Abstract
Heteranthery has been largely associated with a division of labour between anthers. Most species of Stigmaphyllon (Malpighiaceae) present heteromorphic anthers and glandular connectives of different development; yet, the functional meaning of this condition has never been explored in the genus. The aims of this study were to provide a comparative description of the structure and development of anthers and their connective glands in S. bonariense and S. jatrophifolium and to assess the existence of division of functions. Natural populations were selected to collect flowers at different stages. Anthers were subjected to morpho-anatomical, histochemical and pollen viability studies. For both species, abundance of pollen grains and size of anther and their connective glands were estimated. Three types of stamens are recognized: stamen with small, intermediate and large anthers. Anthers of both species exhibit a similar glandular tissue in the connective, and the histochemical analysis revealed that it produce a mucilagous secretion. The pattern of anther wall development, stainability and release of pollen grains was identical among anther types. For both species, we observed a positive relationship between anther size and abundance of pollen grains, but an inverse relationship between area of anthers and size (area and thickness) of connective glands in small anthers vs. intermediate and large ones. Our results evidence a specialization of anthers related to division of labour between heteromorphic stamens in two species of Stigmaphyllon. Thus, one set of anthers produces large amount of pollen grains for pollination and another sets large quantities of mucilage, which would improve pollen transport (better adherence to pollinator body and dampness maintenance). Nevertheless, heteranthery in both Stigmaphyllon species would represent a transitional state towards the division of labour rather than a stable state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The stamen consists of two morphologically distinct parts, the filament and the anther. The latter contains the reproductive and non-reproductive tissues that are responsible for pollen grain development so that pollination and fertilization processes can occur in the flower (Goldberg et al. 1993). In a typical anther, sporogenous cells are involved in the formation of pollen grains, whereas other cell types (epidermis, endothecium, middle layers and tapetum) contribute to pollen maturation, protection or release (Bhandari 1984; Scott et al. 2004). However, although viable pollen is formed, in some cases, it is incapable of fertilization due to some barriers, such as anther indehiscence or inability of pollen to reach ovules due to a defective exine or pollen tube formation (Budar and Pelletier 2001; Kaul 2012). In many flowering plants, anther indehiscence is a common mechanism that affects male functionality of morphologically perfect flowers, resulting in functionally unisexual ones (Acevedo-Rodríguez 1993; Ferrucci and Anzótegui 1993; Ainsworth 2000; Solís et al. 2010; Zini et al. 2012; González et al. 2017). The function of stamens can be broader than just containing the structures that produce and release the male gametes for fertilization. For example, in heterantherous species (i.e. presence of stamens with more than one type of anther in the same flower), one set of anthers can be responsible for supplying pollen to feed the pollinators and another set for the production of pollen for fertilization resulting in a division of labour between anthers (Müller 1881; Luo et al. 2009; Vallejo-Marín et al. 2009; Barrett 2010; Paulino et al. 2013, 2016). In addition, heteranthery has been related to visual attraction (Hrycan and Davis 2005; Velloso et al. 2018) and explosive mechanism of pollen release (Paulino et al. 2016). Therefore, detailed morpho-anatomical studies can provide valuable information about processes involved in fertility of male flowers, as well as functionality of different stamens and thus the availability of pollen for pollination.
Malpighiaceae is a pantropical family represented by approximately 1300 species mostly occurring in the New World (Davis and Anderson 2010). Most Neotropical Malpighiaceae species present a specialized interaction with oil-collecting bees (Anderson 1979; Vogel 1990), primarily females of species belonging to the oil-collecting genera Centris, Epicharis (Apidae: Centridini, following the nomenclature of Michener 2007) and Monoeca (Apidae: Tapinotaspidini) (Torretta et al. 2017). In this interaction, bees collect the oil produced by the calyx glands (elaiophores) and use it for larval provisioning (Vogel 1974; Vinson et al. 1996; Sigrist and Sazima 2004; Reis et al. 2007) and/or cell lining (Neff and Simpson 1981; Simpson and Neff 1981; Buchmann 1987; Vogel 1990), whereas species of Malpighiaceae are effectively pollinated. This highly specialized pollination system between Malpighiaceae, and their specific pollinators has influenced the conserved floral morphology (Anderson 1979) and the diversification rates of this family (Renner and Schaefer 2010; Davis et al. 2014).
Due to the interesting interaction between Malpighiaceae species and their pollinators, most of the available works are focused on the plant-pollinator relationship in different ecosystems (Gottsberger 1986; Rego and Albuquerque 1989; Sazima and Sazima 1989; Teixeira and Machado 2000). However, embryological studies are scarce in Malpighiaceae (Johri et al. 1992). Likewise, anther structure and development are known for few species of this family: in Thryallis glauca (Cav.) Kuntze (Singh 1959), Malpighia glabra L. (Miyashita et al. 1964) and M. coccigera L. (Siddiqui 1968). Steiner (1985) reported functional dioecism in Spachea membranacea Cuatrec.; this author found individuals with flowers that have anthers bearing pollen (male), and other individuals with flowers that look similar but without pollen-bearing anthers (females); however, no morphological (except for length of stamens) or anatomical details of androecium were provided. In addition, anatomical features of the floral pieces related to the morphology and/or foraging behaviour of the pollinators are scarce (Possobom et al. 2015; Aliscioni et al. 2018).
Stigmaphyllon A. Juss. comprises about 120 pantropical species (Anderson 2011). Most of the species have yellow flowers, androecia with 10 fertile stamens and tricarpellate gynoecium (Anderson 1986, 1997; Almeida and Mamede 2016). In most species of Stigmaphyllon, the anthers are heteromorphic and present a well-developed glandular connective (Anderson 2011; Almeida and Mamede 2016). Possobom et al. (2015) proposed that the connective glands of Diplopterys pubipetala (A. Juss.) W.R. Anderson & C. Davis act as osmophores and/or mimic pollen, and that their secretion might increase the efficiency in the transfer of pollen grains (by improving pollen adhesion). So far, heteromorphic stamens have been found in 16 families distributed in 12 orders of angiosperms (Vallejo-Marín et al. 2010) and although the presence of heteromorphic anthers is common in Stigmaphyllon, as well as in other genera of Malpighiaceae (Anderson et al. 2006; Aliscioni and Torretta 2017), no evolutionary meaning has ever been proposed to explain this condition in the family.
In the present study, we provide a comparative description of structure and development of anthers and their connective glands in Stigmaphyllon bonariense (Hook. & Arn.) C.E. Anderson and S. jatrophifolium A. Juss. in order to improve our understanding of the ontogeny, availability and export of pollen grains in these species. Although connective glands are part of the anthers, we will refer to these structures separately for a better understanding. Our study is focused on the following questions: (1) What is the function of the connective glands? (2) Are there differences in the anatomy and development of the anthers and pollen grains between the study species and among different types of anthers? (3) Is there division of labour in anthers of the studied Stigmaphyllon species? We predict that (1) the structure and functioning of connective glands and the nature of the secretion would contribute to the adhesion of pollen grains to the pollinator body; (2) both species will have a similar pattern of anther wall and pollen development because they are under similar selection pressures (e.g. they are sympatric and co-flowering, and share pollinators), but that pattern will differ among types of anthers due to their functionality; (3a) some anthers will be pollen donors and others will primarily have a pollen adhesion function and (3b) the former will be located in the same place as the stigmas and will have a larger size, a higher amount of pollen and smaller connective glands than the latter anthers.
Materials and methods
Study species and sampling sites
Stigmaphyllon bonariense and S. jatrophifolium are woody perennial vines with yellow flowers, arranged in pseudo-umbels (20–30 flowers per inflorescence, but only 2–3 ones synchronously opened), solitary or borne in dichasia (Múlgura de Romero 2005). Natural populations of both species (n = 3) were selected from different sites of Misiones and Corrientes provinces, with both species occurring in two of those sites. Sampling was carried out during the flowering seasons for two consecutive years (February to May in 2017, December in 2017 to May in 2018).
Material collection and field data
In each sampled population, floral buds and flowers at pre-anthesis (60 buds of 2–4 mm in length before anthesis), anthesis (100 flowers of 1 to 2 days after anthesis) and post-anthesis development stages (65 flowers of 3 to 4 days after anthesis) were fixed in formalin, acetic acid and alcohol (FAA) for further anatomical and scanning electron microscopy (SEM) examination. Additionally, we censused individuals (n = 120 for S. bonariense and n = 67 for S. jatrophifolium) in each population for each species. In each census, we recorded the proportion of functionally pistillate flowers (flowers with indehiscent anthers) related to the number of hermaphrodite flowers in an individual (i.e. pistillate flowers/hermaphrodite flowers). Each visit consisted of 1–3 days of observation and data collection. The voucher specimens (Avalos 4, 16, 35, 38; Torretta 78, 79) were deposited in the herbarium of the Instituto de Botánica del Nordeste (CTES), Argentina.
Exomorphological analysis
We analysed the architecture of the flower under stereomicroscope, putting special attention to the arrangement of heteromorphic stamens, length of filaments and morphology of anthers and their connective glands. In addition, we calculated the size of anthers and connective glands in flowers in anthesis (n = 10) of each population. The area of anthers in frontal view (Fig. 1a) and the area (Fig. 1b) and thickness of the connective glands (Fig. 1c) were estimated through digital photographs using function area and length of ImageJ program (Schneider et al. 2012). All these flower morphological analyses were performed under a Leica MZ6 stereomicroscope, equipped with a digital camera.
Dimensions of anthers used in both species of Stigmaphyllon. Abbreviations: A1, area of anther; A2, area of connective gland; T, thickness of connective glands. a Polygon drawing on perimeter of one anther (in front view) to calculate its area. b Polygon drawing on perimeter of connective gland to calculate its area. c Line drawing on connective gland (in lateral view) to calculate its thickness. Scale bars: a-c 0.2 mm
For SEM studies, conventional methods were utilized (O’Brien and McCully 1981), and the stamens from the material preserved in FAA were dehydrated through a series of increasing ethanol solutions. Material was then critically point-dried with solvent-substituted liquid carbon dioxide and coated with a thin layer of gold palladium. Micrographs of anthers and connective glands were obtained with a JEOL 5800LV at 10 Kv and JEOL 100c.
Histological analyses
Permanent slides were prepared for anatomical studies as follows: the fixed material was processed by dehydration through an ethanol series with a pre-impregnant rinsing of tertiary butyl alcohol (Gonzalez and Cristóbal 1997) and infiltration in paraffin Histoplast® (Biopack, Buenos Aires, Argentina), according to Johansen (1940). Buds and flowers were sectioned transversely and longitudinally (10–12 μm thick) with a rotary microtome; the sections were stained with astra blue-safranin (Luque et al. 1996) and mounted with synthetic Canada balsam (Biopur, Buenos Aires, Argentina). Anatomical analyses were performed under a Leica DM LB2 compound microscope (Leica, Wetzlar, Germany) equipped with a digital camera.
For the histochemical tests, hand sections of fresh material of connective glands were cut. Samples were subjected to the following treatments: Lugol reagent for starch grains, Sudan IV for total lipids, brilliant cresyl blue and ruthenium red for pectine/mucilage, naphtol + dimethyl-paraphenylene-diamine (NADI) reagent for terpenes and neutral red for showing cells with open cuticle (Johansen 1940; David and Carde 1964; D'Ambrogio de Argüeso 1986; Zarlavsky 2014; Barrios et al. 2015). Two flowers per treatment were used for each of the five individuals analysed per species. The control samples were tested according to the specifications for each analysis. Micrographs were obtained using stereomicroscope and compound microscope, both equipped with a digital camera.
Pollen grain viability was estimated according to pollen grain stainability, using acetocarmine (Radford et al. 1974). We used pollen of virgin flowers in anthesis (flowers previously covered with cloth bags in order to avoid pollinator visits and contamination with foreign pollen grains). Three randomly selected flowers per individual (n = 10 individuals of S. bonariense and n = 9 individuals of S. jatrophifolium) were studied. For each flower, pollen grains from different anther types were studied separately. Pollen stainability was assessed based on the percentage of 100 well-stained pollen grains, which were counted using an optical microscope (Radford et al. 1974). We also counted the number of pollen grains in each anther type in 36 flowers per species (n = 3 from 12 individuals of each species) according to standardized techniques (Harder 1990). The pollen grains were suspended in 5 ml of a 70% alcohol solution and detergent (100 ml of alcohol and 5 ml of detergent). An aliquot was placed in a Neubauer chamber, and pollen grains were counted using an optical microscope with a × 40 magnification objective lens. The number of pollen grains contributed by each different set of anthers in a flower was calculated by multiplying the number of pollen in each anther type by the number of anthers of each set in a flower (see “Results”).
Statistical analyses
For S. bonariense, we evaluated the relationship between hermaphrodite and pistillate flowers via a linear regression using a generalized linear mixed model (GLMM), performed with lmer function (R Development Core Team 2013). The model used was (pistillate ~ (1|population/individual); log (number of pistillate flowers +1) was used as response variable, and number of hermaphrodite flowers as explanatory variable.
For both species, we analysed the different response variables using GLMMs. In these models, we considered ‘species’ (S. bonariense and S. jatrophifolium) and ‘anther type’ (large, intermediate, and small) as fixed factors. The ‘individuals’ (from which the anthers were obtained) were considered as random factors, nested in ‘population’; the interaction among fixed variables was also included. The GLMMs were performed using the glmer function (lme4 package, R Development Core Team 2013) and negative binomial distribution was used to avoid overdispersion (see GLMMs in the supplementary material). Firstly, we evaluated the number of pollen grains in each set of anthers and used as response variable the number of pollen grains per anther type. The model used was (grain ~ (1|population/individual) + anther*species) (with a negative binomial distribution). Secondly, we evaluated pollen viability (measured as pollen grain stainability) in anther types and species and used the proportion of stained pollen grains as response variable. The model used was (proportion ~ (1|population/individual) + anther*species) (with a binomial distribution). In order to evaluate variations in anther size (measured as area of anther), and area and thickness of connective gland, we conducted GLMMs (with Gaussian distributions) and used area of anther and area and thickness of connective gland as response variables, respectively. The models used were (anther ~ (1|population/individual) + anther*species), (gl-size ~ (1|population/individual) + anther*species) and (gl-thickness ~ (1|population/individual) + anther*species), respectively.
Results
Floral architecture
Flowers of both Stigmaphyllon species are oblique zygomorphic (Figs. 2a, b and 3a, h). Calyx is 5-merous; the anterior sepal is eglandular, whereas the remaining four lateral ones are abaxially biglandular. Corolla is 5-merous with five clawed yellow petals; the claw of the posterior petal is conspicuously thicker than in the lateral ones (Fig. 3a, h). Androecium comprises two pentamerous obdiplostemonous whorls of stamens; the outer whorl is antepetalous and the inner one is antesepalous. At pre-anthesis stage, the two whorls of stamens are easy to recognize; in anthesis, the stamens change their position, and recognition of both whorls becomes difficult (Fig. 2a, b). Syncarpous gynoecium is 3-carpellate. The three styles are free and have laterally one (posterior styles) or two (anterior style) foliaceous expansions at the apex. Stigma is located on the adaxial internal angle, facing the interior of the flower. On the first day of anthesis, the three styles are located close together in the centre of the flower, whereas on the second and third days, they separate, with four stamens (antesepalous) with small anthers and one stamen (antepetalous) with intermediate anther (see more details below) remaining in the centre of the flower (Figs. 2a, b and 3f).
Floral diagram of Stigmaphyllon studied species. a Pre-anthesis stage, note that the outer and inner whorls of anthers are easy to recognize and stigmas positioned close together in the centre of the flower. b Anthesis stage, note that the anthers and stigmas change their position. The connective glands are gridded. Abbreviations: b, s, sepal; p, petal; pf, flag petal; la, large anther; ia, intermediate anther; b, bract, sa, small anther; as, anterior stigma; ps, posterior stigma. Arrow points to orientation of the monosymmetry in the flower
Morphology of androecium in two species of Stigmaphyllon. a–gS. bonariense.a Flower at the anthesis stage. b Arrangement of large and small anthers showing the connective glands (black arrows) and the thecae (white arrows), the small anthers have large connective glands. c Three intermediate anthers showing connective glands (black arrow) and the thecae (white arrow). d Large anther with a small connective gland (black arrow) and a small one with a large connective gland (black arrow). e Small anther in frontal view showing a single theca (white arrow), note its large connective gland (black arrow). f Anthers of functionally pistillate flower; note the indehiscent theca in front view (white arrow), with functional connective glands (black arrows). g Detail of a large indehiscent anther in frontal view. h–kS. jatrophifolium.h Flower at the anthesis stage i Large anther in lateral view showing the small connective gland (black arrow) and the theca (white arrow). j Three intermediate anthers showing their connective glands (black arrows). k Detail of a small anther in frontal view (white arrows), note the connective gland (black arrow). Abbreviations: la, large anther; sa, small anther. Scale bars: f 0.8 mm; b 0.7 mm; c, d, i 0.5 mm; g, j 0.4 mm; and e, k 0.2 mm
Androecium
Heteromorphic stamens differ in length of filaments and size of anthers and connective glands (Fig. 2a, b): two stamens have large anthers and long filaments (hereafter: large anthers), four stamens have intermediate anthers and short filaments (hereafter: intermediate anthers), and four stamens have small anthers and filaments of intermediate length (hereafter: small anthers) (Figs. 3b–k and 4a–f). Filaments are glabrous and fused at their base. Anthers are basifix, bithecal, oblong and longitudinally dehiscent and have connective glands of different sizes (Fig. 4a–f). The outer whorl consists of two stamens with large anthers and three with intermediate ones, whereas the inner whorl presents one stamen with intermediate anther and four with small ones (Fig. 2b). The two large anthers are located at the same site as the two posterior stigmas, whereas three intermediate anthers are positioned at the same site as the anterior stigma; the remaining anthers (i.e. one intermediate anther and four small ones) are located in the centre of the flower (Figs. 2b and 3f). We observed that the four small anthers were variable in relation to the number of thecae, ranging from two small thecae to absence of thecae (Figs. 3b, e, k and 4c, f). Subtle differences between species are the presence of stomata in the filaments of some stamens and the glabrous anthers in S. jatrophifolium and variable pubescence of the anthers in S. bonariense (Fig. 4a–f).
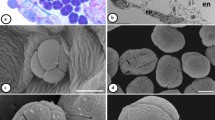
SEM micrographs of mature androecium in two species of Stigmaphyllon. a–cS. bonariense. a Large anther, note the pubescence (white arrow). b Intermediate anther with conspecific (white arrows) and heteroespecifc (black arrow) pollen grains adhered to the connective gland. c Small anther, note the reduced thecae and the presence of the coespecific (white arrows) and heteroespecific (black arrows) pollen grains glued to the connective gland. d–fS. jatrophifolium. d Large anther. e Intermediate anther. f Small anther. Scale bars: d 400 μm; a 300 μm; b, e, f 100 μm; c 50 μm
Morpho-anatomy of connective glands
Anthers of both species exhibit similar glandular tissue in the connective. The latter is positioned abaxially and is composed of apically large globular cells that are light yellow to translucent and flat cells on the basal and lateral regions (Figs. 3b, d, f and 5a–l). Connective glands vary in size, with large anthers having smaller glands, intermediate anthers having glands of intermediate size, and small anthers having larger glands (Fig. 5a–f).
SEM micrographs of the connective glands in Stigmaphyllon. a–c, g–kS. bonariense. a Detail of the small connective gland in large anther. b Intermediate connective gland in intermediate anther. c Large connective gland in small anther with pollen grains adhered to the secretion (arrows). d–f, l–mS. jatrophifolium. d Detail of the small connective gland in large anther. e Intermediate connective gland in intermediate anther. f Large connective gland in small anther with pollen grains adhered to the secretion (arrows). g Epidermis of the connective glands at pre-secretory stage, observe the striated cuticle. h Developing of bubbles in cells contacting areas (arrows). i Detail of secretory cells turgid and globular, with their surface completely bubbled. j The bubbles break down and release a slightly viscous exudate and the cells become flattened. k Detail of broken bubbles (arrows) present on the globular cells and the released secretion. l Connective gland of small anther at post-secretory stage with coespecific (white arrows) and heteroespecific (black arrow) pollen grains. (M) Detail of mucilage secretion. Scale bars: a–f, l 200 μm; h, j 20 μm; g 10 μm; and i, k, m 5 μm
Secretory cells are flat and display a striate cuticle at pre-secretory stage (Fig. 5g). In areas where these cells become in contact, they begin to develop “bubbles” on their surface (Fig. 5h); later, they become turgid and globular, with their surface completely bubbled (Fig. 5i). Finally, in the secretory stage, the bubbles break down and release a slightly viscous exudate and the cells become flattened (Fig. 5j, k). We observed conspecific and heterospecific pollen grains adhered to the secretion of the connective gland in both species at post-secretory stage (Figs. 4c and 5c, f, l).
In cross section, the connective glands are dorsally lined with one layer (rarely two) of globular cells that are directly responsible for secretion; on both sides, there is one layer of flat cells, which are intensely stained (Fig. 6a–d). Subjacent to the epidermis, one or two layers of endothecium cells and several layers of parenchymal cells supplied with xylem and phloem in the connective tissue are observed (Fig. 6d). The globular cells are filled with a dense droplet-like substance, and its bubbled surface is due to differential thickening of the cell wall (Fig. 6e, f).
Cross sections of the anthers in Stigmaphyllon species showing structure of connective glands. a, bS. bonariensea Intermediate and large anthers showing the connective gland cells with globular (black arrows) and flat cells (white arrows); note many pollen grains in the locules. b Two small anthers with large connective glands (black arrows); note only one theca and few grains in both. c–fS. jatrophifolium.c Three small anthers with large connective glands in S. jatrophifolium showing globular cells and few pollen grains (black arrows). d Detail of the connective gland showing globular cells (black arrow) and flat cells (white arrow); cell wall thickening of the endothecium (white arrowheads), parenchyma cells with druses (black arrowheads) and the vascular bundle. e Detail of mucilage-filled and empty globular cells; note the wall thickenings of the endothecium. f Detail of a globular cell showing a thick-walled region (black arrow) and thin-walled protuberant regions (white arrow). Scale bars: a 300 μm; c 250 μm; b 200 μm; d 100 μm; e 50 μm; and f 20 μm
Connective glands and the secretion produced reacted positively with ruthenium red and brilliant cresyl blue (Fig. 7a, b). Likewise, the glands reacted positively with NADI and neutral red (Fig. 7c, e) and the cytoplasm of globular cells presented small droplets that reacted positively with Sudan IV (Fig. 7d), whereas they did not stain with Lugol (Table 1).
Histochemical analysis of the connective glands in S. bonariense. a Cross section of connective glands treated with ruthenium red; note a globular cell that reacted positively (black arrow). b Cross section of the connective gland treated with brilliant cresyl blue, detail of the secretion (arrow) stained. c Cross section of connective gland treated with NADI; note that the cells filled of secretion and cuticle react positively (arrows). d Cross section of connective gland cells showing small droplets (arrows) that reacted positively with Sudan IV. e Anther treated with neutral red reagent; note that only the connective gland reacted positively. Scale bars: a–c 50 μm; d 25 μm; e 0.2 mm
Anther structure, microsporogenesis and microgametogenesis
The study species share similar developmental patterns in anther wall, microsporogenesis and microgametogenesis; however, some differences were observed.
Stages with shared characters
At an early stage of development, the sporogenous tissue differentiates into microspore mother cells (MMCs), which are polygonal and surrounded by callose. Later, MMCs become rounded and separated from one another. At this moment, the anther wall consists of an epidermis, an endothecium with thin-walled cells and conspicuous nuclei, two middle layers and a binucleate tapetum (Fig. 8a). Each MMC undergoes simultaneous reduction division, resulting in a tetrahedral tetrad (Fig. 8b, d). This stage is characterized by microspores in tetrads that are uniformly surrounded by callose; the tapetal cells elongate radially and become multinucleate, and the middle layers begin to crush, whereas the remaining layers of the anther wall are still not modified (Fig. 8c, e).
Microsporangium at different developmental stages in Stigmaphyllon species. a–dS. bonariense. a Cross section of the young anther, showing epidermis, endothecium, middle layers, tapetum and microspore mother cells. b Detail of MMCs undergoing meiosis. c Microspores tetrads stage showing the anther wall with epidermis, endothecium, middle layers and binucleate tapetal cells (white arrow). d Microspore tetrads surrounded by callose. e–hS. jatrophifolium. e Anther wall at early free microspores stage, the epidermis appears more compressed, endothecium and uninucleate tapetal cells. f Anther wall at late free microspores stage; note the position of tapetal cells (black arrows), endothecium cells with fibrous thickenings and compressed epidermis cells. g Dehiscent anther with some pollen grains (black arrows). h Detail of mature pollen grains. i–oS. bonariense. i Anther wall at early free microspores stage; note the tapetal cells (white arrows) invading the locule. j Tapetal cells surrounding free microspores without loss individuality (white arrows). k Detail of young pollen grains aggregated by “viscine” threads (black arrows). l Dehiscent anther with mature pollen grains showing the release of pollen grains (black arrows). m Mature bicellular pollen grains. n Indehiscent anther in pistillate flower with non-functional stomium (black arrows) at post-anthesis stage. o Pollen grains collapsed, endothecium with fibrous thickenings (white arrow), remnants of tapetal cells and epidermis. Abbreviations: epidermis (e), endothecium (en), middle layers (ml) and secretory tapetum (t). Scale bars: l 500 μm; g, n 200 μm; a, o 50 μm; i 30 μm; k 25 μm; and b–f, h, j, m 20 μm
Stages with different characters between species
Following degradation of the callosic tetrad walls, the microspores present a dense cytoplasm, and the layers of anther wall undergo changes. In both species, the epidermis is slightly compressed, the endothecium cells increase in length and begin to develop fibrillar thickenings, and the middle layers degenerate. Conversely, tapetal cells undergo a different process. In S. jatrophifolium, the tapetal cells maintain their position, which corresponds to the secretory tapetum (Fig. 8e, f), whereas in S. bonariense, those cells invade the locule and surround pollen grains, maintaining their individuality, in correspondence with an invasive non-syncytial tapetum (Fig. 8i, j). Subsequently, the nucleus of the microspore divides by mitosis, giving rise to the generative and vegetative cells (Fig. 8m). Later, the endothecium develops complete fibrillar thickenings, middle layers and tapetal cells are degenerated, the parenchymatous septa separating pollen sacs break down, the anther dehisces through longitudinal slits, and pollen grains are released at two-celled stage (Fig. 8g, h, l, m). Pollen aggregation is by “viscin” threads (Fig. 8k).
No differences in patterns of anther or pollen development were observed among types of anthers in either species. However, in some flowers of S. bonariense, anthers are not functional. Although the anther wall consists of an epidermis and endothecium similar to those previously described, and although tapetum and parenchymatous septa show notable signs of degradation at mature pollen grain stage, all anthers are indehiscent, and these flowers function as pistillate ones. In addition, pollen grains exhibit abnormal shapes, they are completely collapsed and their cytoplasms stained weakly with acetocarmine (Fig. 8n, o). We observed that the number of pistillate flowers was positively and significantly related to that of hermaphrodite ones (R2 = 0.474, P < 2.2e−16, Fig. 9).
Relation between hermaphrodite and pistillate flowers in S. bonariense, based on log (number of pistillate flowers + 1) as response variable, and number of hermaphrodite flowers as explicative variable. R2 = 0.474, P < 2.2e−16. Equations and determination coefficient R2 were determined by linear regression
Dimensions of anthers and stainability and number of pollen grains
Our results indicate that pollen grain stainability was not significantly different among anther types (X2 = 0.73, P = 0.693; Fig. 10a); however, it was significantly higher in S. jatrophifolium than in S. bonariense (X2 = 125.39, P < 2e−16). On the other hand, anther area was significantly different among anther types (X2 = 5057.19, P < 2e−16). The large anthers had a higher area, followed by intermediate and small ones (Fig. 10b). The amount of pollen grains significantly varied among anther types in both species (X2 = 5733.45, P < 2e−16). The large anthers had a higher number of pollen grains, followed by intermediate and small ones (Fig. 10c); by contrast, no difference in number of pollen grains between large and intermediate anthers was recorded when considering all anthers of each type per flower (× 2 and × 4, respectively) (Fig. 10d). In addition, the area (X2 = 670.83, P < 2e−16) and thickness (X2 = 1353.50, P < 2e−16) of connective glands in small anthers were significantly higher than in intermediate and large ones (P < 0.05), with the latter two types not differing significantly from each other (Fig. 10e, f). For both species, we observed an inverse relationship between area of anthers and size of connective glands.
Discussion
Architecture and morphology of androecium
Our results show that the androecium in both species consists of heteromorphic stamens that differ mainly in size of anthers and connective glands. The particular position of anthers in anthesis suggests their specialized role. Thus, the two large anthers are located in the same position as the two posterior stigmas, whereas three intermediate anthers are located together with the anterior stigma. The other anthers (one intermediate and four small ones) lie in the centre of the flower. Therefore, these intermediate and small anthers would not become in contact with the same sites on the body of pollinators as those that the stigmas touch. Several works on heterantherous species have shown that the anthers involved in pollination and the stigmas touch the same areas of the pollinator body (Forbes 1882; Müller 1882; Dulberger 1981; Hrycan and Davis 2005; Luo et al. 2009; Paulino et al. 2016; Velloso et al. 2018). This functional partition of the pollinator body is needed to allow specialization of the anther functions (Vallejo-Marín et al. 2010).
On the other hand, we recorded variability in number of thecae in small anthers with large connective glands, in accordance with other species of this genus (Anderson 1997; Almeida and Mamede 2016). Apparently, the presence of heteromorphic anthers is a common and variable character in Stigmaphyllon and could have originated on different occasions within the genus (Anderson 1997; Almeida and Mamede 2016), probably due to the absence of selection pressure to retain them.
What is the function of the connective glands?
In both species, the anthers have similar glandular tissue in the connective in terms of structure, secretion and functioning, suggesting an important role in pollination, as previously proposed (Anderson 1979; Gates 1982; Vogel 1990; Possobom et al. 2015). Globular cells of connective glands are responsible for releasing the secretion. This feature is consistent with findings reported for species of Banisteriopsis C.B. Rob. and Diplopterys A. Juss. (Gates 1982; Possobom et al. 2015).
Mechanism by which connective glands release the secretion is still not clear. Previous works proposed that the presence of endothecial-like cells layers underlying connective glands would facilitate the release of secretion by the centripetal tension generated through anther dehiscence (Hufford and Endress 1989; Possobom et al. 2015). Similarities in tissue organisation of species analysed in this study support this viewpoint. We observed that one or two layers of endothecial cells are subjacent to this specialized epidermis and could contribute to secretion release. On the other hand, several species of Centris and Epicharis have been reported as effective pollinators for S. bonariense and S. jatrophifolium in Argentina (Torretta et al. 2017). During the stereotyped oil-collecting foraging, these bees become in contact with the anthers with the ventral area of the body; this fact probably produces a mechanical rupture of connective gland bubbles, and the secretion released is adhered directly to the pollinator body. For others species of Malpighiaceae, pollinators were found to provoke the mechanical rupture of the stigma cuticle and exposure of the stigmatic surface (Sigrist and Sazima 2004; Aliscioni et al. 2018). Thus, pollinators could cause a similar rupture in connective glands. Nevertheless, it is certain that mixed mechanisms are involved in secretion release, on the one hand, facilitated by tension generated through anther dehiscence and on the other hand, provoked by mechanical actions of pollinators.
The histochemical analysis of connective glands indicates the presence of mucilage because the connective gland cells and their secretion were stained with ruthenium red and brilliant cresyl blue. On the other hand, the positive results of NADI test may suggest that cells of connective glands produce essential oil or/and volatile terpenoids. The neutral red staining result shows that cuticle of cells of connective glands has open structure and allows secretion release. Thus, probably, connective gland plays the role as osmophore. Staining of the same gland with different chemical substances may result from a staining reaction with different cellular components that occur together in gland cells (Stern et al. 1986). In this case, it may be expected that the glands produce a mixed secretion that could suggest more than one function. Gates (1982) proposed that secretion of connective glands in Banisteriopsis (Malpighiaceae) acts as a glue to adhere pollen to the ventral surface of the body of pollinators. Later, Possobom et al. (2015) suggested a complex function for the glandular connectives in Diplopterys (Malpighiaceae), associating them with pollen mimicry (visual attraction), osmophores (chemical attraction) and adherence of pollen grains to pollinator body maintaining the dampness of the pollen grains by mucilage secretion during foraging trip. Although our results also suggest a mixed function, the primary function would be that of viscous substance (mucilage) allowing adhesion of pollen grains to the ventral part of the pollinator and protection from dehydration, favouring adherence to the stigma and subsequent germination, as Possobom et al. (2015) previously suggested. In this sense, we observed pollen grains of both species of Stigmaphyllon and other non-Malpighiaceae ones adhered to connective glands through the secretion. Likewise, the presence of a lipophilic droplet-like substance in cells of the connective gland could be interpreted as an energy-rich system that enables a high activity of the glands to produce the secretion (Stern et al. 1986). These results support our prediction that structure and functioning of connective glands and the nature of the secretion would contribute to the adhesion of pollen grains to the body of pollinator. Nevertheless, we do not discard the possibility that the glands can also play a role in chemical attraction of pollinators, as suggested by Possobom et al. (2015).
Are there differences in the anatomy and development of the anthers and pollen grains between the study species and among different types of anthers?
Characters shared by these species are anther wall development, which follows the basic type (Davis 1966), simultaneous divisions of the MMCs, tetrads with tetrahedral arrangement and pollen grains shed at the two-celled stage. Tetrad arrangement is a variable feature among few of the studied species of Malpighiaceae (Singh 1959; Siddiqui 1968; Johri et al. 1992). In contrast, the two-celled dispersal stage of pollen grains is a character shared by all the species studied so far (Singh 1959; Miyashita et al. 1964; Siddiqui 1968; Johri et al. 1992).
The tapetum cells exhibit differences: in S. jatrophifolium, the tapetal cells maintain their position and the tapetum is of secretory type, whereas in S. bonariense, they invade the locule and surround pollen grains without losing individuality; this type of tapetum is invasive non-syncytial (Pacini et al. 1985). By contrast, a tapetum forming a periplasmodium has been reported in other species of Stigmaphyllon (Johri et al. 1992). It is noteworthy that, although Malpighiaceae is a diverse family, general embryological descriptions have focused on a very limited number of species, and hence, there are no distinctive features that may be of systematic value (Singh 1959; Miyashita et al. 1964; Siddiqui 1968; Johri et al. 1992).
We observed that all flowers are hermaphrodites (i.e. with functional anthers) in S. jatrophifolium, whereas S. bonariense presents two floral morphs, hermaphrodites and functionally pistillate flowers (i.e. with indehiscent anthers). Some flowers have indehiscent anthers at mature pollen grains stage, and pollen grains may exhibit abnormal shapes and/or be completely collapsed. This event reveals that S. bonariense is a gynomonoecious species in all three studied populations. Hermaphroditism is a common condition in Neotropical Malpighiaceae (Anderson 1990); however, pseudo-hermaphroditism or functional dioecy has been reported in various genera and species (Anderson 1981, 1982; Steiner 1985). The number of pistillate flowers is positively related to perfect ones. It is known that geitonogamy (i.e. fertilization between flowers of the same individual) should increase with the number of open flowers (Geber 1985; Klinkhamer and de Jong 1993; Harder and Barrett 1995); however, the presence of pistillate flowers could reduce self-pollen availability in monoecious and gynomonoecious plants, thus, in these species, would be expected to be less selfed than hermaphrodites (Collin and Shykoff 2003). Therefore, we speculate that the condition observed in S. bonariense would be a strategy to reduce geitonogamy.
Despite the remarkable variation in size and morphology of heteromorphic stamens, the ontogeny and development of the anther wall and pollen grains reveal no variances among the three types of anthers in both species. In addition, we found no significant differences in stainability of pollen grains among types of anthers; hence, characteristics in the cytoplasm of pollen grains appear to be similar. Conversely, several authors have reported important differences related to anther dehiscence, endothecium cells and pollen viability between heteromorphic anthers in other families, revealing the diverse abilities of pollen to reach stigma, germinate and fertilize the ovules (Hrycan and Davis 2005; Luo et al. 2009; Paulino et al. 2016; Velloso et al. 2018).
The present results do not provide support for our second prediction due to the important differences found in tapetal cell development and sexuality of flowers between species and to identical ontogeny and development of pollen grains among the three types of anthers in each species. Although the variation in sexuality features between the studied Stigmaphyllon species might respond to different reproductive strategies, it would be premature to confirm this assumption.
Is there a division of labour of anthers of the studied Stigmaphyllon species?
Our results reveal differences in size and number of pollen grains among anther types in both species. The number of pollen grains produced in large anther is higher than in intermediate and small anthers. In several species with heteromorphic anthers, a greater amount of pollen was reported for larger anthers (Dulberger 1981; Hrycan and Davis 2005; Luo et al. 2009; Paulino et al. 2016; Velloso et al. 2018), with amount of pollen having been related to anther size (Smith and Evenson 1978; Rodríguez-Riaño et al. 1999; Paulino et al. 2016). However, considering the total number of pollen grains in each set of anthers per flower (four small, four intermediate, and two large anthers), there are no significant differences between large and intermediate anthers. Therefore, the latter two anther types contribute much more to pollen production than small anthers.
On the other hand, the size of connective glands varied inversely with anther size. These results reveal that small anthers with large connective glands could be more engaged in the production of the secretion (mucilage) than in supplying pollen for fertilization. This finding would be supported by the reduction or total absence of thecae, which is common in this type of anther.
So far, heterostemony has been suggested as result of functional differences between heteromorphic stamens (Müller 1881, 1882, 1883; Luo et al. 2009; Vallejo-Marín et al. 2009; Barrett 2010; Paulino et al. 2013, 2016; Velloso et al. 2018). These functional differences have been proposed as mechanisms for the solution of conflict known as the “pollen dilemma” (Westerkamp 1996; Luo et al. 2009; Vallejo-Marín et al. 2009; Barônio et al. 2016), related to explosive pollen release (Paulino et al. 2016) and/or visual attraction to pollinators (Hrycan and Davis 2005; Velloso et al. 2018). Our results showed that morphological and functional differences observed among stamens may reflect a specialization of anthers related to division of labour within androecium as strategies to improve pollen transport (better adherence and dampness maintenance). Therefore, there would be two sets of anthers depending on their functions: those destined for pollen production (large and intermediate anthers) and those intended to improve pollen adhesion through mucilage production (anthers with large connective glands). Nonetheless, the fact that we did not observe differences in anther wall and pollen grain development or stainability of pollen grains between anthers may reflect similarities in the capacity of fertilization of pollen grains produced by any anther. Therefore, all anthers may eventually contribute to pollen for pollination and mucilage secretion, although unevenly. Therefore, this condition would represent a transitional rather than a stable state in the evolution towards division of labour of anthers, and differences in anther wall development and viability of pollen grains between different sets of anthers might become more pronounced in a derived condition. In an evolutionary context, it would be difficult to test this because Stigmaphyllon is a very diverse genus and not fully phylogenetical solved and it belongs to stigmaphylloids clade which needs a better phylogenetic resolution (Davis et al. 2001; Davis and Anderson 2010).
According to the differential allocation of energy to reproductive structures of plants, it is known that different amounts of energy are invested in different parts of the stamen, androecium and/or the flower (Smith and Evenson 1978). Consequently, the differential abundance of pollen source among anthers in flowers of Stigmaphyllon may be explained by investment of more energy in the production of higher amount of pollen in the same places where the stigmas reside, the proper position to allow pollination on successive visits (Buchmann 1983; Michener 2007; Luo et al. 2009). Likewise, differential size of connective glands among anthers may be explained by investment of more energy in secretion production to improve adhesion of pollen to the pollinator body rather than the production of a large amount of pollen that, in any case, would not become in contact with the stigmas in next visits.
Conclusions
This is the first work that contributes with empirical evidence suggesting a specialization of anthers related to division of labour between heteromorphic stamens in two heterantherous species of Malpighiaceae. The main reasons explaining division of labour among different anthers are their size, amount of pollen produced by each anther and the size of their connective glands. Thus, each flower presents two sets of anthers according to their functions, those intended for the production of a large amount of pollen grains for pollination purposes and those engaged in the production of greater quantities of mucilage, which would improve pollen transport (better adherence and dampness maintenance). Nevertheless, in contrast to our predicted pattern of anther wall development, stainability and release of pollen grains were identical in the three anther types, suggesting that pollen grains of any anther present the same capacity of fertilization. Thus, heteranthery in both Stigmaphyllon species would be a transitional state towards the division of labour rather than a stable state in which these traits may display more pronounced differences.
References
Acevedo-Rodríguez P (1993) Systematics of Serjania (Sapindaceae). Part I: a revision of Serjania sect. Platycoccus. Mem New York Bot Gard 67:1–93
Ainsworth C (2000) Boys and girls come out to play: the biology of dioecious plants. Ann Bot 86:211–221
Aliscioni SS, Torretta JP (2017) Malpighiaceae. In: Zuloaga FO, Belgrano MJ (eds) Flora Argentina, vol 17. Sigma, Buenos Aires, pp 163–205
Aliscioni SS, Gotelli M, Torretta JP (2018) Structure of the stigma and style of Callaeum psilophyllum (Malpighiaceae) and its relation with potential pollinators. Protoplasma 255:1433–1442
Almeida RF, Mamede MCH (2016) Sinopse de Malpighiaceae no Estado do Espírito Santo, Brasil: Stigmaphyllon. A. Juss. Hoehnea 43:601–633
Anderson WR (1979) Floral conservatism in Neotropical Malpighiaceae. Biotropica 11:219–223
Anderson WR (1981) Malpighiaceae: the botany of Guayana Highland. Part XI. Mem New York Bot Gard 32:21–305
Anderson C (1982) A monograph of the genus Peixotoa (Malpighiaceae). Contr Univ Michigan Herb 15:1–92
Anderson C (1986) Novelties in Stigmaphyllon (Malpighiaceae). Syst Bot 11:120–130
Anderson WR (1990) The origin of the Malpighiaceae-the evidence from morphology. Mem New York Bot Gard 64:210–224
Anderson CE (1997) Monograph of Stigmaphyllon (Malpighiaceae). Syst Bot Monogr 51:1–313
Anderson CE (2011) Revision of Ryssopterys and transfer to Stigmaphyllon (Malpighiaceae). Blumea 56:73–104
Anderson WR, Anderson C, Davis CC (2006) Malpighiaceae. http://herbarium.lsa.umich.edu/malpigh/index.html [January 2019]
Barônio GJ, Maciel AA, Oliveira AC, Kobal RO, Meireles DA, Brito VL, Rech AR (2016) Plantas, polinizadores e algumas articulações da biologia da polinização com a teoria ecológica. Rodriguésia 67:275–293
Barrett SC (2010) Darwin’s legacy: the forms, function and sexual diversity of flowers. Philos Trans R Soc Lond Ser B Biol Sci 365:351–368
Barrios D, Flores J, González-Torres LR, Palmarola A (2015) The role of mucilage in the germination of Leptocereus scopulophilus (Cactaceae) seeds from Pan de Matanzas, Cuba. Botany 93:251–255
Bhandari NN (1984) The microsporangium. In: Jhori BM (ed) Embriology of angiosperms. Springer-Verlag, Berlin, pp 53–121
Buchmann SL (1983) Buzz pollination in angiosperms. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York
Buchmann SL (1987) The ecology of oil flowers and their bees. Annu Rev Ecol Evol Syst 18:343–369
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. C R Acad Sci 324:543–550
Collin CL, Shykoff JA (2003) Outcrossing rates in the gynomonoecious-gynodioecious species Dianthus sylvestris (Caryophyllaceae). Am J Bot 90:579–585
D'Ambrogio de Argüeso A (1986) Manual de técnicas en histología vegetal. Hemisferio Sur S.A, Buenos Aires
David R, Carde JP (1964) Coloration différentielle des pseudophylles de Pinmaritime au moyen du réactif de Nadi. C R Acad Sci 258:1338–1340
Davis G (1966) Systematic embryology of the angiosperms. Wiley, New York
Davis CC, Anderson WR (2010) A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. Am J Bot 97:2031–2048
Davis CC, Anderson WR, Donoghue MJ (2001) Phylogeny of Malpighiaceae: evidence from chloroplast ndhF and trnL-F nucleotide sequences. Am J Bot 88:1830–1846
Davis CC, Schaefer H, Xi Z, Baum DA, Donoghue MJ, Harmone LJ (2014) Long-term morphological stasis maintained by a plant–pollinator mutualism. Proc Natl Acad Sci U S A 111:5914–5919
Dulberger R (1981) The floral biology of Cassia didymobotrya and Cassia auriculata (Caesalpiniaceae). Am J Bot 68:1350–1360
Ferrucci MS, Anzótegui LM (1993) El polen de Paullinieae (Sapindaceae). Bonplandia 6:211–243
Forbes HO (1882) Two kinds of stamens with different functions in the same flower. Nature 26:386
Gates B (1982) Banisteriopsis, Diplopterys (Malpighiaceae). In: Flora Neotropica Monograph, vol 30. Hafner, New York, pp 1–237
Geber MA (1985) The relationship of plant size to self-pollination in Mertensia ciliata. Ecology 66:762–772
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Gonzalez AM, Cristóbal CL (1997) Anatomía y ontogenia de semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 9:287–294
González VV, Solís SM, Ferrucci MS (2017) Embryological studies of Magonia pubescens (Dodonaeaeae, Sapindaceae): development of male and female gametophytes in both floral morphs and its phylogenetic implications. Aust Syst Bot 30:279–289
Gottsberger G (1986) Some pollination strategies in Neotropical savannas an forest. Pl Syst Evol 152:29–45
Harder LD (1990) Pollen removal by bumble bees and its implications for pollen dispersal. Ecology 71:1110–1125
Harder LD, Barrett SC (1995) Mating cost of large floral displays in hermaphrodite plants. Nature 373:512
Hrycan WC, Davis AR (2005) Comparative structure and pollen production of the stamens and pollinator-deceptive staminodes of Commelina coelestis and C. dianthifolia (Commelinaceae). Ann Bot 95:1113–1130
Hufford LD, Endress P (1989) The diversity of anther structures and dehiscence patterns among Hamamelididae. Bot J Linn Soc 99:301–346
Johansen DA (1940) Plant Microtechnique. McGraw-Hill BooK Co, New York
Johri BM, Ambegaokar KB, Srivastava PS (1992) Comparative embriology of angiosperms, vol 1. Springer–Verlag, Berlin, pp 1–614
Kaul ML (2012) Male sterility in higher plants, vol 10. Springer Science & Business Media
Klinkhamer PG, de Jong TJ (1993) Attractiveness to pollinators: a plant's dilemma. Oikos 66:180–184
Luo ZL, Gu L, Zhang DX (2009) Intrafloral differentiation of stamens in heterantherous flowers. J Syst Evol 47:43–56
Luque R, Sousa HC, Kraus JE (1996) Métodos de coloração de Roeser (1972) modificado-E Kropp (1972), visando a substituição do Azul de Astra por Azul de Alcião 8GS ou 8GX. Acta Bot Bras 10:199–212
Michener CD (2007) The bees of the world, 2nd edn. The Johns Hopkins University Press, Baltimore
Miyashita RK, Nakasone HY, Lamoureux CH (1964) Reproductive morphology of acerola (Malpighia glabra L.). University of Hawaii. Hawaii Agricultural Experiment Station 63:1–31
Múlgura de Romero ME (2005) Malpighiaceae. In: Burkart AE, Bacigalupo NM (eds) Flora Ilustrada de Entre Ríos (Argentina): dicotiledóneas arquiclamídeas. Geraniales a Umbeliflorales, Instituto Nacional de Tecnología Agropecuaria, Secretaría de Agricultura, Ganadería, Pesca y Alimentos, Buenos Aires, pp 71–86
Müller H (1881) Two kinds of stamens with different functions in the same flower. Nature 24:307–308
Müller H (1882) Two kinds of stamens with different functions in the same flower. Nature 26:30
Müller F (1883) Two kinds of stamens with different functions in the same flower. Nature 27:364–365
Neff JL, Simpson BB (1981) Oil-collecting structures in the Anthophoridae (Hymenoptera): morphology function and use in systematics. J Kansas Entomol Soc 54:95–123
O’Brien TP, McCully ME (1981) The study of plant structure principles and selected methods. Termarcarphi Pty. Ltd., Melbourne
Pacini E, Franchi GG, Hesse M (1985) The tapetum: its form, function, and possible phylogeny in Embryophyta. Pl Syst Evol 149:155–185
Paulino JV, Mansano VF, Teixeira SP (2013) Elucidating the unusual floral features of Swartzia dipetala (Fabaceae). Bot J Linn Soc 173:303–320
Paulino JV, Mansano VF, Prenner G (2016) Evidence for division of labor and division of function related to the pollen release in Papilionoideae (Leguminosae) with a heteromorphic androecium. Int J Bot 177:590–607
Possobom CCF, Guimaraes E, Machado SR (2015) Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora 211:26–39
R Core Team (2013) R: a language and environment for statistical computing. (R Foundation for Statistical Computing: Vienna, Austria) Available at: http://www.r-project.org
Radford AE, Dickison WC, Massey JR, Bell CR (1974) Vascular plant systematics. Harper & Row Publishers, New York
Rego MMC, Albuquerque PMC (1989) Comportamento das abelhas visitantes de múrice, Byrsonima crassifolia (L.) Kunth, Malpighiaceae. Bol Mus Para Emílio Goeldi Série Zoologia 5:179–193
Reis MG, Faria AD, Alves-dos-Santos I, Amaral MCE, Marsaioli AJ (2007) Byrsonic acid—the clue to floral mimicry involving oil-producing flowers and oil-collecting bees. J Chem Ecol 33:1421–1429
Renner SS, Schaefer H (2010) The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philos Trans R Soc Lond Ser B Biol Sci 365:423–435
Rodríguez-Riaño T, Ortega-Olivencia A, Devesa JA (1999) Types of androecium in the Fabaceae of SW Europe. Ann Bot 83:109–116
Sazima M, Sazima I (1989) Oil-gathering bees visit flowers of eglandular morphs of the oil-producing Malpighiaceae. Bot Acta 102:106–111
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16:46–60
Siddiqui SA (1968) The microsporangium and the male gametophyte in Malpighia coccigera Linn. Beitr Biol Pflanzen 44:361–364
Sigrist MR, Sazima M (2004) Pollination and reproductive biology of twelve species of Neotropical Malpighiaceae: stigma morphology and its implications for the breeding system. Ann Bot 94:33–41
Simpson BB, Neff JL (1981) Floral rewards: alternatives to pollen and nectar. Ann Missouri Bot Gard 68:301–322
Singh B (1959) Studies in the Malpighiaceae I. morphology of Thryallis glauca Kuntze. Hort Adv 3:1–19
Smith CA, Evenson WE (1978) Energy distribution in reproductive structures of Amaryllis. Am J Bot 65:714–716
Solís SM, Galati B, Ferrucci MS (2010) Microsporogenesis and microgametogenesis of Cardiospermum grandiflorum and Urvillea chacoensis (Sapindaceae, Paullinieae). Aust J Bot 58:597–604
Steiner KE (1985) Functional dioicism in the Malpighiaceae: Spachea membranacea Cuatr. Am J Bot 72:1537–1543
Stern WL, Curry KJ, Whitten WM (1986) Staining fragrance glands in orchid flowers. Bull Torrey Bot Club 113:288–297
Teixeira LAG, Machado IC (2000) Sistema de polinização e reprodução de Byrsonima sericea DC (Malpighiaceae). Acta Bot Bras 14:347–357
Torretta JP, Aliscioni SS, González-Arzac A, Avalos AA (2017) Is the variation of floral elaiophore size in two species of Stigmaphyllon (Malpighiaceae) dependent on interaction with pollinators? Plant Ecol Divers 10:403–418
Vallejo-Marín M, Manson JS, Thomson JD, Barrett SC (2009) Division of labour within flowers: heteranthery, a floral strategy to reconcile contrasting pollen fates. J Evol Biol 22:828–839
Vallejo-Marín M, Silva EM, Sargent RD, Barrett SC (2010) Trait correlates and functional significance of heteranthery in flowering plants. New Phytol 188:418–425
Velloso MSC, Brito VLG, Caetano APS, Romero R (2018) Anther specializations related to the division of labor in Microlicia cordata (Spreng.) Cham. (Melastomataceae). Acta Bot Bras 32:349–358
Vinson SB, Frankie GW, Williams HJ (1996) Chemical ecology of bees of the genus Centris (Hymenoptera: Apidae). Fla Entomol 79:109–129
Vogel S (1974) Ölblumen und ölsammelndeBienen. Trop Subtrop Pflanzenwelt 7:1–267
Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Mem New York Bot Gard 55:130–142
Westerkamp CH (1996) Pollen in bee-flower relations some considerations on melittophily. Botanica Acta 109:325–332
Zarlavsky GE (2014) Histología Vegetal: técnicas simples y complejas. Sociedad Argentina de Botánica, Buenos Aires
Zini ML, Galati GB, Solís SM, Ferrucci MS (2012) Anther structure and pollen development in Melicoccus lepidopetalus (Sapindaceae): an evolutionary approach to dioecyin the family. Flora 207:712–720
Acknowledgments
We thank H. J. Marrero for his support on statistical analysis, N. A. Ramirez for helping with the statistical figures and S. S. Aliscioni for the constructive comments on our work.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Adan Alberto Avalos. The first draft of the manuscript was written by Adan Alberto Avalos, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Funding information
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP N° 11220170100429C) and the Universidad Nacional del Nordeste (PI N° 15-A002), Argentina.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Dorota Kwiatkowska
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avalos, A.A., Pablo, T.J., Lattar, E.C. et al. Structure and development of anthers and connective glands in two species of Stigmaphyllon (Malpighiaceae): are heteromorphic anthers related to division of labour?. Protoplasma 257, 1165–1181 (2020). https://doi.org/10.1007/s00709-020-01497-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-020-01497-x