radioactivity accumulation. Bioconjug Chem 21:2013–2022
Tolmachev V, Velikyan I, Sandström M, Orlova A (2010c) A HER2-binding Affibody molecule labelled with 68Ga for PET imaging: direct in vivo comparison with the 111In-labelled analogue. Eur J Nucl Med Mol Imaging 37:1356–1367
Tran T, Engfeldt T, Orlova A et al (2007a) In vivo evaluation of cysteine-based chelators for attachment of 99mTc to tumor-targeting Affibody molecules. Biocojug Chem 18:549–558
Tran T, Engfeldt T, Orlova A et al (2007b) 99mTc-maEEE-ZHER2:342, an Affibody molecule-based tracer for detection of HER2-expression in malignant tumors. Bioconjug Chem 18:1956–1964
Tran T, Ekblad T, Orlova A et al (2008) Effects of Lysine-containing mercaptoacetyl-based Chelators on the Biodistribution of 99mTc-labeled anti-HER2. Bioconjug Chem 19:2568–2576
Tran TA, Rosik D, Abrahmsén L et al (2009) Design, synthesis and biological evaluation of a HER2-specific affibody molecule for molecular imaging. Eur J Nucl Med Mol Imaging 36:1864–1873
Wållberg H, Orlova A (2008) Slow internalization of anti-HER2 synthetic affibody monomer 111In-DOTA-ZHER2:342-pep2: implications for development of labeled tracers. Cancer Biother Radiopharm 23:435–442
Wållberg H, Ahlgren S, Widström C, Orlova A (2010) Evaluation of the radiocobalt-labeled [MMA-DOTA-Cys61]-Z HER2:2395(-Cys) affibody molecule for targeting of HER2-expressing tumors. Mol Imaging Biol 12:54–62
Wållberg H, Orlova A, Altai M, Widström C, Hosseinimehr SJ, Malmberg J, Ståhl S, Tolmachev V (2011a) Molecular design and optimization of 99mTc-labeled recombinant affibody molecules improves their biodistribution and imaging properties. J Nucl Med 52:461–469
Wållberg H, Löfdahl PÅ, Tschapalda K, Uhlén M, Tolmachev V, Nygren PÅ, Ståhl S (2011b) Affinity recovery of eight HER2-binding affibody variants using an anti-idiotypic affibody ligand. Protein Exp Purif 76:127–135
Acknowledgments
This research was financially supported by grants from the Swedish Cancer Society (Cancerfonden) and the Swedish Research Council (Vetenskapsrådet).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Altai, M., Wållberg, H., Orlova, A. et al. Order of amino acids in C-terminal cysteine-containing peptide-based chelators influences cellular processing and biodistribution of 99mTc-labeled recombinant Affibody molecules. Amino Acids 42, 1975–1985 (2012). https://doi.org/10.1007/s00726-011-0927-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0927-x