Abstract
Lophopterys floribunda is a Neotropical species of Malpighiaceae endemic to Brazil, occurring in both the Amazon and Atlantic Forest. Instead of the typical bi-glandular sepals reported for Neotropical Malpighiaceae, this species presents a single, large gland on the lateral sepals. In addition, ant patrolling was observed at the apex of bracts and bracteoles during fieldwork. Thus, this work aimed to describe the sepalar gland of L. floribunda and other secretory structures in its flowers and inflorescence. Samples of bracts, bracteoles, sepals, petals, and anther were collected and submitted to usual anatomical techniques. Unexpected nectaries at the apex of bracts and bracteoles, not visible to the naked eye, were described and represent a new type of structure for the family due to both their position and size. Mutualistic ants consume the exudate produced by these tiny nectaries, and such structures enable a specific visitation pattern for Lophopterys. Typical epithelial elaiophores occur on the lateral sepals, formed by an invaginated epidermis, which predominantly produce lipid secretion. The petal marginal glands are anatomically similar to the standard type of colleter, which exude mucilaginous substances. The exudate produced by the petal marginal glands was considered to have an additional role of contributing to the maintenance of the closed bud during the beginning of development. The globose epidermal cells containing lipids, proteins, and polysaccharides observed in the connective may be responsible for the typical aroma emitted by these flowers. The diversity of secretory structures reported here has application in both systematic and ecological studies of Malpighiaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lophopterys floribunda is an endemic species of Brazil with a large, single gland on its lateral sepals, distinguishing it from other Neotropical species of Malpighiaceae, which have well-preserved floral morphology with a pair of sepal glands (Anderson and Davis 2001). Sepal glands of Neotropical Malpighiaceae are described as elaiophores, which are glands that produce non-volatile oils that are predominantly lipophilic or mixed secretion (Vogel 1990; Araújo et al. 2010; Guesdon 2017; Possobom and Machado 2017). The secretion produced by elaiophores is assumed to be attractive to specialized oil-collecting bees that act as pollinators (Renner and Schaefer 2010; Mello et al. 2013; Davis et al. 2014). Therefore, in this work, we evaluate the functional role of the exceptional sepal gland and other floral glands heretofore unexplored for L. floribunda.
The presence of elaiophores and zygomorphic flowers has been emphasized as a striking feature of Neotropical Malpighiaceae (Anderson 2004; Davis and Anderson 2010). Such conservation of floral morphology has been related to the oil-collecting bees that are endemic to the Neotropics (Anderson 1979; Vogel 1990; Davis et al. 2014). Most species of Neotropical Malpighiaceae have pairs of elaiophores that occur on five or four sepals (Vogel 1990; Davis and Anderson 2010). Due to the absence of oil-collecting bee species in the Paleotropics, Malpighiaceae species (≈ 150 species) there exhibit diverse floral morphologies, generally lacking floral zygomorphy with the absence, or reduced number and size, of sepal glands (Davis and Anderson 2010; Davis et al. 2014; Guesdon et al. 2019). It is interesting to note that, even when present, the sepal glands of Paleotropical species, such as those of Acridocarpus and Hiptage, act as nectaries, since they secrete nectar and not oils (Anderson 1979; Vogel 1990; Guesdon et al. 2019; Ren et al. 2013).
In addition to elaiophores, osmophores and nectaries have also been reported for flowers and inflorescences within Malpighiaceae (Anderson 1990; Possobom et al. 2015; Guesdon 2017; Guesdon et al. 2019; Possobom and Machado 2017, 2018). Clarification of the types of floral secretory structures contributes to the understanding of ecological relationships and supports taxonomic and evolutionary studies. Several insects have already been reported as visitors to Malpighiaceae flowers, such as pollen-collecting bees, ants, and beetles, whose behavior is not directly related to pollination (Alves-Silva et al. 2014; Barônio et al. 2017). In general, floral visitors of Malpighiaceae seek a reward, which can be oil-resin, nectar, or even mucilage (Pearse 1980; Subramanian et al. 1990; Possobom et al. 2015; Araújo and Meira 2016; Nery et al. 2017; Guesdon 2017; Anderson and Anderson 2018; Matos and Araújo 2021). The unusual behavior of an ant patrol route was observed in the inflorescence of L. floribunda during fieldwork and suggests the presence of secretory structures not yet registered for the species.
Anatomical and histochemical analyses are essential to define the chemical characteristics of secretion and ensure the correct characterization of glands, especially in floral secretory structures of Malpighiaceae (Possobom et al. 2015; Guesdon 2017; Possobom and Machado 2017, 2018). Therefore, this study aimed to anatomically describe in detail the floral secretory structures of Lophopterys floribunda, focusing on: (a) the description of morphological floral structures and patrolling visitors; (b) morpho-functional characterization of the sepal gland; (c) investigation of the existence of floral secretory structures not yet reported for the species and describing them; and (d) identifying the secretory structures responsible for exudates that attract visitors to the inflorescence and flowers.
Material and methods
Field observations and sample collection
Lophopterys floribunda WR Anderson & CC Davis (Malpighiaceae) has a disjunct distribution, occurring in the Amazon Forest, dryland forests, and the Atlantic Forest of the states of Bahia, Espírito Santo, Minas Gerais, and Rio de Janeiro in Brazil (BFG 2015). Fieldwork was performed at the Reserva Particular do Patrimônio Natural Feliciano Miguel Abdala (RPPN-FMA), located between the cities of Caratinga and Ipanema (Minas Gerais, Brazil). Collections were carried where the highest occurrence of individuals of the species was observed (S 19° 44′ 42.1″ W 41° 49′ 15.4″). Voucher material was herborized and deposited in the herbarium of the Federal University of Viçosa, UFV (VIC), under numbers 53553–53,556. Representative samples of L. floribunda (Mota, C. D. A. da 66,954; Lima, E. S 115432) from distinct populations were also obtained from vouchers to assess anatomical variation in gland structure.
Field expeditions were performed during the flowering period (September/October 2017 and 2018). The pattern of inflorescence visitation by insects was observed on 4 days, in the morning and afternoon from 6 am to 5 pm. Visitor behavior was described based on field observations of control branches from a subsample of five L. floribunda plants. Visitor activities were recorded as images and videos using a Nikon D7000 photographic camera (Japan). Collected visitors were identified and incorporated into the Myrmecological Collection of the Community Ecology Laboratory (LABEL) of the Entomology Department at UFV.
Sample processing for light microscopy and histochemistry
Inflorescence type was classified according to Anderson (1981). Collected material was analyzed and photographed under a stereomicroscope (Stemi 2000-C Zeiss, Gottingen, Germany) equipped with a digital camera (AxioCam ERc; Zeiss, Gottingen, Germany). Anatomical and histochemical characterizations of secretory structures used flower buds and flowers collected at different stages of development from three specimens. The collected material was fixed in neutral buffered formalin (NBF) (Johansen 1940) or ferrous sulfate in neutral formalin (FSF), for the detection of phenolic compounds (Johansen 1940), for 48 h and stored in 70% ethanol. Some of the samples were kept in NBF fixative for histochemical tests. The presence of glucose in sepal gland exudate was tested by urinalysis tape (DFI CO Ltd. Republic of Korea).
Based on the visitation pattern of insects and observations made in the field and laboratory, fixed samples were dissected, and the fragments dehydrated in an ethanolic series and included in methacrylate (Historesina Leica Microsystems Nussloch GmbH, Heidelberg, Germany) for anatomical characterization. Transverse and longitudinal 5-µm-thick sections of these samples were obtained with a rotary microtome with automatic advance (RM2155, Leica Microsystems Inc., Deerfield, USA) and stained with toluidine blue at pH 4.7 (O’Brien et al. 1964 modified). Permanent slides were mounted on Permount synthetic resin (Permount, Fisher Scientific, New Jersey, USA).
The secretory structures found on bracteoles, sepals, petals, and connective of anthers were subjected to histochemical tests. Sections were obtained by freehand cuts or using a rotary microtome. The following tests were performed: xylidine ponceau (Clark 1981) and Coomassie blue (Fisher 1968) for total proteins; periodic acid–Schiff reagent (PAS) for total polysaccharides (McManus 1948); ruthenium red for either mucilage or pectin (Johansen 1940); Lugol reagent for starch (Johansen 1940); Fehling’s reagent for reducing sugars (Purvis et al. 1964); Sudan black B and/or Sudan red for total lipids (Pearse 1980), NADI reagent for oil/oil-resin (David and Carde 1964); metachromasia in Toluidine blue (O’Brien et al. 1964); and ferrous sulfate in neutral formalin—FSF (Johansen 1940)—for structural and non-structural phenolic compounds. Observations and photographic documentation were performed using a light microscope (AX70TRF, Olympus Optical, Japan) equipped with an image capture system (Ax Cam, Zeiss, Germany).
Results
Morphological description and patrolling visitors in floral structures of Lophopterys floribunda
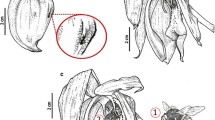
The inflorescence of Lophopterys floribunda has a brown to dark brown peduncle and is composed of elongated axillary thyrsi of thyrsi (Fig. 1A). Each thyrsi contains approximately 50 single flowers with acropetal maturity, occurring from the base to the apex (Fig. 1B). The calyx is brownish in color (Fig. 1C–D), due to the dense coverage of malpighiaceous trichomes and has an eglandular anterior sepal and a large-yellow gland that occupies most of the sepal (Fig. 1E). The activity of these glands is precocious, with secretion accumulated in protuberances present on the surface of flowers, without overflow, in pre-anthesis (Fig. 1E). The sepal glands show a change of color and surface conformation according to phenophase. From bud to open flower, the glands are yellow, and the surface is straight to slightly convex (Fig. 1E–F) with small projections dilated fully with secretion (Fig. 1E). After pollination, the glands acquire a green color and a concave surface, showing whitish traces due to scaling of the cuticle by oil-collecting bees (Fig. 1G), while the convex surface with the secretion of the previous phase is no longer evident (Fig. 1G).
Floral morphology and visitors of Lophopterys floribunda. A Inflorescence of thyrsi of thyrsi type. Note the dark brown color of the stem. B Thyrsi with buds, flowers, and fruits, showing the acropetal maturity of the flowers. Note a bee of the genus Trigona visiting the open flower (square). C–D Ants visit under entire inflorescence in exploratory and random behavior. The red arrows indicate the route of the ants, from the most proximal to the most distal part of the inflorescence. E Floral bud showing the yellow sepal gland with a bulge due to the accumulation of secretion (white arrow). F Open flower showing the anterior eglandular sepal (ES) and the large gland occupying more than half of the sepal (SG). G Post-anthesis phase with the beginning of fruit formation. Note the green color of the sepal gland with whitish streaks due to the scaling of the cuticle (white arrow). H Open flower showing the zygomorphic symmetry. Note the reduced posterior petal (PP) with glandular fimbriae, a pair of latero-posterior petals (LP), and another pair of latero-anterior petals (LA). I Floral bud showing red coloration resulting from a positive reaction for lipids on the sepal gland; note the small projections fully dilated of secretion (white arrow). J–K Positive reaction for lipids in the sepal gland and connective gland (arrows). L Mark of the buccal apparatus of oil collecting bees on the posterior petal (circle). M Stamen with yellowish gland present in the connective, the arrow indicates the anther gland. N Pair of bracteoles at the base of the pedicel and bract at the base of the peduncle, inserted between the bracteoles; the tinny glands are indicated by white arrows. O Ant feeding on the secretion produced by the bracteole. Bars: A–D: 30 mm; E, G: 3 mm; F, H–J, N–O: 2 mm; K–M: 1 mm
The petals are always yellow, with the two cucullate latero-anterior petals, two flat latero-posterior petals, and a reduced posterior petal with glandular fimbriae only on the margins of the basal portion of the limb, near the claw, conferring bilateral symmetry to the flower (Fig. 1H). The outermost petal is slightly more concave than the remaining petals, which are concave to flat (Fig. 1H). In this arrangement, the outermost petal covers the other petals during the bud phase. Dipping pre-anthesis and open flowers in the histochemical reagents Sudan red and Sudan black revealed strong staining of the sepal gland (Fig. 1I–K) and the apparently secretory portion of the connective in the open flower (Fig. 1J–K), showing the presence of lipid secretion.
Bees of the tribe Centridini were observed visiting the flowers. Marks on the posterior petal (Fig. 1L) evidence the visitation of these bees, which are attached in this region by their oral apparatus at the time of oil collection. Bees of the genus Trigona were also observed visiting the flowers and collecting pollen (Fig. 1B). The aroma perceived in the field can be directly related to the attraction of bees. The connective tissue of the anther possesses a small yellowish and dilated region with a secretory aspect (Fig. 1M).
At the base of the pedicel of each single-flowered cincinnus is a pair of bracteoles in opposite position and a small bract at the base of a short peduncle that is inserted between the bracteoles (Fig. 1N). Bracteoles and bracts are persistent, triangular and with numerous malpighiaceous trichomes, which confer a brownish color; glands visible to the naked eye were not observed (Fig. 1N–O). Many ants of the genera Azteca, Camponotus, Cephalotes, and Crematogaster were observed in intense activity of visitation to entire inflorescences (supplementary document), from the most proximal to the most distal part but especially in apices of bracteoles (Fig. 1O), throughout the investigated period (Fig. 1C–D). The feeding behavior of the ants was exploratory and random, indicating possible secretory structures in the regions, which were investigated by anatomical and histochemical analyses. The main change of development in the sepalar glands was morphological. We did not notice differences between all other floral glands according to phenophase.
Anatomical and histochemical descriptions of the sepal glands of Lophopterys floribunda
Sessile glands are present on all but the anterior sepal, which is eglandular (Fig. 2A). The median region is slightly concave in cross-sectional view (Fig. 2A) and no signs of fusion were observed. The secretory epidermis of the sepal gland is composed of columnar cells, with dense cytoplasm and a prominent central nucleus, organized in a single palisade layer (Fig. 2B–D). The cuticle is thick and the epidermis forms countless invaginations that make the gland surface irregular (Fig. 2B–C). Below the epidermis, the secretory parenchyma consists of isodiametric cells of the underlying region where idioblasts accumulate. Both regions exhibit strongly greenish-blue stained contents using toluidine blue (Fig. 2C), indicating the presence of phenolic compounds (Fig. 2E, F), as confirmed by FSF (ferrous sulfate in neutral formalin). Xylem vascularizes the gland in the subepidermal region (Fig. 2D). The adjacent parenchymal cells are larger than the cells in the subepidermal region, and phenolic compounds (Fig. 2E–F), druses (Fig. 2G–H), and starch (Fig. 2G) were found in randomly scattered idioblasts.
Anatomical description of sepal glands in Lophopterys floribunda. A Cross-section of the calyx stained with toluidine blue, showing the position of the sessile sepal glands. Note the eglandular anterior sepal (ES). B Sessile sepal gland in the longitudinal section of the sepal stained with toluidine blue. C Detail of the invaginations of the secretory epidermis and thin cuticle. Note the greenish-blue strongly stained region of the subepidermal and underlying parenchyma. D Section stained with toluidine blue showing the epidermal secretory gland with columnar cells, dense cytoplasm, and a prominent central nucleus. Note the vascularization of the underlying tissue by the xylem (xy). E–F Positive reaction for phenols with FSF (ferrous sulfate in neutral formalin) in the parenchymatic tissue of the gland. G Reaction with toluidine blue against Lugol reagent staining. Note the starch grains near the vascular cylinder of the floral bud (black arrow), the phenolic compounds in greenish-blue, and the druse (white arrow). H Druses under polarized light present in the underlying tissue of the gland. I–J Positive reaction for PAS, cuticular extract strongly marked by magenta color, note secretion accumulated in the subcuticular space (arrows) and the separation of the apical portion of the cells of the secretory epidermis. K Positive reaction for proteins with Coomassie blue note the blue secretion accumulated (arrow). L Positive reaction for Sudan red for detection of lipids, note the red secretion accumulated (arrow). M–N Positive reaction for the NADI reagent. Note the blue/violet secretion in N (arrow). Bars: A: 700 μm; B: 300 μm; C: 100 μm; D–E, K–L, N: 50 μm; F: 400 μm; G: 30 μm; H: 200 μm; I: 100 μm; J: 30 μm; M: 20 μm
Secretion accumulates from bud to anthesis in large subcuticular spaces of the secretory epidermis (Fig. 2I–M), as well as in the cytoplasm (Fig. 2I–N). This secretion reacted positively for polysaccharides (Fig. 2I–J), proteins (Fig. 2K), lipids (Fig. 1I–K; 2L), and oil-resin (Fig. 2M–N). The thick cuticle was also strongly stained by Sudan (Fig. 2L). Cuticle detachment reveals a separation of the apical portion of the cells of the secretory epidermis (Fig. 2J–M). No glucose were detected in the exudate of the sepal gland for urinalysis tape tests.
Description of floral secretory structures with new records for Lophopterys floribunda
Bracts and bracteoles are covered by a dense tomentose indument consisting of malpighiaceous trichomes, except at the apex (Fig. 3A). In the field, ants were found constantly visiting these apices, which support a complete patrol of the entire inflorescence. For this reason, the apex of bracts and bracteoles was evaluated in detail. Unexpected glands, imperceptible to the naked eye, were unveiled at the apex, which are formed by columnar secretory epidermal cells (Fig. 3B–C). The secretory cells have thin walls and dense cytoplasm and cover a short non-secretory parenchymal axis that is vascularized by phloem (Fig. 3C). The cuticle is thick and secretion accumulation is observed on both wall and cuticle (Fig. 3C). The secretion accumulated reacting to the histochemical test for polysaccharides (Fig. 3D) and protein (Fig. 3E). Starch (Fig. 3F–G) and reducing sugars (glucose and fructose) (Fig. 3H–I) were detected in the cells of bracts and bracteoles.
Anatomical description of the glands on bracteoles, petals, and connective in Lophopterys floribunda, visualized in longitudinal section of glands. A–C, J–L, P–Q were stained with toluidine blue. A Pedicel showing the glands located at the apex of each bracteole (arrows). B Bracteole apex gland with short parenchymal axis and adaxial face covered by trichomes. C Bracteole gland with thick cuticle, epidermis with columnar cells, and subepidermal cells vascularized by the phloem (pl). D Positive reaction to PAS for polysaccharides, note the magenta color of the secretion (arrows). E Bracteole gland showing the positive reaction to Coomassie blue for protein evidenced by blue color (arrows). F–G Positive reaction to Lugol for starch blackened stained. H–I Positive reaction to Fehling test for reducing sugar, note the dark content inside cells. J Floral bud showing the latero-anterior petals (LA), the latero-posterior petals (LP), and the posterior petal (PP) with the petal gland (PG) on the margin (arrow). K–L Marginal petal gland. K The gland consists of a parenchyma axis vascularized by xylem (xy) and a palisade secretory epidermis. Note the subcuticular space with secretion (*) and short peduncle (Pe). L Detail of secretory epidermal cells with dense cytoplasm, large nucleus, and intercellular spaces with secretion (*). Note the phenolic idioblasts (PI). M Positive reaction to PAS in secretory epidermis showing polysaccharides stained in magenta color. N Positive reaction for mucilage stained in light red with Ruthenium red. O Positive reaction for protein stained light red with xylidine ponceau. P Stamen showing the gland (arrow) location in the connective. Q Cross-section of the connective with juxtaposed cells of the gland containing numerous vesicles. Observe endothecium (en), phenolic idioblasts (PI), and druses (arrow). R Positive reaction to xylidine ponceau in connective cells above of the endothecium (en). Note the evident nucleus in a central position. S Positive reaction to PAS for polysaccharides. Note the evident magenta secretion in the vesicles (arrows) inside the connective cells, which are placed above the endothecium (en). T Positive reaction to Sudan black for lipids, note small black dots (arrows) in the connective gland. U Positive reaction to NADI for volatile oils. Note the blue/violet secretion (arrows). Bars: A: 400 μm; B: 200 μm; C–E: 30 μm; F, K: 50 μm; G, H, I: 30 μm; I: 100 μm; J: 6 mm; L: 300 μm; M, O: 50 μm; N: 30 μm; P: 300 μm; Q–T, U: 20 μm
The posterior petal is more internally positioned during the bud phase, which is followed by the latero-posterior petals and finally by the latero-anterior petals (Fig. 3J). Only the posterior petal has marginal glands at the apex of fimbriae (Fig. 3J). This structure is claviform, short pedunculated, and composed of a palisade-like secreting epidermis that covers a central axis vascularized by xylem (Fig. 3K). The cuticle is thin and is detached by the accumulation of secretion in subcuticular spaces (Fig. 3K–L). The secretory epidermal cells are elongated, have dense cytoplasm, a large nucleus, and thin walls (Fig. 3K–L). Intercellular spaces containing secretion were also observed (Fig. 3K–L). The parenchyma underlying the secretory epidermis is composed of isodiametric cells, mostly containing phenolic compounds (Fig. 3L). The secretory epidermis reacted positively for polysaccharides (Fig. 3M), mucilage (Fig. 3N), and proteins (Fig. 3O).
The anther connective gland was strongly stained with both red and black Sudan during fieldwork (Fig. 1J–K). Stereomicroscopy revealed the Sudan-stained region to be dilated (Fig. 1M), which is placed at the portion of the connective in the open flower (Fig. 3P). The connective cells are large, compressed, and bear thin walls, densely stained cytoplasm with numerous vesicles, and a large central nucleus (Fig. 3Q). The endothecium is formed by voluminous cells with wall thickenings that show a transversal bar pattern (Fig. 3Q). Idioblasts containing both phenolics and druses are common in the region underlying the endothecium (Fig. 3Q). Histochemical tests showed the presence of proteins (Fig. 3R), polysaccharides (Fig. 3S), lipids (Fig. 1J–K; 3T), and volatile oils (Fig. 3U) in the cytoplasm and inside the vesicles.
Discussion
The sepal glands of Lophopterys floribunda are elaiophores
The positive histochemical results for lipids, oil-resins, proteins, and polysaccharides in the secretion produced by the sepal glands of L. floribunda (Table 1) allow us to characterize them as elaiophores. Similar results have been reported for species of Banisteriopsis, Burdachia, Byrsonima, Diplopterys, Glandonia, Mcvaughia, Stigmaphyllon, and Peixotoa (Possobom et al. 2015; Araújo and Meira 2016; Possobom and Machado 2017; Guesdon 2017; Guesdon et al. 2018; Almeida et al. 2019; Matos and Araújo 2021). Using the NADI reagent, the present work demonstrated the oil-resinous nature of the secretion of L. floribunda. Similar results were reported in species of the Mcvaughioide clade (Guesdon 2017; Almeida et al. 2019). The parenchyma adjacent to the secretory epidermis was strongly stained for phenolic compounds and possessed many druses. This may indicate a protection mechanism against herbivory, as the relationship with visiting ants has been studied in Malpighiaceae before (Fernandes et al. 2005; Alves-Silva et al. 2014).
There is structural diversity of elaiophores within Malpighiaceae, and they may be an adaptation to improve interactions with pollinators (Aliscioni et al. 2022). Although the sepal gland of L. floribunda has a reduced number of glands per calyx and a different external morphology, especially in size when compared to other groups of Malpighiaceae (Anderson and Davis 2001), it does not differ anatomically from the elaiophores described for some species of the family. These glands correspond to the epithelial type, as they are made up of a layer of palisade secretory epidermal cells, with a generally thick cuticle, below of which parenchyma is present. Vascular bundles emerge from the sepal and gradually fuse until reaching the secretory epidermis of the elaiophores. This anatomical structure is common to elaiophores and has been reported for many species of Malpighiaceae (Possobom et al. 2015; Araújo and Meira 2016; Guesdon 2017; Possobom and Machado 2017, 2018).
The elaiophore of L. floribunda is sessile, as in Burdachia spp. (Guesdon 2017), but differs from those of Banisteriopsis spp. (Araújo and Meira 2016; Possobom and Machado 2017), Diplopterys spp. (Possobom et al. 2015), Glandonia spp., and Mcvaughia spp. (Almeida et al. 2019; Guesdon et al. 2018), which are subsessile. Although having this distinction, apparently no differential functional role is evident, being only part of the diversity of this trait, which acquires taxonomical importance, as recorded for the Mcvaughioide clade (Guesdon 2017; Guesdon et al. 2018; Almeida et al. 2019). The irregular secretory epidermis with invaginations observed in the elaiophores of L. floribunda has also been registered in several Neotropical species, such as those of Banisteriopsis, and Paleotropical species, such as those of Hiptage (Araújo and Meira 2016; Possobom and Machado 2017; Subramanian et al. 1990). On the other hand, these invaginations are absent from the Mcvaughioide clade (Almeida et al. 2019), which may reflect some phylogenetic relationship or differentiated secretion dynamics for this group.
Nectar, pollen, oil-resins, and flower essences are examples of flower resources that may be used by animals that repeatedly visit flowers and inflorescences, leading to pollination (Simpson and Neff 1981). Therefore, oil production combined with bilateral symmetry in L. floribunda reinforces the existing correlation between Neotropical species of Malpighiaceae and their effective pollinators—the oil-collecting bees (Renner and Schaefer 2010; Mello et al. 2013; Barônio et al 2017). Bees of the tribe Centridini are endemic to the Neotropics and are regular consumers of exudate sepal glands of species of Malpighiaceae (Simpson and Neff 1981; Vogel 1990). As observed for Lophopterys, these bees are recurrent visitors to species of Banisteriopsis, Byrsonima, Heteropterys, and Peixotoa (Barônio and Torezan-Silingardi 2017).
As previously explored, most Neotropical Malpighiaceae have elaiophores (Possobom et al. 2015; Araújo and Meira 2016; Guesdon 2017; Guesdon et al. 2018; Almeida et al. 2019; Possobom and Machado 2017), as for L. floribunda of the present study. However, the sepal glands of species of the Paleotropical Acridocarpus were anatomically described as nectaries (Guesdon et al. 2019). A floral trend of the lack or reduction of sepal glands is noticed for Paleotropical species, which was interpreted as a response to the absence of selective pressure from oil collecting bees in the Paleotropics (Vogel 1990). The single sepal elaiophore of L. floribunda (numerical reduction) might have been compensated for by its larger size, since it occupies more than 50% of the sepal surface. Although no morphoanatomical evidence of fusion has been observed, the single large sepalar gland suggests a fusion event of two small glands, as observed in Acridocarpus (Guesdon et al. 2018). This characteristic was also reported in taxonomic works on other species of Lophopterys (Anderson and Davis 2001) and for species of Jubelina and Mezia, two Amazonian climbing genera (Anderson 1990; Anderson and Anderson 2018). In this case, it seems that the reduction in the number, the increase in the size, and the yellow color of the sepalar gland might be a strategy to maximize oil resource performance, making the gland more visible and allowing more secretion to be collectible by pollinating bees. In addition, the presence of only one gland in each sepal of L. floribunda, the climbing species, can still represent a trait derived from the ancestral condition of two glands per sepal in climbing Neotropical Malpighiaceae.
The tiny glands of the apex of bracts and bracteoles in Lophopterys floribunda are nectaries
The presence of tiny glands at the apex of bracts and bracteoles of L. floribunda is registered here for the first time, which correspond to nectaries. The presence of glands on the adaxial face of bracts and bracteoles is a characteristic of extreme importance in Malpighiaceae and has been extensively used to better delimitate genera, such as Acridocarpus, Amorimia, Bunchosia, Burdachia, Glandonia, and Mcvaughia (Guesdon 2017; Guesdon et al. 2018; 2019; Almeida et al. 2016, 2018, 2019). The presence of such glands was not mentioned in a taxonomic review of Lophopterys (Anderson and Davis 2001; Almeida et al. 2016) and was probably overlooked given that detailed anatomical study is necessary to best detect them and provide additional taxonomic information and insight into the phylogenetic evolution of features.
The glands of the apex of the bracts and bracteoles of L. floribunda are comprised of a short peduncle that is vascularized by phloem and covered by secretory epidermis with elongated columnar cells, which exude polysaccharides, protein, and reducing sugars secretion. Such characteristics allow these structures to be classified as nectaries and are in accordance with other records of extrafloral nectaries (EFNs) in Malpighiaceae (Araújo et al. 2010; Possobom et al. 2010; Nery et al. 2017; Guesdon 2017). Species of the Mcvaughioide clade also have bracteolar glands that are nectaries (Guesdon 2017). The nectaries of bracts and bracteoles of L. floribunda are anatomically like those of the Mcvaughioide clade (Guesdon 2017) and leaves of several species of Banisteriopsis (Araújo and Meira 2016) and other species present in the Brazilian Cerrado (Machado et al. 2008), being composed of a palisade secretory epidermis and vascularized nectary parenchyma. The abaxial bracteoles of Burdachia, Glandonia, and Mcvaughia bear one conspicuous centrally positioned nectary (Guesdon 2017), which differs from those of the bracteoles of Lophopterys, which are tiny in size (not being perceptible to the naked eye) and positioned on the apex of bracts and bracteoles; glands on bracts have not been recorded for the Mcvaughioide clade (Guesdon 2017).
According to location, the nectaries of bracts and bracteoles of L. floribunda can be classified as floral (Caspary 1848), even though they seem to bear no direct relationship with pollination. The observed visitation of ants to the nectaries of L. floribunda suggests a mutualistic protection interaction against herbivory of reproductive organs by insects, as reported for the Mcvaughioide clade (Guesdon 2017) and Byrsonima crassifolia (Fernandes et al. 2005). The presence of proteins might indicate a supply to the dietary needs of the ants, thus ensuring the successful interaction with nectaries (floral and extrafloral) reported in the family (Possobom et al. 2010; Araújo and Meira 2016; Guesdon 2017). Starch is considered a product of the storage stage that allows the release of nectar by the nectaries throughout the day (Pacini et al. 2003). Therefore, the starch detected in all tissues of the gland of the bracts and bracteoles of Lophopterys floribunda, as well as the observation in the field of an intense visitation activity of ants with feeding behavior (complementary document). Both the ant behavior and the presence of reducing sugar (glucose and fructose) are arguments for classifying this gland as a nectary. Tests for mucilage were negative for Lophopterys, similar to those observed for the bracteoles glands of Burdachia, Glandonia, and Mcvaughia (Guesdon 2017).
Ants of the genera Cephalotes, Crematogaster, and Camponotus were visitors of the inflorescences of L. floribunda and consumed nectar from bracts and bracteoles to be continuous until after senescence of the flowers in inflorescences. Ants were more evident in number and species diversity compared to bees. The behavior of the ants followed the same repeated pattern, starting at the base of the inflorescence, stooping to bracts and bracteoles, petals, and ending in the young shoots. This behavior is equivalent to what was reported for the Mcvaughioide clade, with ants stooping to feed on bracteolar nectaries and patrolling the entire inflorescence (Guesdon 2017). For Malpighiaceae, nectar consumption by leaf-visiting ants has been observed in species of Banisteriopsis, Burdachia, Byrsonima, Glandonia, Diplopterys, Mcvaughia, and Heteropterys (Araújo et al. 2010; Possobom et al. 2010; Guesdon 2017; Nery et al. 2017). Species of Camponotus were registered in leaf nectaries of D. pubipetala (Possobom et al. 2010) and inflorescences of species of Burdachia and Mcvaughia. Ants of the genus Crematogaster also visit species of Glandonia and Burdachia (Guesdon 2017). The presence of species of Cephalotes, Crematogaster, and Camponotus at leaf nectaries of Chamaecrista spp. (Leguminosae) demonstrated the strong relationship and importance of nectar produced by nectaries in increasing fruit generation (Baker-Méio and Marquis 2012). The registration of visitors, as evidenced in the present study, is a strong indication of the presence of secretory structures. The biological importance of nectaries of Malpighiaceae needs to be better understood.
Could be the petal glands Lophopterys floribunda classified as colleters?
The margin gland on the posterior petal of L. floribunda exhibits a typical morpho-anatomical structure of a standard colleter, as described by Lersten and Horner (1967). Although the same anatomical structure was recorded in other genera, such as Burdachia, Diplopterys, Glandonia, and Mcvaughia, these were recognized as osmophores (floral scent glands) (Possobom et al. 2015; Guesdon et al. 2018; Almeida et al. 2019). Yet, in taxa belonging to different lineages of Malpighiaceae, such as Burdachia, Diplopterys, and Mcvaughia, there is a similar structure to that of Lophopterys, but in the genus Glandonia the secretory epidermis occurs only on the upper surface of the petal (Guesdon 2017). The descriptions of osmophores in Malpighiaceae were based on ultrastructural analyses (Possobom et al. 2015), secretory phase, and positive histochemical results for both lipids and oil/resin compounds (Guesdon 2017). Scanning electron microscopy analysis of petal glands in pre-anthesis of Burdachia, Glandonia, and Mcvaughia showed that secretion begins to accumulate in the subcuticular space and is only released when the flower is in anthesis through cuticle disruption (Guesdon 2017).
Here, we did not detect evidence of lipid production, in contrast with other works on petal glands. Although previous works have related the posterior petal to pollinator signaling, the inner position with reproductive whorls suggests that the secretion released in the early stages of development may play a protective role against desiccation. As is known, colleters are secretory structures that have early activity with the exudate protecting developing meristems and young organs against desiccation (Thomas 1991) and can be present in vegetative and reproductive organs (Fahn 1979).
We suggest that these glands could be acting as colleters in the pre-anthesis phase. In addition, the production of polysaccharides and mucilage associated with secretory activity and the release of secretion in stages of bud development prior to anthesis suggests that these glands could also play a protective role against desiccation. However, in Burdachia, Diplopterys, Glandonia, and Mcvaughia, genera of Malpighiaceae, it was suggested that petal glands anatomically similar to those of L. floribunda may act as an osmophore (Possobom et al. 2015; Guesdon 2017). Considering that we performed the histochemical tests in the fixated samples, we did not reject the hypothesis that the petal gland of L. floribunda may also act as an osmophore. Further ultrastructural analysis may help to clarify this hypothesis.
Could be the anther glands of Lophopterys floribunda classified as osmophore?
The epidermis of the connective of L. floribunda consists of a single layer of bulky cells with dense cytoplasm with many vesicles filled with content that reacted to tests for proteins, polysaccharides, and lipids. These results agree with Diplopterys pubipetala (Possobom et al. 2015), Sigmaphyllum bonariense, and S. jatrophifolium (Avalos et al. 2020), for which osmophores formed by non-stratified epithelium in the anther connective tissue were described. Polysaccharides detected in the exudate of L. floribunda can contribute to the adhesion of pollen grains to the body of bees, as suggested by Gates (1982) and reinforced by Possobom et al. (2015) and Avalos et al. (2020). The sticky secretion released by the connective gland meets the visitor’s ventral portion, increasing the adhesion of pollen grains to the visitor’s body. It is worth noting that, based in herborized-reversed material, the glandular connective tissues in anthers of representatives of the Stigmaphylloid clade were described as elaiophores, while osmophores were observed on the abaxial surface of the anther of Byrsonima spicata, Camarea affnis, C. humifusa, and Cottsia gracilis (Arévalo-Rodrigues et al. 2020).
The perceptive aroma emitted by flowers of L. floribunda should be produced by the osmophores on the anther connective. Structures that secrete fragrances have been mostly investigated in works on the pollination biology of Orchidaceae and Solanaceae, being engaged with the attraction of pollinating bees (Vogel 1990; Pansarin et al. 2009, 2014; Wiemer et al. 2009; De Melo et al. 2010). Records of osmophores in anthers are rare. Osmophores have been reported on the floral structures of only a few groups within Malpighiales (Gagliardi et al. 2016; Tölke et al. 2018; Arévalo-Rodrigues et al. 2020). In Malpighiaceae, osmophores were also reported for the anther connective in the genus Diplopterys. Although the test with Nadi’s reagent had a very weak reaction, the anther glands of L. floribunda are similar both anatomically and functionally to the osmophores described for Diplopterys (Possobom et al. 2015). The reaction for lipids compounds were less evident compared to those tests performed in the fieldwork, probably due the volatile nature of the secretion.
The finding of the presence of osmophores in anthers of Lophopterys reinforces the presence of these structures in Malpighiaceae, suggesting attention in future works to the occurrence in other genera and how their anatomical structure originates. Such approaches might expand the set of data, contributing to a better knowledge of the floral biology of Malpighiaceae.
Conclusions
Tiny nectaries located at the apex of bracteoles and bracts of L. floribunda are a novelty for Malpighiaceae due to their size and location. The individual, large gland present on sepals of L. floribunda is probably the result of the fusion of two elaiophores and thus contributes as a strategy to maximize oil resource performance and making the structure more visible to pollinators in this vine species from the Amazon and Atlantic forests. The marginal glands of the posterior petal show standard colleter morphology, with early activity and mucilage/protein exudate possibly contributing to the maintenance of the closed bud during development onset. The presence of polysaccharides in the exudate of anther gland contributes to the adhesion of pollen grains to the body of bees, while the oil/resin detected by histochemical test indicate that this gland may also act as osmophore. Ecological studies to verify the functional role of the aroma emitted by the flowers in L. floribunda in the pollination of the species are necessary. Knowledge about these floral secretory structures and their relationship with their visitors expands the study of floral biology in Malpighiaceae and has taxonomic and phylogenetic potential.
References
Aliscioni SS, Gomiz NE, Agüero JI, Torretta JP (2022) Structural diversity of elaiophores in Argentine species of Malpighiaceae: morphology, anatomy, and interaction with pollinators. Protoplasma 259:789–807
Almeida RF, Francener A, Amorim AM (2016) A generic synopsis of Malpighiaceae in the Atlantic Forest. Nord J Bot 34:285–301. https://doi.org/10.1111/njb.01016
Almeida RF, Pessoa C, Francener A (2018) Sinopse de Malpighiaceae Juss. do Estado da Bahia, Brasil: chave para gêneros e monografias dos gêneros Alicia, Aspicarpa, Galphimia, Lophopterys, Mcvaughia e Verrucularia. Boletim Do Museu De Biologia Mello Leitão 40:55–91
Almeida RF, Guesdon IR, Pace MR, Meira RMSA (2019) Taxonomic revision of Mcvaughia WR Anderson (Malpighiaceae): notes on vegetative and reproductive anatomy and the description of a new species. PhytoKeys 117:45
Alves-Silva E, Bächtold A, Barônio GJ, Torezan-Silingardi HM, Del-Claro K (2014) Ant–herbivore interactions in an extrafloral nectaried plant: are ants good plant guards against curculionid beetles? J Nat Hist 49:841–851. https://doi.org/10.1080/00222933.2014.954020
Amorim M, De Marco P (2011) Pollination of Byrsonima coccolobifolia: short-distance isolation and possible causes for low fruit production. Braz J Biol 71:709–717. https://doi.org/10.1590/S1519-698420110004000166
Anderson WR (1979) Floral conservation in Neotropical Malpighiaceae. Biotropica 11:219–223
Anderson WR (1981) A botânica do altiplano de Guayana: parte 11. Malpighiaceae. Mem N Y Bot Gard 32:21–305
Anderson WR (1990) The origin of the Malpighiaceae - The evidence from morphology. Mem N Y Bot Gard 64:210–224
Anderson WR (2004) Malpighiaceae. In: Smith, N. & al. (ed.) Flowering Plants of the Neotropics 1:229–232
Anderson C, Anderson WR (2018) Revision of mezia (malpighiaceae). Edinb J Bot 75(3):321–376. https://doi.org/10.1017/S096042861800015X
Anderson WR, Davis CC (2001) Monograph of Lophopterys (Malpighiaceae). Contributions University Michigan Herbarium 23:83–105
Araújo JS (2014) Anatomia de espécies de Banisteriopsis c. B. Rob. (Malpighiaceae) ocorrentes no Brasil. 2014. 73f. Tese (Doutorado em Botânica) - Universidade Federal de Viçosa, Viçosa, MG
Araújo JS, Meira RMSA (2016) Comparative anatomy of calyx and foliar glands of Banisteriopsis CB Rob. (Malpighiaceae). Acta Botanica Brasilica 30:112–123. https://doi.org/10.1590/0102-33062015abb0248
Araújo JS, Azevedo AA, Silva LC, Meira RMSA (2010) Leaf anatomy as an additional taxonomy tool for 16 species of Malpighiaceae found in the Cerrado area (Brazil). Plant Syst Evol 286:117–131. https://doi.org/10.1007/s00606-010-0268-3
Arévalo-Rodrigues G, de Almeida RF, Cardoso-Gustavson P (2020) Anatomy of staminal glands in the Stigmaphylloid clade sheds light into new morphotypes of elaiophores and osmophores in Malpighiaceae. Plant Syst Evol 306:1–9. https://doi.org/10.1007/s00606-020-01680-w
Avalos AA, Pablo TJ, Lattar EC, Ferrucci MS (2020) Structure and development of anthers and connective glands in two species of Stigmaphyllon (Malpighiaceae): are heteromorphic anthers related to division of labour? Protoplasma 257:1165–1181
Avalos AA, Marrero HJ, Ferrucci MS, Torretta JP (2021) Stigmas arrangement, reproductive system, and maternal reproductive success in two species of Stigmaphyllon (Malpighiaceae): does pollinator size matter? Plant Ecol 222:1263–1279
Baker-Méio B, Marquis RJ (2012) Context-dependent benefits from ant–plant mutualism in three sympatric varieties of Chamaecrista desvauxii. J Ecol 100:242–252. https://doi.org/10.1111/j.1365-2745.2011.01892.x
Barônio GJ, Torezan-Silingardi HM (2017) Temporal niche overlap and distinct bee ability to collect floral resources on three species of Brazilian Malpighiaceae. Apidologie 48:168–180. https://doi.org/10.1007/s13592-016-0462-6
Barônio GJ, Haleem MA, Marsaioli AJ, Torezan-Silingardi HM (2017) Characterization of Malpighiaceae flower-visitor interactions in a Brazilian savannah: How do floral resources and visitor abundance change over time. Flora 234:126–134. https://doi.org/10.1016/j.flora.2017.07.015
BFG—Brazilian Flora Group (2015) Growing knowledge: an overview of Seed Plant diversity in Brazil. Rodriguésia 66:1085–1113
Caspary R (1848) De Nectariis. PhD Thesis. Elverfeldae, Bonn
Castro MA, Vega AS, Mulgura ME (2001) Structure and ultrastructure of leaf and calyx glands in Galphimia brasiliensis (Malpighiaceae). Am J Bot 88:1935–1944. https://doi.org/10.2307/3558420
Clark SG (1981) Staining procedures Williams & Wilkins. Baltimore
Cocucci AA, Holgado AM, Anton AM (1996) Estudio morfologico y anatomico de los eleoforos pedicelados de Dinemandra ericoides, Malpighiacea endemica del desierto de Atacama, Chile. Darwiniana 34:183–192
David R, Carde JP (1964) Coloration differentialle dês pseudophylles de Pin maritime au moyen reactif de Nadi. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences 258:1338–1340
Davis CC, Anderson WR (2010) A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Am J Bot 97(12):2031–2048. https://doi.org/10.3732/ajb.1000146
Davis CC, Bell CD, Mathews S, Donoghue MJ (2002) Laurasian migration explains Gondwanan disjunctions: evidence from Malpighiaceae. Proc Natl Acad Sci 99(10):6833–7683. https://doi.org/10.1073/pnas.102175899
Davis CC, Schaefer H, Xi Z, Baum DA, Donoghue MJ, Harmon LJ (2014) Long-term morphological stasis maintained by a plant-pollinator mutualism. Proc Natl Acad Sci 111:5914–5919. https://doi.org/10.1073/pnas.1403157111
De Melo MC, Borba EL, Paiva EAS (2010) Morphological and histological characterization of the osmophores and nectaries of four species of Acianthera (Orchidaceae: Pleurothallidinae). Plant Syst Evol 286:141–151. https://doi.org/10.1007/s00606-010-0294-1
Del-Claro K (2010) Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a neotropical savanna. Flora 205:754–756. https://doi.org/10.1016/j.flora.2009.12.040
Del-Claro K, Marquis RJ (2015) Ant Species Identity has a Greater Effect than Fire on the Outcome of an Ant Protection System in Brazilian Cerrado. Biotropica 47:459–467. https://doi.org/10.1111/btp.12227
Delpino F (1868) Ulteriori osservazioni e considerazioni sulla dicogamia nel regno vegetale. Atti Della Societa Italiana Di Scienze Naturali e Dei Museo Civico Di Storia Et Naturale Di Milano 11:265–332
Elias TS, Gelband H (1976) Morphology and anatomy of floral and extrafloral nectaries in Campsis (Bignoniaceae). Am J Bot 63:1349–1353
Fahn A (1979) Secretory Tissues in Plants. Academic Press, London
Fahn A (1988) Secretory tissues in vascular plants. New Phytol 108:229–257
Feio AC, Ricarda R, Renata MSAM (2016) Secretory structures in leaves and flowers of two Dragon’s blood Croton (Euphorbiaceae): new evidence and interpretations. Int J Plant Sci 177:511–522. https://doi.org/10.1086/685705
Fernandes, G. Wilson, Fagundes, Marcílio, Greco, Magda K. Barcelos, Barbeitos, Marcos Soares e Santos, Jean Carlos (2005) Ants and their effects on an insect herbivore community associated with the inflorescences of Byrsonima crassifolia (Linnaeus) H.B.K. (Malpighiaceae). Revista Brasileira de Entomologia 49:264-269. https://doi.org/10.1590/S0085-56262005000200011
Fisher DB (1968) Protein staining of ribboned epon sections for light microscopy. Histochemie 16:92–96. https://doi.org/10.1007/BF00306214
Foster AS (1950) Practical plant anatomy. D. Van Nostrand Company Inc, Toronto
Gagliardi KB, Cordeiro I, Demarco D (2016) Protection and attraction: bracts and secretory structures in reduced inflorescences of Malpighiales. Flora - Morphol Distrib Funct Ecol Plants 220:52–62. https://doi.org/10.1016/j.flora.2016.02.003
Gates B (1982) Banisteriopsis and Diplopterys (Malpighiaceae). Flora Neotropica Monograph 30:1–237
Gonçalves-Souza P, Schlindwein C, Dötterl S, Paiva EAS (2017) Unveiling the osmophores of Philodendron adamantinum (Araceae) as a means to understanding interactions with pollinators. Ann Bot 119:533–543. https://doi.org/10.1093/aob/mcw236
Guesdon I (2017) Estruturas secretoras em linhagens Neo e Paleotropicais de Malpighiaceae: morfoanatomia, evidências funcionais e contribuições taxonômicas e evolutivas. 2017. 144f. Tese (Doutorado em Botânica) - Universidade Federal de Viçosa, Viçosa, MG
Guesdon IR, Amorim AM, Meira RMSA (2018) The hydrochorous Amazonian genus Glandonia (Malpighiaceae): new records, morphoanatomy updates and taxonomic contributions. Phytotaxa 345:13–25. https://doi.org/10.11646/phytotaxa.345.1.2
Guesdon IR, Amorim AM, Meira RMSA (2019) Functional role and evolutionary contributions of floral gland morphoanatomy in the Paleotropical genus Acridocarpus (Malpighiaceae). PLoS One 14:9. https://doi.org/10.1371/journal.pone.0222561
Johansen DA (1940) Plant microtechnique. McGraw Hill, New York
Kettler BA, Solís SM, Ferrucci MS (2018) Comparative survey of secretory structures and floral anatomy of Cohniella cepula and Cohniella jonesiana (Orchidaceae: Oncidiinae). New evidences of nectaries and osmophores in the genus. Protoplasma 255:1–18. https://doi.org/10.1007/s00709-018-1330-1
Kowalkowska AK, Pawłowicz M, Guzanek P, Krawczyńska AT (2018) Studies on floral nectary, tepals’ structure, and gynostemium morphology of Epipactis palustris (L.) Crantz (Orchidaceae) (L.) Crantz (Orchidaceae). Protoplasma 255:1811–1825. https://doi.org/10.1007/s00709-018-1274-5
Lersten NR, Horner HT Jr (1967) Development and structure of bacterial leaf nodules in Psychotria bacteriofila Val. (Rubiaceae). J Bacteriol 94:2027–2036
Machado SR, Morellato LPC, Sajo MG, Oliveira PS (2008) Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado. Plant Biol 10:660–673. https://doi.org/10.1111/j.1438-8677.2008.00068.x
Matos RRD, Araújo JS (2021) Morfoanatomia das glândulas foliares e calicinais de Stigmaphyllon A. Juss. (Malpighiaceae): evidências funcionais, contribuições taxonômicas e evolutivas. Hoehnea 48:e282021. https://doi.org/10.1590/2236-8906-28/2021
Mayer JLS, Carmello-Guerreiro SM, Mazzafera P (2013) A functional role for the colleters of coffee flowers. Plantas AoB 5. https://doi.org/10.1093/aobpla/plt029
McManus JFA (1948) Histological and histochemical uses of periodic acid. Stain Technol 23:99–108. https://doi.org/10.3109/10520294809106232
Mello MAR, Bezerra ELS, Machado IC (2013) Functional roles of Centridini oil bees and Malpighiaceae oil flowers in biome-wide pollination networks. Biotropica 45:45–53. https://doi.org/10.1093/aobpla/plt029
Nery LA, Vieira MF, Ventrella MC (2017) Leaf glands of Banisteriopsis muricata (Malpighiaceae): distribution, secretion composition, anatomy and relationship to visitors. Acta Botanica Brasilica 31:459–467. https://doi.org/10.1590/0102-33062017abb0108
O’Brien TP, Feder N, Mccully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Pacini E, Nepi M, Jl V (2003) Nectar biodiversity: a short review. Plant Syst Evol 238:7–22
Paiva EAS (2009) Occurrence, structure and functional aspects of the colleters of Copaifera langsdorffii Desf. (Fabaceae, Caesalpinioideae). CR Biol 332:1078–1084. https://doi.org/10.1016/j.crvi.2009.08.0033
Pansarin LM, Castro MM, Sazima M (2009) Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Bot J Linn Soc 159:408–415. https://doi.org/10.1111/j.1095-8339.2009.00953.x
Pansarin LM, Pansarin ER, Sazima M (2014) Osmophore structure and phylogeny of Cirrhaea (Orchidaceae, Stanhopeinae). Bot J Linn Soc 176:369–383. https://doi.org/10.1111/boj.12206
Pearse AGE (1980) Histochemistry: theoretical and applied, vol. 2, 4th edn. Churchill Livingstone, Edinburgh
Płachno BJ, Swiaztek P, Szymczak G (2010) Can astench be beautiful? Osmophores in stem-succu-lent stapeliads (Apocynaceae–Asclepiadoideae–Cero-pegieae–Stapeliinae). Flora 205:101–105. https://doi.org/10.1016/j.flora.2009.01.002
Possobom CCF, Machado SR (2017) Elaiophores: their taxonomic distribution, morphology and functions. Acta Botanica Brasilica 31:503–524. https://doi.org/10.1590/0102-33062017abb0088
Possobom CCF, Machado SR (2018) Elaiophores in three Neotropical Malpighiaceae species: a comparative study. Plant Syst Evol 304:15–32. https://doi.org/10.1007/s00606-017-1443-6
Possobom CCF, Guimarães E, Machado SR (2010) Leaf glands act as nectaries in Diplopterys pubipetala (Malpighiaceae). Plant Biol 12:863–870. https://doi.org/10.1111/j.1438-8677.2009.00304.x
Possobom CCF, Guimarães E, Machado SR (2015) Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora: Morphology. Distrib Funct Ecol Plants 211:26–39. https://doi.org/10.1016/j.flora.2015.01.002
Purvis MJ, Collier DC, Walls D (1964) Laboratory Techniques in Botany. Butterwoths, London
Ren MX, Zhong YF, Song XQ (2013) Mirror-image flowers without buzz pollination in the Asian endemic Hiptage benghalensis (Malpighiaceae). Bot J Linn Soc 173:764–774
Renner SS, Schaefer H (2010) The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philos Trans Royal Soc b: Biol Sci 365:423–435. https://doi.org/10.1098/rstb.2009.0229
Rosumek FB, Silveira FA, Neves FDS, Barbosa NPDU, Diniz L, Oki Y, Cornelissen T (2009) Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160:537–549. https://doi.org/10.1007/s00442-009-1309-x
Sazima M, Vogel S, Cocucci A, Hausner G (1993) The perfume flowers of Cyphomandra (Solanaceae): Pollination by euglossine bees, bellows mechanism, osmophores, and volatiles. Plant Syst Evol 187:51–88
Schmid R (1988) Reproductive versus extra-reproductive nectaries—historical perspective and terminological recommendations. Bot Rev 54:179–227
Simpson BB, Neff JL (1981) Floral rewards: alternatives to pollen and nectar. Ann Mo Bot Gard 68:301–322
Subramanian RB, Arumugasamy K, Inamdar JS (1990) Studies in secretory glands of Hiptage sericea (Malpighiaceae). Nord J Bot 10:57–62. https://doi.org/10.1111/j.1756-1051.1990.tb01753.x
Teixeira LAG, Machado IC (2000) Sistema de polinização e reprodução de Byrsonima sericea DC (Malpighiaceae). Acta Botanica Brasilica 14:347–357
Thomas V (1991) Structural, functional and phylogenetic aspects of the colleter. Ann Bot 68:287–305. https://doi.org/10.1093/oxfordjournals.aob.a088256
Tölke ED, Bachelier JB, de Lima EA, Ferreira MJP, Demarco D, Carmello-Guerreiro SM (2018) Osmophores and floral fragrance in Anacardium humile and Mangifera indica (Anacardiaceae): an overlooked secretory structure in Sapindales. Plantas AoB 10:1–14. https://doi.org/10.1093/aobpla/ply062
Vogel S (1990) History of the Malpighiaceae in the Light of Pollination Ecology. Mem N Y Bot Gard 55:130–142
Wiemer AP, More M, Benitez-Vieyra S, Cocucci AA, Raguso RA, Sersic AN (2009) A simple floral fragrance and unusual osmophore structure in Cyclopogon elatus (Orchidaceae). Plant Biol 11:506–514. https://doi.org/10.1111/j.1438-8677.2008.00140.x
Acknowledgements
This work was carried out with support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Financing Code 001. We thank the funding agencies FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) and Proap (Programa de Apoio à Pós-graduação) for supporting collection and field work. Renata Meira (DEB #307987/2022-1) thanks CNPq for her productivity grants. We also thank Roberto Paulino Pereira, the guide of the RPPN, for accompaniment in the field and Júlio Cézar Mário Chaul for the identification of the collected insects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Dorota Kwiatkowska
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 2735 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanches, M.M., Guesdon, I.R. & Alves Meira, R.M.S. Diversity and functional roles of floral glands in Malpighiaceae: insights in Lophopterys floribunda W.R. Anderson & C. Davis. Protoplasma 260, 1555–1567 (2023). https://doi.org/10.1007/s00709-023-01871-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-023-01871-5