Abstract
Exine, this complex sporopollenin-containing and highly variable among taxa envelope of the male gametophyte, consists of two layers, ectexine and endexine. We traced in detail the pollen wall development in Lysimachia vulgaris (Primulaceae), with emphasis on driving forces and critical ontogenetic time. By observation on the sequence of the emergent patterns and by analysis of their substructure with TEM, we intended to clarify the obvious and not-obvious ways of exine construction and to find out the common features in pattern development in other representatives in living nature. The ectexine and endexine ontogeny follows the main stages observed in many other species: first, the appearance of microspore plasma membrane invaginations with isotropic contents within, changed later to anisotropic state; then successive appearance of spherical, rod-like, and lamellate units in the periplasmic space. The lamellate endexine appears unusually early in the exine development. All these elements and their aggregations are manifestation of well-known physical phenomena: phase separation and micellar self-assembly. A consideration of similar surface patterns in very remote taxa suggests the participation in their development of some general nature phenomena as the lows of space-filling operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen wall, this complex male gametophyte envelope, gives us a rare possibility to study pattern formation and the key for the pollen morphological diversity in the frames of a single cell. Heslop-Harrison (1972) astutely called pollen ontogeny “morphogenesis in miniature.” Exine, sporopollenin-containing outer part of the envelope, develops in the periplasmic space, between the microspore plasma membrane and callose envelope. The periplasmic space is still absent at the early tetrad stage, gradually increasing in the course of exine development, as the constructive substances appear beyond the plasma membrane. The precise chemical composition of substances, located inside the microspore periplasmic space, is species-specific and difficult to be determined, but the classes of chemical substances—complex polysaccharides, most probably glycoproteins and lipopolysaccharides—were tested histochemically (Rowley 1973; Pettitt and Jermy 1974; Rowley and Dahl 1977; Pettitt 1979). These substances, their concentrations, and sporopollenin precursors, monomers and regulatory mechanisms, are determined by genome (Herminghaus et al. 1988; Gubatz and Wiermann 1992; Wiermann and Gubatz 1992; Collinson et al. 1993; Wilmesmeier and Wiermann 1997; Van Bergen et al. 2004; Hemsley et al. 1996a; Wilmesmeier and Wiermann 1997; Grienenberger et al. 2010; Wang et al. 2013; Quilichini et al. 2015; Li et al. 2019; Hou et al. 2023) which are delivered into the periplasmic space at the definite ontogenetic time. All these substances are surface active (surfactants) and are capable to form colloidal solutions in the periplasmic space.
Many works and some reviews have shown many genes playing a role in the establishment of the exine (e.g., Ariizumi and Toriyama (2011); Dobritsa et al. (2011); Shi et al. (2015); Wang and Dobritsa (2018); Xiong et al. (2020); Liu and Wang (2021); Xu et al. (2022); Zhou and Dobritsa (2023); Suh et al. (2024)). But in which way all these constructive substances are arranged into different intricate patterns of pollen and spore envelopes? Mixtures of components should undergo some transformations to be integrated and to form finally 3D heterogeneous patterns such as pollen walls.
Here, we find ourselves in the physical–chemical field of space-filling operations. Wodehouse (1935) was the first palynologist who understood this. But even earlier, Thompson (1917), a physicist, mathematician, and biologist, pointed out that different microarchitecture patterns in nature (e.g., Foraminifera and Heliozoa skeletons, diatomeae frustules, beetle elytrons, surfaces of seeds, spores and pollen walls) are formed by physical forces, such as structure-forming mechanisms, e.g., self-assembly. This idea was picked up and developed by biologists, physicists, and mathematicians (Heslop-Harrison 1972; Mandelbrot 1982; Dickinson and Sheldon 1986; Newman and Comper 1990; Newman and Forgacs 2009; Kauffman 1993; Ingber 1993; Scott 1994; Kurakin 2005; Blackmore et al. 2007, 2010; Benítez 2013; Lintilhac 2014; Stillman and Mayor 2023). Heslop-Harrison (1972) mentioned that genomic control must work at strategic points in development, then physical processes similar to crystallization must do the rest of work to complete the space-filling operation. Physicists and mathematicians occurred to be more inquisitive than biologists, looked to the neighboring discipline (biology, palynology) and concluded that there was no sense for nature to overload genetic code with huge information where simple physical mechanisms, working in tandem with genome, were capable to do the constructive work (Mandelbrot 1982; Kauffman 1993).
In the case of pollen wall development, colloidal systems of the surface-active exine constructive substances in the microspore periplasmic space are capable to micellar self-assembly where hydrophobic interactions play the main role. The importance of looking through colloidal chemistry “window” was suggested in some papers (Hemsley et al. 1992; Collinson et al. 1993; Gabarayeva 1993; Gabarayeva and Hemsley 2006; Hemsley and Gabarayeva 2007).
Primexine, a blue-print stage, bearing all the main features of the future mature exine, is far not being an ephemeral structure, existing only for a short period of time. It was shown in a series of destruction experiments with exines in several species that after sporopollenin precursor accumulation, the primexine matrix (glycocalyx) occurred embedded and gradually sealed into sporopollenin, preserved in mature pollen/spore walls, and its proteins and polysaccharides can be revealed again after severe oxidation of sporopollenin (Rowley and Prijanto 1977). Such experimental destruction of exines with severe damaging properties—chemical and physical—has shown unexpectedly that secondary sporopollenin, accumulated in the free microspore period, is more vulnerable to oxidation and physical destruction than initial constructive substances of primexine (complex polysaccharides, probably glycoproteins and lipopolysaccharides), sealed into primary sporopollenin, accumulated in the end of the tetrad period (Rowley and Prijanto 1977).
All the microstructures observed in mature sporoderm (granules, rod-like columellae, hexagonally packed into layers, and lamellae with central “white lines”) represent the history of their formation as the micellar progressing sequence (spherical micelles, their columns, arranged into cylindrical micelles, bilayers with central gap), immortalized by sporopollenin as “stiffened history” (Gabarayeva et al. 2020). The idea about micellar self-assembly as the driving force of exine emergence came from the observation on the coincidence of exine developmental stages with micellar self-assembling stages (mesophases) in colloidal systems, subject to increasing concentrations of ingredients (Gabarayeva and Hemsley 2006; Hemsley and Gabarayeva 2007; see also English abstract in Gabarayeva and Hemsley (2010)).
Lavrentovich et al. (2016) suggested that the diversity of exine patterns could be explained by phase transitions to spatially modulated phases. These authors proposed a general theory for surface patterning in many different biological systems, including mite and insect cuticles, pollen grains, fungal spores, and insect eggs. This theory extends Brazovskii’s (1975) ideas on such transitions on a flat, infinite sheet to transitions on spheres (including most of pollen grains). Lavrentovich et al. (2016) also showed that the membrane undulations (the common feature of the microspore plasma membrane at the tetrad stage in any species) are a function of physical parameters. Further development of these ideas was carried out in the next theory of this group (Radja et al. 2019).
The confirmation of these ideas came from modelling artificial exines in vitro, first simulated by the flocculation of polystyrene particles (Hemsley et al. 1996b, 1998, 2003; Hemsley and Griffiths 2000; Griffiths and Hemsley 2002; Moore et al. 2009), then via mixed colloidal systems with anther-like medium components (Gabarayeva and Grigorjeva 2016, 2017; Gabarayeva et al. 2019) and by computer modelling (Radja et al. 2019). The final joint conclusion was that both physical–chemical processes—phase separation and micelle self-assembly—dominate in the course of the exine development and carry out the ultimate 3D microarchitectural pattern of exines, following genomic control under chemical composition of the exines’ building substances (Gabarayeva et al. 2020).
Thus, this is exactly the primexine template at the middle tetrad stage that defines the final structure of mature exines. The key role of the tetrad stage in exine development was emphasized many times earlier, e.g., in numerous studies of Rowley (see his full list of papers in Blackmore and Skvarla 2012), followed by other investigations (Taylor and Osborn 2006; Blackmore et al. 2007, 2010; Galati et al. 2012; Taylor et al. 2013, 2015; 2018; Zini et al. 2017) and in recent studies (Wang and Dobritsa 2018; Wang et al. 2021).
Pollen grains in Lysimachia vulgaris is 3-colporate, with reticulate sculpture. There are some works on Lysimachia pollen morphology (Nowicke and Skvarla 1977; Wrońska-Pilarek and Morozowska 2009; Yang et al. 2012; Odabaşi 2021). However, we have not found any studies on pollen wall development of the representatives of the genus Lysimachia; besides, some our previous tests on the species have shown the unusually early start of the endexine development, what was the reasons for choosing this species for this study.
Our goal in this work is to clarify the underlying developmental mechanisms of L. vulgaris pollen wall development. We are going to reveal the complete sequence of processes leading to the appearance of exine pattern in this species with TEM and SEM methods. We also want to determine the most critical time in Lysimachia exine development and to compare our findings with those of earlier ontogenetic studies in other taxa, and also to determine whether our hypothesis on the role of physical processes in spore and pollen wall development also applies in Lysimachia vulgaris.
Material and methods
Flower buds at different developmental stages of Lysimachia vulgaris L. (Primulaceae) were obtained from the Botanical Gargen of Komarov Botanical Institute, St. Petersburg, during the seasons of 2022–2023 years, 40 buds every year—to catch most of the developmental stages. Fragments of stamens were placed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, de-aerated and fixed overnight at 4 °C. The samples were then rinsed in cacodylate buffer, postfixed with 1% OsO4 for 2 h at room temperature, rinsed in distilled water, and dehydrated through a graded ethanol series, embedded in Epon-acetone mixture overnight and put in pure Epon (Epon-medium, DDSA, MNA, DMP-30 mixture). The material was kept in Epon for a day at room temperature and then for 2 days at 62 °C. Sectioning was carried out using an LKB instrument. Ultrathin sections were contrasted with 1% aquatic solution of uranyl acetate and 0.2% lead citrate and examined with a Libra 120 plus TEM instrument.
For scanning electron microscopy (SEM), pollen samples of L. vulgaris from opened flowers were collected and air dried. Dry specimens were attached to a SEM stub by double-sided stick tape, then were coated with gold/palladium fusion at vacuum. Specimens were observed with a JEOL JSM-6390 instrument in the Core Facility ‘Cellular and Molecular Technologies in Plant Science’ of the Komarov Botanical Institute of RAS (Saint Petersburg).
Results
Meiosis
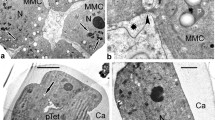
Well-pronounced synaptonemal complexes with central elements are observed in the nuclei of microspore mother cells at the pachytene stage of prophase I (Fig. 1a, b, arrows). The nuclei envelopes are still preserved. Meiocytes are surrounded with a thick callose envelope.
Prophase I of meiosis (pachytene stage) and two types of cytokinesis in Lysimachia vulgaris L. a, b Microspore mother cells (MMC) with synaptonemal complexes (arrows). c Initiation of simultaneous cytokinesis by the appearance and further fusion of small vacuoles (asterisks). d Successive types of cytokinesis, which is carried out by furrowing (arrowhead). Ca callose, Dy dyad, MMC microspore mother cell, N nucleus, Nu nucleolus. Scale bars: a, c 2 µm; b 5 µm, d 1 µm
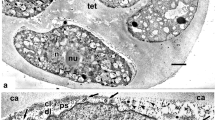
After the completion of meiosis, two types of cytokinesis are evident. Simultaneous cytokinesis is initiated with the appearance of the rows of small vacuoles (Fig. 1c, asterisks), their membranes fuse later with each other, forming the plasma membrane of the tetrad’s microspores. However, successive cytokinesis is also observed, bringing about the appearance of dyads (Fig. 1d), the latter form tetrads by furrowing (Figs. 1c and 2a, arrowheads).
Successive cytokinesis and young tetrad stage in Lysimachia vulgaris. a Formation of dyads by furrowing (arrowheads). b Early tetrads, surrounded by thick callose envelope. c Slightly later stage, the microspore plasma membrane is invaginated. d Invaginations are more pronounced (asterisks), note anisotropic contents inside invaginations. e, f Well-pronounced phase separation of the contents inside invaginations (arrowheads). g Aperture sites in the tetrad microspores. Note the initial endexine lamellae (laminate micelles—arrows). h Higher magnification shows initial endexine lamella (arrowhead) and other different microstructures inside invaginations (asterisk). Ca callose, MC microspore cytoplasm, N nucleus, Tet tetrad. Scale bars: a, g 1 µm; b 5 µm, c–f, h 0.5 µm
Tetrad stages
Young tetrad stage
A young tetrad (Fig. 2b) is surrounded with a thick callose envelope. Initially even, the plasma membrane starts to form small invaginations (Fig. 2c, arrowheads). A bit later, first signs of phase separation are observed as anisotropic distribution of substances in the medium inside the plasma membrane invaginations and the callose envelope (Fig. 2d, asterisks). Somewhat later phase separation is more prominent (Fig. 2e, f, arrowheads).
Further changes have place in the aperture sites: in the periplasmic space, inside plasma membrane invaginations (Fig. 2g, arrows), thin lamella-like formations appear (Fig. 2h, arrowhead), simultaneously with some other membrane-like structures (Fig. 2h, asterisk). These structures are nothing but initial steps of the endexine formation.
Middle tetrad stage
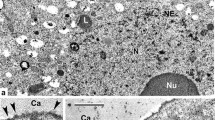
This stage is a key one in the rest of pollen wall development. The intensive process of plasma membrane invagination continues alongside the whole microspore surface (Fig. 3a, arrowheads). Spherical units—micelles—are seen separately (Fig. 3b, arrowheads), in groups, and in strings (Fig. 3c, arrowheads) in the microspore periplasmic space. Somewhat later, columella-like pattern is observed, consisting of string-like thin pro-columellae (Fig. 3d, e, arrowheads). Higher magnification shows more details of these pro-columellae (Fig. 3f, arrowheads) and initial tectum (PT); some pro-columellae show clear spiral substructure (Fig. 3f, double arrowheads).
Middle tetrad stage in Lysimachia vulgaris. a Overview of a tetrad. Small invaginations are evident alongside the microspore surface (arrowheads); thin endexine lamellae is seen in the aperture site (asterisk). b, c Nanostructures as spherical micelles (b) and their arrangement into columns and strings (c) are observed in the periplasmic space (arrowheads). d, e Pro-columellae, based on columns of spherical micelles, show clearly their string-like form (arrowheads). f Higher magnification reveals more distinctly the substructure of pro-columellae (arrowheads). Note that some pro-columellae have clearly spiral substructure (double arrowheads). Ca callose, MC microspore cytoplasm, N nucleus, Nu nucleolus, PM plasma membrane, PT protectum, PS periplasmic space, Tet tetrad. Scale bars: a 2 µm; b, c, d, f: 0.2 µm, e 0.5 µm
Late tetrad stage
After initial sporopollenin accumulation on the primexine, the structure of the future exine is defined (Fig. 4a). Young tectum, columellae, and the foot layer are evident. The spiral substructure of columellae is well-pronounced (Fig. 4a, arrowheads). Note distinct signs of phase separation inside plasma membrane invaginations (Fig. 4a, asterisks – condensed subvolume, stars – depleted subvolume). These sites correspond to lacunae of the reticulate exine pattern.
Late tetrad stage in Lysimachia vulgaris pollen wall and tapetum development. a Proximal side of two adjacent microspores of a tetrad. After initial sporopollenin accumulation tectum, columellae and foot layer of exine are evident. The substructure of the developing exine reveals spiral nature of young columellae, based on twisted cylindrical micelles (arrowheads). Signs of phase separation—concentrated (asterisks) and depleted (stars) areas in places of the former plasma membrane invaginations—are distinct. b Tapetal cells at the middle tetrad stage. Note pro-orbicules (arrows) alongside the cells’ surface. c Tapetal cells at the late tetrad stage. Mature orbicules (arrows) alongside the tapetal cells. d A border of tapetum and of a free microspore. Orbicules (arrowheads) alongside the tapetal cell. e Degenerating tapetum in the vicinity of free microspores. Orbicules are preserved (arrowheads). AL anther loculus, Ap aperture, Ca callose, DTa degenerating tapetum, LG lipid globule, MC microspore, Co columella, Ex exine, FL foot layer, T tectum, Ta tapetum. Scale bars: a 0.5 µm; b–d 1 µm; e 2 µm
The inner, locular sides of the tapetal cells, being covered with pro-orbicules at the previous middle tetrad stage (Fig. 4b, arrows), bear actually mature orbicules at the late tetrad stage (Fig. 4c, arrows).
Disintegrating tetrads and early free microspores
At the early free microspore stage, orbicules keep their location around the tapetal cells (Fig. 4d, arrowheads). Somewhat later, the tapetal cells start to degenerate, but orbicules appear intact, probably saved by the peritapetal membrane (Fig. 4e, arrowheads).
The overviews (Fig. 5a, b) show the tetrad on the point of disintegration (Fig. 5a) and the tetrads with remnants of callose envelope (Fig. 5b).
Disintegrating tetrad stage and early free microspores in Lysimachia vulgaris. a Late tetrad on the point of disintegration. b Disintegrating tetrad, callose envelope is in remnants. c Free microspores. Well-pronounced endexine lamellae in the aperture sites (arrows) started to develop at the middle tetrad stage on the base of laminate micelles. d Initiation of the stage of vacuolization. Note that the endexine is present not only in the aperture sites (arrow), but also in inter-aperture regions (white asterisks). AL anther loculus, Ca callose, MC microspore cytoplasm, N nucleus, Nu nucleola. Scale bars: a, d 2 µm; b 5 µm, c 1 µm
Young free microspores have well-developed lamellae of the endexine in the aperture sites (Fig. 5c, arrows), which were initiated as early as at the young tetrad stage. At this stage, the endexine is observed in the aperture sites only, whereas later, at the beginning of the vacuolation stage, the endexine is present over the whole microspore surface (Fig. 5d, asterisks). In further development, the central vacuole increases in size (Fig. 6a, b), displacing the cytoplasm with all its organelles apart.
The stage of vacuolization (a, b) and two-celled pollen grains (c–f) in Lysimachia vulgaris. a, b Gradual enlargement of the vacuole, the latter displaces the cytoplasm to the periphery. c, d Two-celled pollen grains after completion of the microspore mitosis. Note distinct envelope between generative and vegetative cells. e Pollen grain with well-pronounced intine disposed under apertures and numerous starch grains in the cytoplasm of the vegetative cell. f Two-celled pollen grain, note fragments of pollenkitt (asterisks). Ap aperture site, Ect ectexine, End endexine, Ex exine, DV developing vacuole, GC generative cell, GN generative nucleus, Int intine, MC microspore cytoplasm, N nucleus, SG starch grains, V vacuole, VC vegetative cell, VN vegetative nucleus, WGC wall of generative cell. Scale bars: a, c, e, f 5 µm; b, d 1 µm
Mature pollen grains
After microspore mitosis two-celled pollen grains appear, with vegetative and generative cells (Fig. 6c, d). The intine develops at the aperture sites (Fig. 6e). Note (increasing magnification in Fig. 6e) that lamellae of the endexine are intermixed with intine in aperture sites. Multiple starch grains crowd in the cytoplasm of the vegetative cell (Fig. 6c–f). No orbicules are seen around pollen grains; they evidently persist in the forming peritapetal membrane. Instead, portions of pollenkitt are observed sticking to the surface of pollen grains (Fig. 6f, asterisks).
SEM images in Fig. 7 show pollen grains in polar view (Fig. 7a) and in equatorial view, where intermixed intine protrudes through apertures (Fig. 7b). The surface view shows the character reticulate sculpture with heads of atectate columellae on the bottoms of lacunae (Fig. 7c).
SEM images of pollen grains in Lysimachia vulgaris. a Polar view. All the three apertures are seen in this view. b Equatorial view. The aperture with protruding intermixed intine is evident. c Surface view. Typical reticulate sculpture with lacunae and heads of atectate columellae on the bottoms of lacunae. Scale bars: a–c 1 µm
Discussion
It is clear from our results that the exine formation in Lysimachia vulgaris proceeds according to already well-known way: after genomic control under synthesis and delivery of the species-specific building substances to the microspore periplasmic space at the tetrad period, the constructive processes of the exine are triggered off by physical forces (Gabarayeva et al. 2009a, b, 2023, 2024; Gabarayeva 2023): first, phase separation acts, changing isotropic contents in the microspore periplasmic space to anisotropic (condensed and dilute regions appear inside plasma membrane invaginations), then the sequence of self-assembling micellar structures unfolds, starting with spherical micelles and their strings. Later at the middle tetrad stage, distinct columellate pattern is seen which still bears clear signs of columns of spherical micelles. First signs of the apertures appear at the young tetrad stage.
The probable mechanism of apertures’ localization was shown with the model plant Arabidopsis (Dobritsa and Coerper 2012; Dobritsa et al. 2018; see also a review (Albert et al. 2022)). However, an unusual feature in Lysimachia is the ontogenetic time of the endexine formation: first signs of the endexine appear in the aperture sites strikingly early in exine ontogeny, when the aperture sites start to be evident at the young tetrad stage. Inside plasma membrane invaginations, building blocks as short membranous fragments, circles and strings, with one large lamina appear, corresponding to laminate micella (and its precursors). This laminate micella is the base for the future first lamella of the endexine. Later in development, the number of lamellae increases, and up to the end of the tetrad period the lamellate endexine is well-developed and consists of 5–6 lamellae, separated in aperture sites but fused between the apertures. The usual ontogenetic time of the endexine appearance for most angiosperms is the young post-tetrad period. But exclusions always exist: for example, such near-basal angiosperms as Magnolia species, where the endexine lamellae form at the transition from the late tetrad stage to early free microspore (Gabarayeva and Grigorjeva 2012, Fig. 9) or one of the basal (or next to basal) angiosperms, Victoria (Nymphaeales), where the endexine lamellae appear at the late tetrad stage (Taylor et al. 2013). In gymnosperms, the endexine develops also at the late tetrad stage (Juniperus and Larix: Gabarayeva et al. 2014; Gabarayeva and Grigorjeva 2017).
New wave of phase separation evidently proceeds in the periplasmic space at the late tetrad stage, followed by the appearance of the order-interval pattern of the concentrated-diluted regions. This pattern was called “Golden Gates” (Wang et al. 2021) because of similarity with the eponymous bridge. In 3D projection, this pattern determines the future reticulate sculpture of the pollen grains, where diluted areas correspond to lacunae of the reticulum. Figure 4a is expressive of many substructural spiral elements, described first by Rowley as fundamental exine units—tufts (Rowley 1990) and later considered being tightly packed cylindrical micelles, arranged to bundles/tufts (Gabarayeva and Hemsley 2006).
Another unusual feature of L. vulgaris is the early formation of orbicules (Ubish bodies) in the tetrad period. Pro-orbicules appear alongside the tapetal cells at the middle tetrad stage and are seen mature at the late tetrad stage. However, orbicules are not observed around mature pollen grains; they most probably remain incrusted into the peritapetal membrane.
There are many well-known examples of self-assembling processes in living and non-living nature. One of such examples is a striking similarity between the surface patterns of pollen exines and spores of myxomycete (Eukaryota, phylum Amoebozoa). These ancient organisms, slime molds, are classified as Protista—neither plants, animals, nor fungi normally take the form of amoeba but also develop fruit bodies that release spores. It is enough to look at pictures from the papers on myxomycete spore wall development (Mims 1972; Aldrich 1974) and ornamentation (García-Cunchillos et al. 2021) to be puzzled by similarity between spore/pollen sculpture of these very remote taxa. It is difficult to suspect these two taxa in genomic similarity; however, every palynologist would immediately recognize a certain pollen taxon, the pattern of which is similar to the pattern of some myxomycete spores (compare, e.g., ridged hexagonal spore surface pattern in Fig. 56 from García-Cunchillos et al. 2021 with that of pollen in Scorzonera hispanica – see Blackmore and Claugher (1987)). This phenomenon is, in essence, not surprising; it is in accordance with the laws of nature, with general rules of space-filling operation. Moreover, the plasma membrane undulations are observed in myxomycete spore wall development (Fig. 17 in Aldrich 1974) as it is in microspore wall development.
It is highly probable that another physical mechanism—tensegrity—participates to the formation of plasma membrane undulations, producing compression membrane wrinkles and spontaneously contracting malleable extra-cellular gel—glycocalyx (Ingber 1993, 2003). In the case of microspore development, tensegrity mechanism could produce a deeply, periodically invaginated cell surface, with periodically contracted, wrinkled glycocalyx.
Refrains (iterations) are characteristic for most features in biological variety and have the universal character in nature (Meyen 1984; Pozhidaev 1993, 1995, 1998, 2000, 2002; Chaikovsky 2018; Pozhidaev and Petrova 2023).
The data on other species have shown that the underlying mechanisms of exine development are general (Gabarayeva et al. 2024).
Two types of cytokinesis
Though the type of post-meiotic cytokinesis is accepted as a marker for a species and more higher taxa (see, e.g., a review (Albert et al. 2022)), the simultaneous presence of two types of meiotic cytokinesis—successive and simultaneous—in Lysimachia vulgaris is not a single case: the same phenomenon is observed, for instance, in Cymbalaria muralis (Polevova et al. 2023). In general, many variations are observed in the course of cytokinesis in a wide range of species. Microsporogenesis is highly labile in basal angiosperms (see Gabarayeva and Grigorjeva (2014) and references in). For example, the resulting tetrads may range from tetragonal to symmetric or asymmetric tetrahedral, with occasional rhomboidal tetrads (Nadot et al. 2006). Numerous intraspecific variations in aperture pattern and location were shown for many species and regarded as nature regularities (Pozhidaev 1998, 2000, 2002). In Juniperus communis, the type of simultaneous cytokinesis is also unusual: semi-furrows appear at telophase-I, and the final centripetal cleavage proceeds gradually at telophase-II (Gabarayeva et al. 2014). Simultaneous cytokinesis is a very primitive mode, known as Magnolia type.
Conclusions
The most critical ontogenetic time in Lysimachia vulgaris exine development is the tetrad period.
The underline mechanisms of pollen wall development are physical forces—phase separation and micellar self-assembly—which act in colloidal mixture of the periplasmic space after genomic control under precise chemical composition and increasing concentrations of exine building substances. These mechanisms are evidently universal for exine development in other species and many other patterns in living nature.
The unusual features in exine development in L. vulgaris are very early start of the endexine development. Orbicules which are produced by the tapetum in the tetrad period, are not associated with pollen grains.
Data availability
All relevant data are included in this manuscript and its supporting information is acceptable if this is the case.
References
Albert B, Matamoro-Vidal A, Prieu C, Nadot S, Till-Bottraud I, Ressayre A, Gouyon P-H (2022) A review of the developmental processes and selective pressures shaping aperture pattern in Angiosperms. Plants 11:357. https://doi.org/10.3390/plants11030357
Aldrich HC (1974) Spore cleavage and the development of wall ornamentation in two myxomycetes. Proc Iowa Acad Sci 8:28–36
Ariizumi T, Toriyama K (2011) Genetic regulation on sporopollenin synthesis and pollen exine development. Ann Rev Plant Biol 62:1–24. https://doi.org/10.1146/annurev-arplant-042809-112312
Benítez M (2013) An interdisciplinary view on dynamic models for plant genetics and morphogenesis: scope, examples and emerging research avenues. Front Plant Sci 4:7. https://doi.org/10.3389/fpls.2013.00007
Blackmore S, Claugher D (1987) Observations on the substructural organization of the exine in Fagus sylvatica L. (Fagaceae) and Scorzonera hispanica L. (Compositae: Lactuceae). Rev Palaeobot Palynol 53:175–184
Blackmore S, Skvarla J (2012) John Rowley (1926–2010), palynologist extraordinaire. Grana 51:77–83. https://doi.org/10.1080/00173134.2012.661454
Blackmore S, Wortley AH, Skvarla JJ, Rowley JR (2007) Pollen wall development in flowering plants. New Phytol 174:483–498. https://doi.org/10.1111/j.1469-8137.2007.02060.x
Blackmore S, Wortley AH, Skvarla JJ, Gabarayeva NI, Rowley JR (2010) Developmental origins of structural diversity in pollen walls of Compositae. Plant Syst Evol 284:17–32. https://doi.org/10.1007/s00606-009-0232-2
Brazovskiǐ SA (1975) Phase transition of an isotropic system to a nonuniform state. ZhETF 68(1):175–185
Chaikovsky JV (2018) Autopoiesis. Society of scientific editions KMK, Moscow
Collinson ME, Hemsley AR, Taylor WA (1993) Sporopollenin exhibiting colloidal organization in spore walls. Grana Suppl 1:31–39
Dickinson HG, Sheldon JM (1986) The generation of patterning at the plasma membrane of the young microspore of Lilium. In: Blackmore S, Ferguson IK (eds) Pollen and Spores: form and function. Academic Press, London, pp 1–18
Dobritsa AA, Coerper D (2012) The novel plant protein INAPERTURATE POLLEN1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. Plant Cell 24:4452–4464
Dobritsa AA, Geanconteri A, Shrestha J et al (2011) A large-scale genetic screen in Arabidopsis to identify genes involved in pollen exine production. Plant Physiol (lancaster) 157:947–970. https://doi.org/10.1104/pp.111.179523
Dobritsa AA, Kirkpatrick AB, Reeder SH, Li P, Owen HA (2018) Pollen Aperture factor INP1 acts late in aperture formation by excluding specific membrane domains from exine deposition. Plant Physiol 176:326–339
Gabarayeva NI (1993) Hypothetical ways of exine pattern determination. Grana 33(Suppl 2):54–59
Gabarayeva NI (2023) The role of physical processes in pollen wall morphogenesis: hypothesis and experimental confirmation. Russian J Dev Biol 54(5):283–305. https://doi.org/10.1134/S1062360423050053
Gabarayeva N, Grigorjeva V (2012) Sporoderm development and substructure in Magnolia sieboldii and other Magnoliaceae: an interpretation. Grana 5:119–147
Gabarayeva N, Grigorjeva V (2014) Sporoderm and tapetum development in Eupomatia laurina (Eupomatiaceae). An Interpretation Protoplasma 251:1321–1345. https://doi.org/10.1007/s00709-014-0631-2
Gabarayeva N, Grigorjeva V (2016) Simulation of exine patterns by self-assembly. Plant Syst Evol 302:1135–1156. https://doi.org/10.1007/s00606-016-1322-6
Gabarayeva NI, Grigorjeva VV (2017) Self-assembly as the underlying mechanism for exine development in Larix deciduas D. C Plant 246:471–493. https://doi.org/10.1007/s00425-017-2702-z
Gabarayeva NI, Hemsley AR (2006) Merging concepts: the role of self-assembly in the development of pollen wall structure. Rev Palaeobot Palynol 138:121–139
Gabarayeva N, Grigorjeva V, Rowley JR, Hemsley AR (2009a) Sporoderm development in Trevesia burckii (Araliaceae). I. Tetrad period: further evidence for participating of self-assembly processes. Rev Palaeobot Palynol 156:211–232
Gabarayeva N, Grigorjeva V, Rowley JR, Hemsley AR (2009b) Sporoderm development in Trevesia burckii (Araliaceae). II. Posttetrad period: further evidence for participating of self-assembly processes. Rev Palaeobot Palynol 156:233–247
Gabarayeva N, Grigorjeva V, Polevova S (2014) Sporoderm and tapetum ontogeny in Juniperus communis (Cupressaceae). Connective structures between tapetum and microspores. Rev Palaeobot Palynol 206:23–44
Gabarayeva NI, Grigorjeva VV, Shavarda AL (2019) Mimicking pollen and spore walls: self-assembly in action. Ann Bot 123(7):1205–1218. https://doi.org/10.1093/aob/mcz027
Gabarayeva NI, Grigorjeva VV, Lavrentovich MO (2020) Artificial pollen walls simulated by the tandem processes of phase separation and self-assembly in vitro. New Phytol 225:1956–1973. https://doi.org/10.1111/nph.16318
Gabarayeva NI, Grigorjeva VV, Polevova SV, Britski DA (2023) Ontogenesis in miniature Pollen wall development in Campanula rapunculoides. Planta 258:38. https://doi.org/10.1007/s00425-023-04198-w
Gabarayeva NI, Britski DA, Grigorjeva VV (2024) Pollen wall development in Impatiens glandulifera: exine substructure and underlying mechanisms. Protoplasma 261:111–124. https://doi.org/10.1007/s00709-023-01887-x
Gabarayeva NI, Hemsley AR (2010) Pattern formation in microcosm: the role of self-assembly in development of complex envelopes of biological matters. Biol Bull Rev (Zurnal Obschei Biologii) 71(4):310–336
Galati BG, Zarlavsky G, Rosenfeldt S, Gotelli MM (2012) Pollen ontogeny in Magnolia liliflora Desr. Plant Syst Evol 298:527–534. https://doi.org/10.1007/s00606-011-0563-7
García-Cunchillos I, Estébanez B, Lado C (2021) Spore ultrastructural features and significance of their diverse ornamental elements in the evolutionary history of the order Trichiales (Myxomycetes, Amoebozoa). Europ J Protistol 81:125839. https://doi.org/10.1016/j.ejop.2021.125839
Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, Souza Cde A, Heitz T, Douglas CJ, Legrand M (2010) Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell 22:4067–4083. https://doi.org/10.1105/tpc.110.080036
Griffiths PC, Hemsley AR (2002) Raspberries and muffins – mimiking biological pattern formation. Colloids Surf B Biointerfaces 25:163–170. https://doi.org/10.1016/S0927-7765(01)00316-2
Gubatz S, Wiermann R (1992) Studies on sporopollenin biosynthesis in Tulipa anthers. 3. Incorporation of specifically labeled C-14 Phenylalanine in comparison to other precursors. Bot Acta 105:407–413
Hemsley AR, Griffiths PC (2000) Architecture in the microcosm: biocolloids, self-assembly and pattern formation. Phil Trans Royal Soc Lond A 358:547–564. https://doi.org/10.1098/rsta.2000.0545
Hemsley AR, Collinson ME, Brain APR (1992) Colloidal crystal-like structure of sporopollenin in the megaspore walls of recent Selaginella and similar fossil spores. Bot J Linn Soc 108:307–320. https://doi.org/10.1111/j.1095-8339.1992.tb00247.x
Hemsley AR, Scott AC, Barrie PJ, Chaloner WG (1996a) Studies of fossil and modern spore wall biomacromolecules using 13C solid state NMR. Ann Bot (oxford) 78:83–94
Hemsley AR, Jenkins PD, Collinson ME, Vincent B (1996b) Experimental modelling of exine self-assembly. Bot J Linn Soc 121:177–187
Hemsley AR, Vincent B, Collinson ME, Griffiths PC (1998) Simulated self-assembly of spore exines. Ann Bot 82:105–109
Hemsley AR, Griffiths PC, Mathias R, Moore SEM (2003) A model for the role of surfactants in the assembly of exine sculpture. Grana 42:38–42
Hemsley AR, Gabarayeva NI (2007) Exine development: the importance of looking through a colloid chemistry “window”. Plant Syst Evol 263: 25–49. https://www.jstor.org/stable/23655636
Herminghaus S, Gubatz S, Arendt S, Wiermann R (1988) The occurrence of phenols as degradation products of natural sporopollenin – a comparison with “synthetic sporopollenin.” Z Naturf 43:491–500
Heslop-Harrison J (1972) Pattern in plant cell walls: morphogenesis in miniature. Proc R Inst Great Brit 45:335–351
Hou Q, An X, Ma B, Wu S, Wei X, Yan T, Zhou Y, Zhu T, Xie K, Zhang D, Li Z, Zhao L, Niu C, Long Y, Liu Ch, Zhao W, Ni F, Li J, Fu D, Yang Z-N, Wan X (2023) ZmMS1/ZmLBD30-orchestrated transcriptional regulatory networks precisely control pollen exine development. Mol Plant 16:1321–1338. https://doi.org/10.1016/j.molp.2023.07.010
Ingber D (1993) Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci 104:613–627
Ingber DE (2003) Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116:1157–1173
Kauffman SA (1993) The origin of order. Oxford University Press, New York, Oxford
Kurakin A (2005) Self-organization versus watchmaker: stochastic dynamics of cellular organization. Biol Chem 38:247–254. https://doi.org/10.1515/BC.2005.030
Lavrentovich MO, Horsley EM, Radja A, Sweeney AM, Kamien RD (2016) First-order patterning transitions on a sphere as a route to cell morphology. Proc Natl Acad Sci USA 113:5189–5194. https://doi.org/10.1073/pnas.1600296113
Li FS, Phyo P, Jacobowitz J, Hong M, Weng J-K (2019) The molecular structure of plant sporopollenin. Nat Plants 5:41–46. https://doi.org/10.1038/s41477-018-0330-7
Lintilhac PM (2014) The problem of morphogenesis: unscripted biophysical control systems in plants. Protoplasma 251:25–36. https://doi.org/10.1007/s00709-013-0522-y
Liu L, Wang T (2021) Male gametophyte development in flowering plants: a story of quarantine and sacrifice. J Plant Physiol 258–259:153365. https://doi.org/10.1016/j.jplph.2021.153365
Mandelbrot BB (1982) The fractal geometry of nature. WH Freeman and Co., San Francisco
Meyen SV (1984) Basic features of gymnosperm systematics and phylogeny as evidenced by the fossil record. Bot Rev 50:1. https://doi.org/10.1007/BF02874305
Mims CW (1972) Spore-wall formation in the myxomycete Arcyria cinerea. Trans Br Mycol Soc 59(3):477–481
Moore SEM, Gabarayeva N, Hemsley AR (2009) Morphological, developmental and ultrastructural comparison of Osmunda regalis L. spores with spore mimics. Rev Paleobot Palynol 156:177–184
Nadot S, Forchioni A, Penet L, Sannier J, Ressayre A (2006) Links between early pollen development and aperture pattern in monocots. Protoplasma 228:55–64. https://doi.org/10.1007/s00709-006-0164-4
Newman SA, Forgacs G (2009) Biological development and evolution, complexity and self-organization. In: Meyers R (ed) Encyclopedia of Complexity and Systems Science. Springer, NY, pp 524–548. https://doi.org/10.1007/978-0-387-30440-3_35
Newman SA, Comper WD (1990) ‘Generic’ physical mechanisms of morphogenesis and pattern formation. Development 110:1–18. https://doi.org/10.1242/dev.110.1.1
Nowicke JW, Skvarla JJ (1977) Pollen morphology and the relationship of the Plumbaginaceae, Polygonaceae, and Prirnulaceae to the order Centrospermae. Smithsonian Contr Bot 37:1–64
Odabaşi NŞ (2021) Pollen morphology of two Lysimachia L. (Primulaceae) species from Işıklar (Ganos) mountain of Tekirdağ. Eurasian J Forest Sci 9(3):143–150
Pettitt JM (1979) Ultrastructure and cytochemistry of spore wall morphogenesis. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London UK, NY, San Francisco USA, pp 211–252
Pettitt JM, Jermy AC (1974) The surface coats on spores. Biol J Linn Soc 6:245–257
Polevova SV, Grigorjeva VV, Gabarayeva NI (2023) Pollen wall and tapetal development in Cymbalaria muralis: the role of physical processes, evidenced by in vitro modelling. Protoplasma 260:281–298. https://doi.org/10.1007/s00709-022-01777-8
Pozhidaev AE (1993) Polymorphism of pollen in the genus Acer (Aceraceae). Isomorphism of deviant forms of Angiosperm pollen. Grana 32:79–85. https://doi.org/10.1080/00173139309429457
Pozhidaev AE (1995) Pollen morphology of the genus Aesculus (Hippocastanaceae). Patterns in the variety of morphological characteristics. Grana 34:10–20. https://doi.org/10.1080/00173139509429028
Pozhidaev AE (1998) Hypothetical way of pollen aperture patterning. 1. Formation of 3-colpate patterns and endoaperture geometry. Rev Palaeob Palynol 104:67–83
Pozhidaev AE (2000) Hypothetical way of pollen aperture patterning. 2. Formation of polycolpate patterns and pseudoaperture geometry. Rev Palaeobot Palynol 109:235–254. https://doi.org/10.1016/s0034-6667(99)00057-3
Pozhidaev A (2002) Hypothetical way of pollen aperture patterning. 3. A family-based study of Krameriaceae. Rev Palaeobot Palynol 127:1–23
Pozhidaev AE, Petrova NV (2023) Structure of variability of palynomorphological features within and beyond the genus Galeopsis L. Hjl. (Lamiaceae) in the context of divergent morphological evolution. Biol Bull Rev 13:63–80. https://doi.org/10.1134/S2079086423010061
Quilichini TD, Grienenberger E, Douglas CJ (2015) The biosynthesis, composition and assembly of the outer pollen wall: a tough case to crack. Phytochem 113:170–182. https://doi.org/10.1016/j.phytochem.2014.05.002
Radja A, Horsley EM, Lavrentovich MO, Sweeney AM (2019) Pollen patterns form from modulated phases. Cell 176:856–868. https://doi.org/10.1016/j.cell.2019.01.014
Rowley JR (1973) Formation of pollen exine bacules and microchannels on a glycocalyx. Grana 13:129–138
Rowley JR (1990) The fundamental structure of the pollen exine. Plant Syst Evol Suppl 5:13–29
Rowley JR, Dahl AO (1977) Pollen development in Artemisia vulgaris with special reference to glycocalyx material. Pollen Spores 19:169–284
Rowley JR, Prijanto B (1977) Selective destruction of the exine of pollen grains. Geophytology 7(1):1–23
Scott RJ (1994) Pollen exine – the sporopollenin enigma and the physics of pattern. In: Scott RJ, Stead MA (eds) Molecular and cellular aspects of plant reproduction. Society for Experimental Biology Seminar Series 55. Cambridge Univ. Press, Cambridge, pp 49–81
Shi J, Cui M, Yang L, Lim YJ, Zhang D (2015) Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci 20:741–753. https://doi.org/10.1016/j.tplants.2015.07.010
Stillman NR, Mayor R (2023) Generative models of morphogenesis in developmental biology. Semin Cell Dev Biol 147:83–90
Suh D-Y, Sraan DK, Ashton NW (2024) Takakia possesses a key marker of embryophyte sporopollenin. https://doi.org/10.1101/2024.01.17.576121
Taylor ML, Osborn JM (2006) Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Am J Bot 93:344–356
Taylor ML, Hudson PJ, Rigg JM, Strandquist JN, Green JS, Thiemann TC, Osborn JM (2013) Pollen ontogeny in Victoria (Nymphaeales). Int J Plant Sci 174:1259–1276. https://doi.org/10.1086/673246
Taylor ML, Cooper RL, Schneider EL, Osborn JM (2015) Pollen structure and development in Nymphaeales: insights into character evolution in an ancient angiosperm lineage. Am J Bot 102:1–18. https://doi.org/10.3732/ajb.1500249
Taylor ML, Altrichter KM, Aeilts LB (2018) Pollen ontogeny in Ruppia (Alismatidae). Int J Plant Sci 179:217–230. https://doi.org/10.1086/696535
Thompson DAW (1917) On growth and form. Cambridge University Press, Cambridge
van Bergen PF, Blokker P, Collinson ME, Sinninghe Damsté JS, de Leeuw JW (2004) Structural biomacromolecules in plants: what can be learnt from the fossil record? In: Hemsley AR, Poole I (eds) The evolution of plant physiology. Academic Press, Amsterdam, pp 134–154
Wang R, Dobritsa A (2018) Exine and aperture patterns on the pollen surface: their formation and roles in plant reproduction. Ann Plant Rev 1:1–40. https://doi.org/10.1002/9781119312994.apr0625
Wang Y, Lin YC, So J, Du Y, Lo C (2013) Conserved metabolic steps for sporopollenin precursor formation in tobacco and rice. Physiol Plant 149:13–24. https://doi.org/10.1111/ppl.12018
Wang R, Owen HA, Dobritsa A (2021) Dynamic changes in primexine during the tetrad stage of pollen development. Plant Physiol Kiab 426:1–12. https://doi.org/10.1093/plphys/kiab426
Wiermann R, Gubatz S (1992) Pollen wall and sporopollenin. Int Rev Cytol 140:35–72
Wilmesmeier S, Wiermann R (1997) Immunocytochemical localization of phenolic compounds in pollen walls using antibodies against p-coumaric acid coupled to bovine serum albumin. Protoplasma 197:148–159
Wodehouse RP (1935) Pollen grains: their structure, dentification and significance in science and medicine. McGraw-Hill Co., New York
Wrońska-Pilarek D, Morozowska M (2009) Morphological differentiation of pollen of LYSIMACHIA VULGARIS L from Poland. Botanika - Steciana 13:181–190
Xiong S-X, Zeng Q-Y, Hou J-Q, Hou L-L, Zhu J, Yang M, Yang Z-N, Lou Y (2020) The temporal regulation of TEK contributes to pollen wall exine patterning. PLoS Genet 16(5):e1008807. https://doi.org/10.1371/journal.pgen.1008807
Xu M, Yan X, Wang Y, Liu Ch, Yang Q, Tian D, Bednarek SY, Pan J, Wang J (2022) ADAPTOR PROTEIN-1 complex-mediated post-Golgi trafficking is critical for pollen wall development in Arabidopsis. New Phytol 235:472–487. https://doi.org/10.1111/nph.18170
Yang TYA, Grabovskaya A, Illarionova I, Chen C-H (2012) Lysimachia candida Lindl (Primulaceae), an extinct species and also a new record species to Taiwan. Taiwania 57(4):434–442
Zhou Y, Dobritsa AA (2023) Putting the brakes on pollen wall development: a conserved negative feedback loop regulates pollen exine formaton in flowering plants. Mol Plant 17:1376–1378
Zini LM, Galati BG, Zarlavsky G, Ferrucci MS (2017) Developmental and ultrastructural characters of the pollen grains and tapetum in species of Nymphaea subgenus Hydrocallis. Protoplasma 254:1777–1790. https://doi.org/10.1007/s00709-016-1074-8
Acknowledgements
This work was carried out in the framework of the Institutional Research Project of the Komarov Botanical Institute of the Russian Academy of Sciences “Structure-functional and molecular-genetic foundations of development and adaptation of higher plants” No. 124020100138-4 on the equipment of the Core Facilities Centre ‘Cell and Molecular Technologies in Plant Science’ at the Komarov Botanical Institute of RAS (Saint Petersburg).
Funding
Institutional research project of the Komarov Botanical Institute of the Russian Academy of Sciences. No other funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Valentina V. Grigorjeva: collecting and fixation of Lysimachia vulgaris material and preparing of ultrathin sections; fixations and embedding of samples and preparation of ultrathin sections, staining sections; Dmitri A. Britski: taking a part of TEM pictures; Nina I. Gabarayeva: taking a part of TEM pictures, the principal conception, design and analysis of results, writing and submitting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Handling Editor: Alexander Schulz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gabarayeva, N.I., Grigorjeva, V.V. & Britski, D.A. Mechanisms of pollen wall development in Lysimachia vulgaris. Protoplasma (2024). https://doi.org/10.1007/s00709-024-01970-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00709-024-01970-x