Abstract
The migration of nuclei between plant cells (cytomixis) is a mysterious cellular phenomenon frequently observable in the male meiosis of higher plants. Cytomixis attracts attention because of unknown cellular mechanisms underlying migration of nuclei and its potential evolutionary significance, since the genetic material is transferred between the cells that form pollen. Although cytomixis was discovered over a century ago, the advance in our understanding of this process has been rather insignificant because of methodological difficulties. The data that allowed for a new insight into this phenomenon were obtained by examining the migrating nuclei with electron and confocal laser microscopy, immunostaining, and fluorescence in situ hybridization. As has been shown, the chromatin migrating between cells is surrounded by an undamaged nuclear membrane. Such chromatin does not undergo heterochromatization and contains normal euchromatin markers. The condensation degree of the migrating chromatin corresponds to the current meiotic stage, and normal structures of synaptonemal complex are present in the migrating part of the nucleus. The cells involved in cytomixis lack any detectable morphological and molecular markers of programmed cell death. It has been shown that individual chromosomes and genomes (in the case of allopolyploids) have no predisposition to the migration between cells, i.e., parts of the nucleus are involved in cytomixis in a random manner. However, the fate of migrating chromatin after it has entered the recipient cell is still vague. A huge amount of indirect data suggests that migrating chromatin is incorporated into the nucleus of the recipient cell; nonetheless, the corresponding direct evidences are still absent. No specific markers of cytomictic chromatin have been yet discovered. Thus, the causes and consequences of cytomixis are still disputable. This review briefs the recent data on the relevant issues, describes the classical and modern methodological approaches to analysis of the intercellular migration of nuclei, and discusses the problems in cytomixis research and its prospects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytomixis is the migration of nuclei or their fragments between plant cells. This phenomenon is most frequently observed in male meiosis and has been so far described in the microsporogenesis of over 400 higher plant species (Pierre and Sousa 2011; Mursalimov et al. 2013b; Gupta et al. 2017). The migration of nuclei between cells attracts attention because of the yet unknown mechanisms allowing the nuclei to pass through the cell wall and a putative evolutionary significance of cytomixis, since the transfer of genetic material between meiocytes can change the karyotype of produced pollen. Cytomixis was described over a century ago (Arnoldy 1900; Gates 1911) and the numerous attempts to clarify this phenomenon commenced after its discovery. However, this issue has not been considerably clarified until recently. First and foremost, this is associated with methodological difficulties. The key problem when analyzing cytomixis is the lack of the methods to trace a meiocyte from the moment it receives additional chromatin to development of a pollen grain and gametes. The causes thereof stem from limited possibilities to intravitally examine plant male meiocytes, because these cells are poorly culturable and do not form gametes in vitro. The cell layers surrounding meiocytes in an intact anther interfere with examination of meiocytes in vivo. In turn, the observations with fixed cells do not allow analyzing the dynamics of intercellular nuclear migration, and the consequences of such migration cannot be evaluated for these killed cells. The situation is complicated by the fact that any specific markers of cytomictic (migrating) chromatin have not been found so far. In the context of these difficulties, most studies into cytomixis are of a descriptive character and their conclusions rely on indirect data. The deficiency in experimental data explains the fact that the researchers still cannot agree on the causes of this phenomenon and its role in plant development.
The mentioned problems together with a considerable interest to this issue suggest discussing the new data on cytomixis obtained by electron and confocal laser scanning microscopies (CLSM) as well as immunostaining and fluorescence in situ hybridization (FISH).
Several reviews on cytomixis have been published earlier. These reviews focused on a phenomenological description of nuclear migration and the prevalence of this phenomenon in different plant species (Lone and Lone 2013; Mandal et al. 2013; Mursalimov et al. 2013a). In its turn, this review is focused on the methodological approaches that at different moments have been used to study this phenomenon and the experimental data obtained recently. In addition, we compared here the cytomixis in plant meiosis with the analogous phenomenon observable in the animal cell. The key facts about cytomixis, the doubts arising when analyzing this phenomenon, and the prospects in cytomixis research are discussed.
Facts
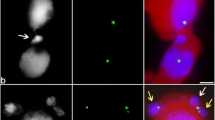
The cytological pattern of cytomixis has been described for many plant species using various methodological approaches (Fig. 1). In general, the migration of nuclei between cells can be described as follows. At the stage of zygo-pachytene in meiotic prophase I, the nucleus leaves the cell central region and shifts to one of the cell walls to the area with clustered cytomictic channels (CCs). As a rule, two such regions of CC clustering, residing at the cell poles, are observed (Kolczyk et al. 2015; Mursalimov et al. 2017a). It is unknown how the nucleus selects the direction of its movement to either pole. When approaching the cell wall, the nucleus elongates and starts to penetrate to a neighboring cell through CC (Fig. 1, arrows). In this process, the nucleus can pass through the cell wall either via one CC (Fig. 1a–e, g, j, l, n) or concurrently via several CCs (Fig. 1m). When passing through a CC, the nucleus is subject to a considerable compression, making the chromatin inside to look as a dark-colored structureless mass; however, the initial chromatin structure is restored after the chromatin leaves the CC and enters the recipient cell cytoplasm (Fig. 1b, j, m). In most cases, the nucleus does not pass to the recipient cell as a whole. Once the CC is passed, parts of the nucleus bud off to form one or several micronuclei in the recipient cell cytoplasm, whereas the larger part of the migrating nucleus remains in the donor cell. The situation when a whole nucleus migrates to the recipient cell to form a binucleated meiocyte is rarer (Sidorchuk et al. 2007a, 2016; Singhal and Kumar 2008; Tsvetova and Elkonin 2013; Mursalimov and Deineko 2015). Interestingly, the size of both the donor and recipient cells does not considerably change during cytomixis between cells of the dicot plants, i.e., only the nucleus migrates in one direction. When a binucleated meiocyte and an empty enucleate cell are formed as a result of cytomixis, they have approximately equal size (Sidorchuk et al. 2007a; Mursalimov and Deineko 2015). However, the cases of unidirectional migration not only of the nucleus, but also of the cell cytoplasm, are frequently observed in the monocot meiosis (Barton et al. 2014; Sidorchuk et al. 2016); correspondingly, the recipient cell considerably increases in its size, while the donor cell becomes smaller (Fig. 1f). As a rule, the nucleus migrates from one donor cell to one recipient cell (Fig. 1). However, several cells can be simultaneously involved in this process in some cases. For example, a nucleus from one donor cell can migrate simultaneously into two cells and as a result, it will be divided into two parts located in different recipient cells. Two nuclei of adjacent donor cells can migrate to one recipient cell that has its own nucleus, forming the cell containing chromatin of three cells. (Li et al. 2009; Kumar et al. 2013; Malik et al. 2017). In some species, cytomixis can shift to later meiotic stages, for example, to the second division (Fig. 1d), or even to the tetrad stage (Ressayre et al. 2003; Kumar et al. 2010). Although individual characteristics of the nuclear migration can considerably differ between plant species, the described cytological pattern of cytomixis is common for all cases.
Cytomixis in plant male meiocytes. a Squashed tobacco meiocytes, light microscopy (LM), carmine staining (Mursalimov and Deineko 2015). b Squashed meiocytes of Brachiaria dura, LM, carmine staining (Risso-Pascotto et al. 2009). c Squashed meiocytes of Crotalaria micans, LM, Giemsa staining (Ferreira et al. 2009). d Squashed meiocytes of Houttuynia cordata, LM, carmine staining (Guan et al. 2012). e A whole anther of the Thinopyrum intermedium/wheat hybrid squashed on a slide, LM, carbol fuchsin staining (Li et al. 2009). f Squashed rye meiocytes, LM, carmine staining (Sidorchuk et al. 2016). g Squashed tobacco meiocytes, fluorescent microscopy (FM); chromatin is blue; H3S10ph, green; H3K27me2, red; and cytoplasm, yellow (Mursalimov et al. 2015). h Squashed wheat meiocytes, FM; chromatin is green and microtubules, orange (Barton et al. 2014). i Squashed rye meiocytes, FM; chromatin is blue and microtubules, green (Sidorchuk et al. 2016). j Unsquashed tobacco meiocytes, CLSM; chromatin is red and cell wall, blue-violet (Mursalimov et al. 2017a). k Tissue section of a tobacco anther embedded in methacrylate, FM; chromatin is blue and cell wall, white (Sidorchuk et al. 2007b). l Tissue section of a tobacco anther embedded in polyethylene glycol, FM; chromatin is blue; cell wall, bright green, and H3S10ph, red (Mursalimov et al. 2015). m Ultrathin section of a tobacco anther embedded in epoxy resin, transmission electron microscopy (Mursalimov et al. 2015). n Lilium meiocytes coated with gold, scanning electron microscopy (Whelan 1974). Arrows denote the part of the nucleus migrating through CC. a–c, e–n Zygo-pachytene stage. d Metaphase II. The figures are adapted for this review (arrows, letters and bars are replaced). Bars, 1 μm in b; 2 μm in d and m; 5 μm in a, c, g, and l; and 10 μm in f, h–j, and n
The following data are the most important among the overall information about this phenomenon obtained so far: (i) the nuclei migrate from cell to cell through CCs rather than plasmodesmata, (ii) all components of the nucleus migrating between cells are surrounded by the nuclear membrane, (iii) cytomictic chromatin displays no signs of inactivation or damage, and (iv) the chromatin migration is a random process.
-
(i)
The nuclei migrate from cell to cell through CCs rather than plasmodesmata
Plasmodesmata are too small and have a complex inner structure for cell organelles to pass through. The intercellular migration of organelles becomes feasible only after CCs are formed; CCs are large (in some cases, up to 3500 nm) channels in the cell wall of plant male meiocytes (Wang et al. 2002). CCs have a simple inner structure, being merely the holes in the cell wall lined with the plasma membrane common for two cells. In the majority of the studied cases, CCs are formed based on plasmodesmata. However, CC formation in the cell wall regions lacking plasmodesmata has been also described. It is assumed that the mechanisms of CC formation are species-specific and depend on the stage of meiosis (Wang et al. 2002; Yu et al. 2004; Wang et al. 2006; Mursalimov et al. 2010).
-
(ii)
All components of the nucleus migrating between cells are surrounded by the nuclear membrane
Many researchers still defined cytomixis as the migration of chromatin/chromosomes between cells (De Storme and Mason 2014; Kumar and Singhal 2016a, b; Qiu et al. 2017). However, this definition is not completely correct. Electron microscopy studies have shown that the chromatin migrates between cells within the nucleus together with other nuclear components, namely, the nucleolus, nuclear matrix, etc., surrounded by the nuclear membrane (Fig. 1m, n). Micronuclei formed as a result of cytomixis also have their individual nuclear membrane with a typical bilayer structure and no visible signs of damage (Feijo and Pais 1989; Mursalimov and Deineko 2011; Qiu et al. 2017). Light microscopy data confirm these observations. For example, visualization of the tubulin cytoskeleton allows for a distinct image of the nuclear zone that encompasses the migrating chromatin (Fig. 1i).
In some cases, cytomixis is detectable in the cells at meta-anaphase I or II, when the nuclear membrane is disintegrated (Fig. 1d). At the first glance, the facts of this kind contradict the statement that the chromatin in cytomixis migrates between cells within the nucleus surrounded by the nuclear membrane. However, we should keep in mind that these facts are observed in fixed material (Fig. 1d), which do not allow the dynamics of the process to be assessed in full. In our view, the nucleus in such cells just has not passed through the CC before disappearance of the nuclear membrane. Thus, the migrating chromatin is stopped in the CC without the possibility to complete migration.
-
(iii)
Cytomictic chromatin displays no signs of inactivation or damage
The fate of migrating chromatin directly depends on its integrity and functional state. The absence of visual differences between the migrating chromatin and the chromatin of intact nuclei has been repeatedly reported (Feijo and Pais 1989; Li et al. 2009; Risso-Pascotto et al. 2009; Mursalimov and Deineko 2011). On the other hand, some authors directly link cytomixis with elimination of damaged chromatin or whole cells (Giorgetti et al. 2007; Kalinka et al. 2010; Kravets 2011; Kravets 2013). However, reliable experimental data describing the functional state and integrity of migrating chromatin were absent until recently. The analysis of posttranslational histone modification as well as a set of experiments on detection of programmed cell death (PCD) in the tobacco male meiocytes involved in cytomixis has demonstrated that cytomictic chromatin is not subject to inactivation or damage and any PCD markers are undetectable in these cells (Mursalimov et al. 2015, 2017b).
Analysis of posttranslational histone modification is widely used for assessing the functional state of chromatin, degree of its condensation, and the presence of DNA breaks (Fuchs and Schubert 2012; He et al. 2014). It is known that damaged and inactivated chromatin, subject to elimination, has its specific characteristics. In particular, such chromatin loses the euchromatin markers (H3K4me2, H3K4me3, etc.) and acquires the heterochromatin ones (H3K9me2, H3K27me2, etc.). Also, eliminated chromatin becomes TUNEL-positive (Goday and Pigozzi 2010; Schoenmakers et al. 2010). Immunostaining has demonstrated that such changes in histone modification are absent in the cytomictic chromatin. The migrating chromatin displays signs of transcriptional activity, as has been shown using six euchromatin markers. Euchromatin markers are also retained in the micronuclei formed after cytomixis in recipient cells. These data are confirmed by analysis of the distribution of three heterochromatin markers in migrating chromatin. Chromatin is neither selectively heterochromatized before migration to another cell nor inactivated in the micronuclei after cytomixis. TUNEL, Comet, and DNA internucleosomal fragmentation assays in tobacco meiocytes have shown the absence of PCD molecular markers in the migrating chromatin and the micronuclei formed as a result of cytomixis as well as in the main nuclei of recipient cells (Mursalimov et al. 2015, 2017b). Ultrastructural examination demonstrated the absence of morphological PCD signs in the cells involved in cytomixis (Feijo and Pais 1989; Mursalimov and Deineko 2011).
-
(iv)
The chromatin migration is a random process
The search of regular patterns in the intercellular migration of individual chromosomes was also for the first time performed in tobacco meiosis (Mursalimov and Deineko 2017). For tobacco karyotyping, a combination of various FISH probes that together make it possible to identify all chromosomes are usually used (Lim et al. 2000; Shibata et al. 2013). For identification of the tobacco chromosomes migrating between meiocytes in cytomixis, a combination of four chromosome-specific markers, NTRS, 5S rDNA, GRS, and HSR60, was used. These markers allowed analyzing the distribution of 15 of the 24 chromosomes of the tobacco haploid genome. The distribution of tobacco S and T genomes was analyzed by genomic in situ hybridization (GISH). The probes for FISH and GISH were hybridized to the nuclei of intact tobacco meiocytes, the migrating nuclei, and the micronuclei formed as a result of cytomixis. Analysis of the distribution of FISH and GISH signals demonstrates that the chromatin migration during cytomixis is not purposeful. Any predisposition to the intercellular migration of individual tobacco chromosomes or genome is absent. Analogous data have been obtained for the male meiosis of polyploid tobacco plants. As has been shown, an increase in the ploidy level does not change the patterns of chromosome distribution in migrating chromatin: migration retains its random character (Mursalimov and Deineko 2017).
Thus, using tobacco plants as a model, it was demonstrated that the chromatin migration during cytomixis is a random process and the migrating chromatin is neither inactivated nor damaged. Assuming that migrating chromatin can be incorporated into the nucleus of recipient cell, cytomixis can be regarded as a mechanism of random recombination rather than a targeted process of the plant karyotype changing.
Doubts
The most important questions in the research into cytomixis are whether (i) cytomixis is a natural process, (ii) the chromatin migrates between cells as intact chromosomes/bivalents, (iii) it is possible to label cytomictic chromatin, (iv) the intercellular migration of nuclei takes place in animal cells, (v) cytomixis is a norm or a pathology, and (vi) cytomixis is a cause of changes in the plant karyotype. We attempted to answer these questions in maximum detail based on the currently available data.
-
(i)
Is cytomixis a natural process?
Cytomixis has been repeatedly described in various plant species from the moment it was discovered. However, some researches encountering this phenomenon still tend to regard it as an abnormality or even as an artifact that emerged during material preparation rather than a natural process. As a rule, the researchers who are skeptic about a natural character of cytomixis refer to experimental works published half a century ago that describe cytomixis in plant cells as a consequence of a mechanical injury (Tarkowska 1965, 1966, 1973). We believe that it is a high time to clarify once and for all whether cytomixis is an artifact or not. For this purpose, it is first and foremost necessary to analyze the data described in the abovementioned papers. Tarkowska (1973) asserts that “Cytomixis is an abnormal phenomenon of a pathological nature” and “It was possible to induce cytomixis patterns experimentally only by some mechanical stimuli applied in a particular way, such as squashing, piercing with a blunt needle and cutting with a blunt blade.” However, the author provides most unconvincing evidences for these statements. The experiments used for the conclusions on an artificial nature of cytomixis were performed in the following way. Unfixed anthers and other plant tissues were squashed between two glass slides and then placed in a fixing agent. As the author saw it, cytomixis in plant tissues appeared after this particular manipulation (Tarkowska 1965, 1966, 1973). Tarkowska (1973) describes the analyzed cells in the following way: “It appears that cells from which chromatin migrates demonstrate all the symptoms of dying and death. Cells to which chromatin has intruded are usually also dead or in very few instances still alive, yet with obvious signs of disorganization.” As we see it, the results of an experiment when unfixed cells are first crushed and then fixed and examined are rather unconvincing; however, even based on such analysis, it is impossible to make any inferences without the control observations. When describing the control material, Tarkowska (1966, 1973) points that cytomixis could be observed in intact (undamaged) cells as well but explains this by that the samples were prepared insufficiently accurately. Moreover, the author explains the fact that cytomixis had been observed by other researchers with the same insufficient accuracy. In addition, Tarkowska admits that cytomixis is detectable only at the stage of prophase I zygo-pachytene even after squashing of the whole anther at different stages of meiosis. This contradicts Tarkowska’s concept that cytomixis is an artifact, since if cytomixis were caused by cell squashing, it would be observable at all meiotic stages with an equal probability. Note in conclusion that the mentioned papers lack any statistical data that would allow for estimation of how comprehensively the experimental and, what is the most important, control materials were examined.
Thus, it is necessary to recognize that the experimental results by Tarkowska are not reliable and the corresponding conclusions are not correct. Hence, we hope that these papers will not be further used as an argument in the discussion of an unnatural character of cytomixis. It is important to emphasize that the results of Tarkowska have not been confirmed by other researchers; on the contrary, hundreds of published research papers demonstrate that cytomixis is not an artifact (Supplemental Table 1).
Supplemental Table 1 briefs different methodological approaches used for studying cytomixis. Most papers on cytomixis are based on squashed preparations and routine staining with carmine, orsein, hematoxylin, etc. These studies have given the insight into the cytological pattern of cytomixis as well as its rate in various plant species and forms (Silva et al. 2006; Li et al. 2009; Kumar and Srivastava 2013; Malik et al. 2017, etc.). Specific staining has allowed for a more detailed analysis of the chromatin and other cell components (cell wall, cytoskeleton, nucleolus, etc.) in the meiocytes involved in cytomixis (Sidorchuk et al. 2007b, 2016; Barton et al. 2014). Examination of the tissues embedded in solid media has given the insight into the native structure of cells during cytomixis that have not been subject to any mechanism impacts. In particular, cytomixis has been studied in the cells embedded in methacrylates (Fig. 1k), polyethylene glycol (Fig. 1l), and epoxy resin (Fig. 1m). The study of cytomixis in sections has not detected any differences in the cytological pattern as compared with the squashed preparations (Sidorchuk et al. 2007b; Mursalimov et al. 2015). Ultrastructural examination of the tissues embedded in epoxy resin has played a special role in the study of cytomixis. Numerous studies of cytomixis in different plant species were performed by transmission electron microscopy. Ultrastructural analysis has allowed for a comprehensive description of the processes underlying CC formation and the migration of nuclei through these channels (Feijo and Pais 1989; Polowick and Sawhney 1992; Wang et al. 2004; Yu et al. 2004; Mursalimov and Deineko 2011). Unfortunately, we know only one single study that used a scanning electron microscope for examination of cytomixis (Whelan 1974).
A serious disadvantage when analyzing squashed cells and tissue sections is the inability to observe a native three-dimensional structure of the whole cell. This problem can be solved using CLSM. Using this approach, a three-dimensional structure of the meiocytes involved in cytomixis was observed in unsquashed preparations (Fig. 1j). The fixed cells were stained with fluorescent dyes, specific for the cell wall and chromatin, and examined under microchamber conditions. The same approach has made it possible to demonstrate that the CCs are located on the cell wall surface in a nonrandom manner (Mursalimov et al. 2017a).
Noteworthy is the only paper describing the analysis of nuclear migration between living cells with the help of time-lapse microscopy (Zhang et al. 1990). The authors observed a nuclear migration similar to that described in male meiocytes in wheat endosperm cells. The authors not only for the first time observed the migration of nuclei between living plant cells, but also demonstrated that the nucleus can return to the initial cell after migration (Zhang et al. 1990). The observation of cytomixis in living cells is the most important evidence demonstrating that cytomixis is a natural process rather than a consequence of sample processing.
It is difficult to say to what degree the nuclear migration in the endosperm cells is similar to the analogous process in male meiocytes. Cytomixis has been also detected in some other tissues, in particular, meristems and tapetum (Guzicka and Wozny 2005; Kuras et al. 2006; Papini et al. 2010; Mandal and Nandi 2017; Silva et al. 2017), but these were accidental findings; no targeted research has been done. Correspondingly, little is known about the specific features of this process. The nuclear migration in somatic cells has no long-term genetic consequences except for the cases of migration in the meristematic tissue further involved in development of generative organs (Guzicka and Wozny 2005). Presumably, the nuclear migration in somatic cells can lead to a mixoploidy and play an important role in tissue grafting. As is shown, hybrid cells displaying characteristics of both parents can appear in the intergrowth of plant grafts belonging to different species. Moreover, viable hybrid plants can be regenerated from such hybrid cells. It is assumed that the intercellular migration of DNA-containing organelles is actively involved in the grafting process (Stegemann and Bock 2009; Stegemann et al. 2012; Thyssen et al. 2012; Fuentes et al. 2014; Gurdona et al. 2016).
Concluding this section, we cannot but brief the history of our studies into cytomixis. At the very beginning of the work on nuclear migration in tobacco male meiosis, we had natural doubts that squashed preparations could provide a correct data. In order to ascertain that the observed picture was adequate, we selected the method that would almost exclude any injuries of tissues or postmortem changes in them. We used instant cryofixation of whole anthers in liquid propane with subsequent freeze substitution with methanol and 0.1% glutaraldehyde. Then, the specimens were embedded in methacrylates, sectioned, and examined (Deineko, unpublished data). The further research into cytomixis was continued only after a comprehensive analysis of the sections that completely confirmed the results obtained with squashed preparations.
Thus, since the works by Tarkowska, cytomixis has been studied by most of the standard cytological methods and none of these studies has shown induction of nuclear migration during sample processing before analysis. We hope that the described data are sufficient to convince that cytomixis is a natural process and that the discussion on an artificial nature of cytomixis has eventually come to its end.
-
(ii)
Does chromatin migrate between cells as intact chromosomes/bivalents?
It is evident that for cytomixis to change the karyotype of produced pollen, whole chromosomes (bivalents in prophase I) with their functional centromeric and telomeric regions should migrate between cells. Only in this case, the chromosomes have any chance to become a constant part of the recipient cell nucleus. If chromosomes are fragmented as a result of migration, their fragments can at best become B chromosomes or, more likely, will be eliminated. Currently, any direct evidence that the migrating chromosomes retain their integrity is absent but certain indirect data suggest such possibility.
Analysis of the posttranslational histone modification has shown that the migrating chromatin is phosphorylated in a correct manner matching the current meiotic stage (Mursalimov et al. 2015), which is of a key importance for chromosome condensation, cohesion, and segregation in dividing cells (Manzanero et al. 2000; Houben et al. 2005; Houben et al. 2007; Kawashima et al. 2010). It is shown that the migrating chromatin is phosphorylated in a correct manner before migration, being in the donor cell, and after migration to the recipient cell. For example, the signal of histone H3 phosphorylation at serine 10, which is normally detectable in the pachytene as individual loci and spreads over the entire chromosome length by metaphase I (Manzanero et al. 2000). As has been demonstrated, analogous changes take place in the migrating chromatin. H3S10ph signal in prophase I is detected as individual loci in the migrating nuclei and micronuclei formed as a result of cytomixis (Fig. 1g, l). In metaphase I, the chromatin in cytomictic micronuclei is phosphorylated over its entire length similar to the chromatin in the main nucleus. Thus, the recipient cell “does not see” the difference between its own chromatin and the chromatin that came from another cell, i.e., the content of cytomictic micronuclei is modified in the same manner as the content of the main nucleus. Analysis of the histone H3 phosphorylation at serine 28, threonine 11 and histone H2A at threonine 121 demonstrates that the migrating chromatin in these characteristics also does not differ from the intact chromatin (Mursalimov et al. 2015). This suggests that the normal processes of chromatin condensation and cohesion of chromosomes take place in the cytomictic chromatin, which should lead to bivalent formation.
The bivalent formation in migrating chromatin is confirmed by the data on histone H2AX phosphorylation at serine 139 (γH2AX), which is a marker for the repaired DNA double-strand breaks (Rogakou et al. 1999; Xiao et al. 2009). Analysis of this type of histone modification demonstrates the presence of γH2AX signals in the migrating chromatin in the zygo-pachytene stage. Similar γH2AX signals are also detectable in the intact nuclei (Mursalimov et al. 2015). The presence of γH2AX signals at this meiotic stage is associated with the recombinant processes (Hunter et al. 2001; Chicheportiche et al. 2007; He et al. 2016; Xue et al. 2016). The amount of γH2AX signals does not increase in the migrating chromatin or cytomictic micronuclei as compared with the control. The fact that γH2AX signal is present in the migrating chromatin at this meiotic stage suggests that the recombinant processes similar to those in the intact nuclei take place there. However, recombination is possible only if bivalents have formed. Electron microscopy examination and immunostaining of the synaptonemal complex (SC) proteins ZIP1 and ASY1 in tobacco meiocytes confirm the presence of SC and bivalent formation in the migrating chromatin (Mursalimov et al. 2015). The functional state of centromeres and telomeres in the migrating chromatin has not been checked yet.
Thus, the migrating chromatin is subject to the modifications that are necessary for correct condensation, cohesion, and segregation of chromosomes matching the current meiotic stage. The migrating chromatin contains SC and is involved in recombination. These data allow us to infer that intact bivalents migrate between cells in cytomixis.
-
(iii)
Is it possible to label cytomictic chromatin?
Selective marking of the migrating chromatin has a key importance for analyzing its fate and clarifying the presence or absence of genetic consequences of cytomixis. However, any specific markers for migrating chromatin have not been discovered so far. Until such markers are found, researchers have the only possibility to directly label chromatin at the moment it migrates between cells. This is a complex yet feasible task. One of the possible approaches to solving this problem is to take advantage of the transgenic plants that express photoactivatable proteins fused to the proteins with nuclear localization (Hedde and Nienhaus 2014). One of the promising proteins for such study is Eos, the photoconvertible protein which irreversibly changes its green fluorescence to red when exposed to the light of a certain wavelength (Wozny et al. 2012; Schattat et al. 2014; Griffiths et al. 2016). Thus, once it is possible to precisely irradiate the migrating chromatin in meiocytes of transgenic plants, the color of its fluorescence is changed, thereby labeling it. This will provide a way to monitor the fate of migrating chromatin and to determine whether it can be incorporated into the recipient cell nucleus. However, the specificity of the analyzed cells should be taken into account along with the evident difficulties associated with targeted irradiation of migrating chromatin. The analysis of this kind should be performed under in vitro conditions, while meiocyte cultivation has certain limitations. In addition, the expression of transgenes during meiosis may considerably differ from the expected one. Standard promoters are inactive at this moment, and the promoters known to work during meiosis are frequently activated only at the microspore stage. Nonetheless, the selective labeling of chromatin in vitro with the help of photoactivatable proteins is most likely the only possibility to trace the fate of migrating chromatin at least to the final stages of meiosis.
-
(iv)
Does the intercellular migration of nuclei takes place in animal cells?
It is known that the contacts between animal cells are most diverse, including the direct cytoplasmic channels. However, as far as we know, any migrations of nuclei between animal cells have not been yet described. The CC analogs in the animal cell are the so-called tunneling nanotubes in mammals and ring channels in insects. Tunneling nanotubes have been found in various tissue types of mammals and shown to provide the migration of small organelles, such as mitochondria and vesicles, between cells (Koyanagi et al. 2005; Gerdes et al. 2007; Zani and Edelman 2010). A more interesting picture is observable in the insect nurse cells united by ring channels. In some drosophila mutants with abnormal formation of actin cytoskeleton, the nuclei of nurse cells lose their cytoskeletal “anchors” and commence passively migrating within the cell with the flow of cytoplasm (Ogienko et al. 2008). The directed flow of cytoplasm gradually shifts the nucleus to ring channels; the nucleus approaches the channel and plugs it but cannot pass through (Fig. 2e). The analogy between the ring channels in insect nurse cells and CCs in plant male meiocytes is evident: both types of channels connect the cytoplasms of neighboring cells involved in gamete formation. When comparing the sizes of nuclei and intercellular channels in drosophila nurse cells and plant meiocytes (for example, of tobacco), it becomes evident that the ring channels are much larger than CCs on the background of approximately same sizes of their nuclei. The average size of the ring channels is about 10 μm (Ogienko et al. 2008) versus the CCs between tobacco microsporocytes, the maximum size of which is about 600 nm (Mursalimov et al. 2010). In other words, both types of nuclei have the same size but the ring channels are more that 15-fold larger than the CCs; however, a passive migration of nuclei through the ring channels is impossible. Migrating nuclei in drosophila cells merely plug the channel and remain in this position, not leaving the channel and not dividing into micronuclei (Fig. 2e). The observed picture suggests that the nuclear migration between cells cannot be an accidental event. The migration of nuclei between cells is an active targeted process. The nucleus should be actively dragged into another cell to successfully path through narrow intercellular CCs.
Nuclear migration in plant and animal cells. a–c Wheat endosperm cells, in vivo time-lapse microscopy; asterisks denote the nucleus and arrow, the part of the nucleus migrating through the cell wall (Zhang et al. 1990). d A nuclear bridge (white arrow) formed as a result of cytomixis in tobacco male meiocytes; white arrowhead denotes the chromatin leaving the nuclear bridge and entering the nuclear space of recipient cell. Insert shows an upscaled nuclear bridge; black arrow denotes the direction of chromatin migration and cw, cell wall (Mursalimov and Deineko 2011). e The nuclei (arrows) migrating through the ring channels (arrowheads) in Drosophila nurse cells (Ogienko et al. 2008). Figures are adapted for this review (arrows, letters, and bars are replaced). Bars, 2 μm in d and 20 μm in e
Note that the nuclear migration in the nurse cells takes place in the absence of normal cytoskeletal structures (Ogienko et al. 2008) versus the situation with cytomixis, when cytoskeleton abnormalities in the plant cells are absent and the nucleus constantly contact the cytoskeletal structures (Zhang et al. 1990; Barton et al. 2014; Sidorchuk et al. 2016). Correspondingly, it would be incorrect to regard the disturbed anchoring of the nucleus as a cause of cytomixis.
In should be emphasized that cytomixis was discovered and named as the process of intercellular chromatin migration (Arnoldy 1900; Gates 1911). Thus, it would be incorrect to use the term “cytomixis” describing migration of cytoplasm and small organelles between animal cells. No cases of cytomixis have been detected yet in animal cells. Presumably, this is associated with a considerably higher sensitivity of the animal cells to any change in chromosome composition. In turn, the plant cell is considerably more tolerant to a change in the karyotype; moreover, polyploidization is one of the main mechanisms in the evolution of higher plants (De Storme and Mason 2014).
-
(v)
Is cytomixis a norm or pathology?
Researchers have different opinions on the causes and consequences of cytomixis. Some of them regard cytomixis as a normal process for male meiosis with a certain role in the evolution, since it putatively contributes to the change in karyotype of the produced pollen (Ghaffari 2006; Negron-Ortiz 2007; Lavia et al. 2011; Pécrix et al. 2011; Farooq et al. 2014). Other researchers believe that cytomixis is a normal phenomenon but regard it as a specific PCD form implementing selective elimination of damaged meiocytes (Kravets 2011, 2013) or as a mechanism for discharge of “surplus” DNA from the cell (Zhou 2003; Kalinka et al. 2010). The opposite opinion is that the migration of nuclei is a kind of pathology induced by either external or internal factors (Bala and Gupta 2011; Singhal et al. 2011; Kumar and Srivastava 2013; Barton et al. 2014).
The former concept stating that cytomixis is a normal phenomenon changing the pollen karyotype so far has got the maximal number of experimental evidences, whereas the idea that cytomixis is a specific type of PCD has not been proved experimentally. As is mentioned above, any PCD markers are undetectable in the migrating chromatin.
The evidences of the pathological nature of cytomixis are also less than sterling. Some authors consider cytomixis as the abnormal process leading to the pollen sterility. In these works, the nuclear migration was observed in meiosis of hybrid, aneuploid, and polyploid plant forms (Li et al. 2009; Bala and Gupta 2011; Singhal et al. 2011) as well as of the plants exposed to external stress impacts, such as temperature stress and chemicals (Alka et al. 2012; Kumar and Srivastava 2013; Barton et al. 2014). However, when making conclusions on a pathological nature of cytomixis, they do not pay any attention to the fact that a high rate of migrating nuclei in experimental plants is always combined with numerous other abnormalities of the meiotic division as well as that cytomixis is also observable in the control plants not subject to stress impacts and displaying a normal fertility rate (Sidorchuk et al. 2007a). In other words, they observe only an increase in the rate of cytomixis under external or internal stress conditions rather than emergence of a novel process absent in the norm. Obviously, an extremely high rate of cytomixis in an abnormal meiosis attracts much more attention than the relatively rare process of nuclear migration that could be found in untreated plants. Correspondingly, it would be more adequate to regard as pathology a drastic increase in the rate of nuclear migration on the background of general disorganization of meiosis in such experimental plants rather that the migration of nuclei per se.
On the other hand, it is known that disorganization of meiosis not always leads to an increase in the rate of cytomixis. For example, it has been shown using the tobacco plants of different ploidies that the rate of cytomixis in male meiosis proportionally increases with the ploidy level (2n = 0.6%, 3n = 18.6%, and 4n = 38.4%); however, the rate of cytomixis does not increase significantly (n = 1.8%) in haploid tobacco (Mursalimov et al. 2016). Thus, although the meiosis of haploid plants has numerous abnormalities and leads to generation of completely sterile pollen, any increase in the rate of cytomixis is unobservable. On the other hand, the meiosis of tetraploid plants is relatively ordered and gives partially fertile pollen yet the rate of cytomixis in such plants is maximal. This pattern has yet to be explained.
Thus, we believe it erroneous to regard cytomixis as a pathology or a specific form of PCD. Taking into account the briefed data and a wide abundance of cytomixis in various systematic groups, we are inclined to regard this phenomenon as a normal process in the cell, which can be a facultative or an obligatory component in the male meiosis of a large number (or, possibly, all) higher plant species.
-
(vi)
Is cytomixis a cause of changes in the plant karyotype?
Unfortunately, any direct evidence for this is absent. Researchers have several opinions on how cytomixis can influence the karyotype of the produced pollen. We have already mentioned the hypothesis that cytomixis may be the mechanism providing selective elimination of the genome part that is “surplus” (in hybrids and polyploids) or damaged (Zhou 2003; Giorgetti et al. 2007; Kalinka et al. 2010; Kravets 2011, 2013; Aksic et al. 2016). However, this theory has not received sufficient experimental confirmation. On the other hand, many researchers believe that the chromatin migrating between cells in cytomixis does not degrade but rather incorporates into the nucleus of the recipient cell and thus changes its karyotype (Ghaffari 2006; Negron-Ortiz 2007; Lavia et al. 2011; Pécrix et al. 2011; Farooq et al. 2014). Incorporation of additional chromatin into the nucleus is possible either when cytomictic micronuclei directly merge to the nucleus of the recipient cell in prophase I or when additional chromosomes move to the poles in anaphase I and getting into newly formed nuclei. Experimental data accumulated so far confirms that the migrated chromatin can be incorporated into the nucleus of the recipient cell. In particular, emergence of additional chromosome copies in tobacco male meiocytes was demonstrated by FISH (Mursalimov and Deineko 2017). Fusion of the nuclear membrane of cytomictic micronuclei and the nuclear membrane of the recipient cell was demonstrated at an ultrastructural level (Mursalimov and Deineko 2015). A special case is the discovery of direct contacts between the migrating nucleus and the nucleus of the recipient cell. As is shown, the nuclear membrane of a migrating nucleus can fuse with the nuclear membrane of a recipient cell, forming a nuclear bridge (Fig. 2a, white arrow). Nuclear bridge is the channel with a diameter of approximately 250 nm confined by the nuclear membrane and directly connecting the nuclei of two cells. The chromatin putatively migrates between the nuclei through this bridge (Fig. 2a, arrowhead). Note that the nuclear membrane retains its integrity over the entire nuclear bridge. The movement of chromatin through the nuclear bridge between two cells can be regarded as its movement within the nucleus, since the chromatin resides in a confined space limited by the nuclear membrane and finds itself in another nucleus without leaving this space. Conceivably, the chromatin involved in this process is not affected by any damaging factors and, correspondingly, its migration may have certain genetic consequences.
We have already mentioned the cases of binucleated meiocytes formed when a whole nucleus migrates to the recipient cell (Sidorchuk et al. 2007a; Singhal and Kumar 2008; Tsvetova and Elkonin 2013; Sidorchuk et al. 2016). As has been shown, both nuclei display no signs of damage or degradation after formation of a binucleated meiocyte at the stage of zygo-pachytene and progressively continue the meiotic division (Mursalimov and Deineko 2015). Such nuclei do not contact each other and have a normal chromosome structure, the degree of their chromatin condensation matching the current meiotic stage. Development of binucleated meiocytes is observable until the nuclear membrane disappears in metaphase I. After this stage, binucleated meiocytes are undetectable. Multipolar anaphases also are undetectable. In this case, it is probable that a single joint spindle is formed for both nuclei. As a result, this can lead to formation of unreduced pollen. This assumption has not been experimentally confirmed; however, it was shown that the pollen exceeds twofold the size of normal pollen produced by the plants with high rate of cytomixis (Ghaffari 2006; Negron-Ortiz 2007; Lavia et al. 2011; Pécrix et al. 2011; Farooq et al. 2014; Kumar and Singhal 2016a, b; Reis et al. 2016; Kumar et al. 2017).
It is much more difficult to determine the fate of donor meiocytes involved in cytomixis that lost part of its chromatin/chromosomes rather than acquired additional material. Unfortunately, it is almost impossible to identify the donor cells that lost a small part of their nuclear volume. The donor cells upon completion of cytomixis can be unambiguously identified only when they completely or almost completely lose their nucleus (Sidorchuk et al. 2007a, 2016). In the products of tobacco meiosis, the cells larger than the norm are detectable, whereas smaller-sized cells are absent (Mursalimov and Deineko 2015). This suggests that donor cells can complete their meiotic division only in the case they have lost a small amount of chromatin/chromosomes. Such aneuploid pollen will insignificantly differ in its size from the normal pollen. Evidently, the cells that lost a major part of all chromatin cannot continue meiotic division.
Thus, numerous indirect evidences obtained so far suggest that cytomixis can change the karyotype of the produced pollen. However, we have to acknowledge that all these data are insufficient to assert that cytomixis is an additional mechanism of genetic recombination in the plant meiosis.
Final remarks and future directions
It is expected that the further research into cytomixis will follow two main directions. First, this is an intravital analysis of the cells involved in cytomixis both in vitro and in vivo. The approach implying creation of transgenic plants that produce various forms of fluorescent proteins provides the opportunity of intravital examination of all cell components. This approach in combination with state-of-the-art microscopy methods has a great potential for gaining the insight into cytomixis as well as other aspects in the male meiosis of higher plants. Second, this is analysis of the functional state and chromosome composition of the migrating chromatin in different plant species and forms. It is assumed that the mechanisms and consequences of this process may be species-specific. This analysis is especially relevant to hybrid plant forms, where cytomixis may play a special role in stabilization of the newly formed genomes.
References
Aksic MF, Cerovic R, Ercisli S, Jensen M (2016) Microsporogenesis and meiotic abnormalities in different ‘Oblacinska’ sour cherry (Prunus cerasus L.) clones. Flora Morphol Distrib Funct Ecol. Plants 219:25–34. https://doi.org/10.1016/j.flora.2015.12.009
Alka AMYK, Bhat TM et al (2012) Induction of polyploid mutant in Linum usitatissimum L. by cytomixis. Golden Res Thoughts 2:1–6. https://doi.org/10.9780/22315063
Arnoldy W (1900) Beiträge zur Morphologie der Gymnospermen. IV. Was sind die ‘Keimbläschen’ oder ‘Hofmeisters-Körperchen. In: der Eizelle der Abietineen? Flora 87, pp 194–204
Bala S, Gupta RC (2011) Effect of secondary associations on meiosis, pollen fertility and pollen size in cape gooseberry (Physalis peruviana L.) Chrom Bot 6(2):25–28. https://doi.org/10.3199/iscb.6.25
Barton DA, Cantrill LC, Law AMK et al (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ 37(12):2781–2794. https://doi.org/10.1111/pce
Chicheportiche A, Bernardino-Sgherri J, de Massy B, Dutrillaux B (2007) Characterization of Spo11-dependent and independent phospho-H2AX foci during meiotic prophase I in the male mouse. J Cell Sci 120(10):1733–1742. https://doi.org/10.1242/jcs.004945
De Storme N, Mason A (2014) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr Plant Biol 1:10–33. https://doi.org/10.1016/j.cpb.2014.09.002
Farooq U, Lovleen, Saggoo MIS (2014) Male meiosis and behaviour of sex chromosomes in different populations of Rumex acetosa L. from the Western Himalayas, India. Plant Syst Evol 300(2):287–294. https://doi.org/10.1007/s00606-013-0881-z
Feijo JA, Pais MS (1989) Cytomixis in meiosis during the microsporogenesis of Ophrys lutea: an ultrastructural study. Caryologia 42(1):37–48. https://doi.org/10.1080/00087114.1989.10796951
Ferreira K, Torres GA, Carvalho IV, Davide LC (2009) Abnormal meiotic behavior in three species of Crotalaria. Pesqui Agropecuária Bras 44(12):1641–1646. https://doi.org/10.1590/S0100-204X2009001200012
Fuchs J, Schubert I (2012) Chromosomal distribution and functional interpretation of epigenetic histone marks in plants. Plant Cytogenetics:231–253
Fuentes I, Stegemann S, Golczyk H, Karcher D, Bock R (2014) Horizontal genome transfer as an asexual path to the formation of new species. Nature 511(7508):232–235. https://doi.org/10.1038/nature13291
Gates RR (1911) Pollen formation in Oenothera gigas. Ann Bot 25:909–940
Gerdes HH, Bukoreshtliev NV, Barroso JF (2007) Tunneling nanotubes: a new route for the exchange of components between animal cells. FEBS Lett 581(11):2194–2201. https://doi.org/10.1016/j.febslet.2007.03.071
Ghaffari SM (2006) Occurrence of diploid and polyploid microspores in Sorghum bicolor (Poaceae) is the result of cytomixis. Afr J Biotech 5:1450–1453
Giorgetti L, Castiglione M, Martini G et al (2007) Methylated DNA sequence extrusion during plant early meiotic prophase. Caryologia 60(3):279–289. https://doi.org/10.1080/00087114.2007.10797949
Goday C, Pigozzi MI (2010) Heterochromatin and histone modifications in the germline-restricted chromosome of the zebra finch undergoing elimination during spermatogenesis. Chromosoma 119(3):325–336. https://doi.org/10.1007/s00412-010-0260-2
Griffiths N, Jaipargas E, Wozny M et al (2016) Photo-convertible fluorescent proteins as tools for fresh insights on subcellular interactions in plants. J Microsc 263(2):148–157. https://doi.org/10.1111/jmi.12383
Guan JZ, Wang JJ, Cheng ZH et al (2012) Cytomixis and meiotic abnormalities during microsporogenesis are responsible for male sterility and chromosome variations in Houttuynia cordata. Genet Mol Res 11:121–130 doi: 0.4238/2012.January.17.2
Gupta H, Gupta RC, Kumar R, Singhal VK (2017) A profile of chromosome counts, male meiosis and pollen fertility in 45 species of Asteraceae from Parvati Valley in Kullu district, Himachal Pradesh. Caryologia 70(2):128–140. https://doi.org/10.1080/00087114.2017.1299976
Gurdona C, Svab Z, Feng Y et al (2016) Cell-to-cell movement of mitochondria in plants. PNAS 113(12):3395–3400. https://doi.org/10.1073/pnas.1518644113
Guzicka M, Wozny A (2005) Cytomixis in shoot apex of Norway spruce [Picea abies (L.) Karst.] Trees 18(6):722–724. https://doi.org/10.1007/s00468-004-0331-1
He S, Yan S, Wang P, Zhu W, Wang X, Shen Y, Shao K, Xin H, Li S, Li L (2014) Comparative analysis of genome-wide chromosomal histone modification patterns in maize cultivars and their wild relatives. PLoS One 9(5):e97364. https://doi.org/10.1371/journal.pone.0097364
He Y, Wang C, Higgins J et al (2016) MEIOTIC F-BOX is essential for male meiotic DNA double strand break repair in rice. Plant Cell 28(8):1879–1893. https://doi.org/10.1105/tpc.16.00108
Hedde PN, Nienhaus GU (2014) Super-resolution localization microscopy with photoactivatable fluorescent marker proteins. Protoplasma 251(2):349–362. https://doi.org/10.1007/s00709-013-0566-z
Houben A, Demidov D, Caperta AD, Karimi R, Agueci F, Vlasenko L (2007) Phosphorylation of histone H3 in plants—a dynamic affair. Biochim Biophys Acta-Gene Struct Expr 1769(5-6):308–315. https://doi.org/10.1016/j.bbaexp.2007.01.002
Houben A, Demidov D, Rutten T, Scheidtmann KH (2005) Novel phosphorylation of histone H3 at threonine 11 that temporally correlates with condensation of mitotic and meiotic chromosomes in plant cells. Cytogenet Genome Res 109(1-3):148–155. https://doi.org/10.1159/000082394
Hunter N, Börner GV, Lichten M, Kleckner N (2001) Gamma-H2AX illuminates meiosis. Nat Genet 27(3):236–238. https://doi.org/10.1038/85781
Kalinka A, Achrem M, Rogalska SM (2010) Cytomixis-like chromosomes/chromatin elimination from pollen mother cells (PMCs) in wheat-rye allopolyploids. Nucleus 53(3):69–83. https://doi.org/10.1007/s13237-010-0002-0
Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327(5962):172–177. https://doi.org/10.1126/science.1180189
Kolczyk J, Tuleja M, Płachno BJ (2015) Histological and cytological analysis of microsporogenesis and microgametogenesis of the invasive species Galinsoga quadriradiata Ruiz & Pav. (Asteraceae). Acta Biol Cracoviensia Ser Bot 57(2):89–97. https://doi.org/10.1515/abcsb-2015-0018
Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S (2005) Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res 96(10):1039–1041. https://doi.org/10.1161/01.RES.0000168650.23479.0c
Kravets E (2013) Cytomixis and its role in the regulation of plant fertility. Russ J Devel Biol 44(3):113–128. https://doi.org/10.1134/S1062360413030028
Kravets E (2011) The role of cell selection for pollen grain fertility after treatment of barley sprouts (Hordeum distichum L.) with UV-B irradiation. Acta Biol Slov 54:31–41
Kumar G, Srivastava N (2013) Induced cytomictic variations in pollen mother cells of Sesbania cannabina Poir. J Cent Eur Agric 14(3):19–27. https://doi.org/10.5513/JCEA01/14.3.1280
Kumar P, Singhal V (2016a) Nucleoli migration coupled with cytomixis. Biologia 71(6):651–659. https://doi.org/10.1515/biolog-2016-0076
Kumar P, Singhal V (2016b) Morphological and ecological adaptations, and cytological studies in Astragalus rhizanthus Royle ex Benth. (Papilionaceae), an endemic to Himalayas. Cytologia 81(2):155–160. https://doi.org/10.1508/cytologia.81.155
Kumar P, Singhal VK, Kaur D, Kaur S (2010) Cytomixis and associated meiotic abnormalities affecting pollen fertility in Clematis orientalis. Biol Plant 54(1):181–184. https://doi.org/10.1007/s10535-010-0031-1
Kumar P, Singhal V, Srivastava S (2017) First detection of cytomixis and its consequences in Thalictrum cultratum Wall. (Ranunculaceae). Cytol Genet 51(5):384–390. https://doi.org/10.3103/S0095452717050061
Kumar S, Jeelani SM, Rani S, Gupta RC, Kumari S (2013) Cytology of five species of subfamily Papaveroideae from the Western Himalayas. Protoplasma 250(1):307–316. https://doi.org/10.1007/s00709-012-0413-7
Kuras M, Nowakowska J, Sliwinska E et al (2006) Changes in chromosome structure, mitotic activity and nuclear DNA content from cells of Allium Test induced by bark water extract of Uncaria tomentosa (Willd.) DC. J Ethnopharmacol 107(2):211–221. https://doi.org/10.1016/j.jep.2006.03.018
Lavia GI, Ortiz AM, Robledo G, Fernández A, Seijo G (2011) Origin of triploid Arachis pintoi (Leguminosae) by autopolyploidy evidenced by FISH and meiotic behaviour. Ann Bot 108(1):103–111. https://doi.org/10.1093/aob/mcr108
Li XF, Song ZQ, Feng DS, Wang HG (2009) Cytomixis in Thinopyrum intermedium, Thinopyrum ponticum and its hybrids with wheat. Cer Res Comm 37(3):353–361. https://doi.org/10.1556/CRC.37.2009.3.4
Lim KY, Matyásek R, Lichtenstein CP, Leitch AR (2000) Molecular cytogenetic analyses and phylogenetic studies in the Nicotiana section Tomentosae. Chromosoma 109(4):245–258. https://doi.org/10.1007/s004120000074
Lone FA, Lone S (2013) Cytomixis – a well known but less understood phenomenon in plants. Int J Recent Sci Res 4:347–352
Malik R, Gupta R, Singh V et al (2017) New chromosome reports in Lamiaceae of Kashmir (Northwest Himalaya), India. Protoplasma 254(2):971–985. https://doi.org/10.1007/s00709-016-1006-7
Mandal A, Datta AK, Gupta S, Paul R, Saha A, Ghosh BK, Bhattacharya A, Iqbal M (2013) Cytomixis—a unique phenomenon in animal and plant. Protoplasma 250(5):985–996. https://doi.org/10.1007/s00709-013-0493-z
Mandal D, Nandi A (2017) Cytomixis with associated chromosomal anomalies and the reproduction of Chlorophytum borivilianum Santapau & R. R Fern Taiwania 62:211–215. https://doi.org/10.6165/tai.2017.62.211
Manzanero S, Arana P, Puertas MJ, Houben A (2000) The chromosomal distribution of phosphorylated histone H3 differs between plants and animals at meiosis. Chromosoma 109(5):308–317. https://doi.org/10.1007/s004120000087
Mursalimov S, Deineko E (2017) Cytomixis in tobacco microsporogenesis: are there any genome parts predisposed to migration? Protoplasma 254(3):1379–1384. https://doi.org/10.1007/s00709-016-1028-1
Mursalimov S, Permyakova N, Deineko E, Houben A, Demidov D (2015) Cytomixis doesn’t induce obvious changes in chromatin modifications and programmed cell death in tobacco male meiocytes. Front Plant Sci 6:1–13. https://doi.org/10.3389/fpls.2015.00846
Mursalimov S, Sidorchuk Y, Deineko E (2013a) The role of spherosome-like vesicles in formation of cytomictic channels between tobacco microsporocytes. Biol Plant 57(2):291–297. https://doi.org/10.1007/s10535-012-0276-y
Mursalimov S, Sidorchuk Y, Deineko E (2017a) Analysis of cytomixis in tobacco microsporocytes with confocal laser scanning microscopy. Protoplasma 254(1):539–545. https://doi.org/10.1007/s00709-016-0973-z
Mursalimov S, Sidorchuk Y, Demidov D, Meister A, Deineko E (2016) A rise of ploidy level influences the rate of cytomixis in tobacco male meiosis. Protoplasma 253(6):1583–1588. https://doi.org/10.1007/s00709-015-0907-1
Mursalimov S, Zagorskaya A, Deineko E (2017b) Evaluation of DNA damage in tobacco male meiocytes involved in cytomixis using comet assay. Protoplasma. https://doi.org/10.1007/s00709-017-1144-6
Mursalimov SR, Baiborodin SI, Sidorchuk YV, Shumny VK, Deineko EV (2010) Characteristics of the cytomictic channel formation in Nicotiana tabacum L. pollen mother cells. Cytol Genet 44(1):14–18. https://doi.org/10.3103/S0095452710010032
Mursalimov SR, Deineko EV (2015) How cytomixis can form unreduced gametes in tobacco. Plant Syst Evol 301(4):1293–1297. https://doi.org/10.1007/s00606-014-1150-5
Mursalimov SR, Deineko EV (2011) An ultrastructural study of cytomixis in tobacco pollen mother cells. Protoplasma 248(4):717–724. https://doi.org/10.1007/s00709-010-0234-5
Mursalimov SR, Sidorchuk YV, Deineko EV (2013b) New insights into cytomixis: specific cellular features and prevalence in higher plants. Planta 238(3):415–423. https://doi.org/10.1007/s00425-013-1914-0
Negron-Ortiz V (2007) Chromosome numbers, nuclear DNA content, and polyploidy in Consolea (Cactaceae), an endemic cactus of the Caribbean Islands. Am J Bot 94(8):1360–1370. https://doi.org/10.3732/ajb.94.8.1360
Ogienko A, Karagodin D, Pavlova N et al (2008) Molecular and genetic description of a new hypomorphic mutation of Trithorax-like gene and analysis of its effect on Drosophila melanogaster oogenesis. Russ J Dev Biol 39(2):108–115. https://doi.org/10.1134/S1062360408020070
Papini A, Tani G, Di Falco P, Brighigna L (2010) The ultrastructure of the development of Tillandsia (Bromeliaceae) trichome. Flora Morphol Distrib Funct Ecol Plants 205(2):94–100. https://doi.org/10.1016/j.flora.2009.02.001
Pécrix Y, Rallo G, Folzer H et al (2011) Polyploidization mechanisms: temperature environment can induce diploid gamete formation in Rosa sp. J Exp Bot 62(10):3587–3597. https://doi.org/10.1093/jxb/err052
Pierre P, Sousa S (2011) Citomixia em plantas: causas, mecanismos e consequências. Brazilian. J Biosci 9:231–240
Polowick PL, Sawhney VK (1992) Ultrastructural changes in the cell wall, nucleus and cytoplasm of pollen mother cells during meiotic prophase I in Lycopersicon esculentum (Mill.) Protoplasma 169(3-4):139–147. https://doi.org/10.1007/BF01323613
Qiu Y, Liao L, Liu S, Mao D, Liu R (2017) Differences on the microsporogenesis and tapetal development of male fertile and cytoplasmic male sterile pepper (Capsicum annuum L.) Grana 56(3):215–227. https://doi.org/10.1080/00173134.2016.1248860
Reis A, Sousa S, Viccini L (2016) High frequency of cytomixis observed at zygotene in tetraploid Lippia alba. Plant Syst Evol 302(1):121–127. https://doi.org/10.1007/s00606-015-1249-3
Ressayre A, Mignot A, Siljak-Yakovlev S, Raquin C (2003) Postmeiotic cytokinesis and pollen aperture number determination in eudicots: effect of the cleavage wall number. Protoplasma 221(3-4):257–268. https://doi.org/10.1007/s00709-002-0075-y
Risso-Pascotto C, Pagliarini MS, do Valle CB (2009) Chromosome number and microsporogenesis of two accessions of Brachiaria dura Stapf (Poaceae). Biota Neotrop 9(2):1–5. https://doi.org/10.1590/S1676-06032009000200024
Rogakou EP, Boon C, Redon C, Bonner WM (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146(5):905–915. https://doi.org/10.1083/jcb.146.5.905
Schattat MH, Barton KA, Mathur J (2014) The myth of interconnected plastids and related phenomena. Protoplasma 252(1):359–371. https://doi.org/10.1007/s00709-014-0666-4
Schoenmakers S, Wassenaar E, Laven JSE, Grootegoed JA, Baarends WM (2010) Meiotic silencing and fragmentation of the male germline restricted chromosome in zebra finch. Chromosoma 119(3):311–324. https://doi.org/10.1007/s00412-010-0258-9
Shibata F, Nagaki K, Yokota E, Murata M (2013) Tobacco karyotyping by accurate centromere identification and novel repetitive DNA localization. Chromosom Res 21(4):375–381. https://doi.org/10.1007/s10577-013-9363-y
Sidorchuk YV, Deineko EV, Shumny VK (2007a) Peculiarities of cytomixis in pollen mother cells of transgenic tobacco plants (Nicotiana tabacum L.) with mutant phenotype. Cell Tiss Biol 1(6):570–576. https://doi.org/10.1134/S1990519X07060144
Sidorchuk YV, Deineko EV, Shumny VK (2007b) Role of microtubular cytoskeleton and callose walls in the manifestation of cytomixis in pollen mother cells of tobacco Nicotiana tabacum L. Cell Tiss Biol 1(6):577–581. https://doi.org/10.1134/S1990519X07060156
Sidorchuk YV, Novikovskaya AA, Deineko EV (2016) Cytomixis in the cereal (Gramineae) microsporogenesis. Protoplasma 253(2):291–298. https://doi.org/10.1007/s00709-015-0807-4
Silva N, Machado MFPS, Mangolin CA, Pagliarini MS (2006) Microsporogenesis in somaclones of Cereus peruvianus Mill. (Cactaceae). J Plant Sci 1(1):8–13. https://doi.org/10.3923/jps.2006.8.13
Silva SL, Magalhaes KM, Carvalho R (2017) Karyotype variations in seagrass (Halodule wrightii Ascherson—Cymodoceaceae). Aquat Bot 136:52–55. https://doi.org/10.1016/j.aquabot.2016.09.005
Singhal VK, Kumar P (2008) Impact of cytomixis on meiosis, pollen viability and pollen size in wild populations of Himalayan poppy (Meconopsis aculeata Royle). J Biosci 33(3):371–380. https://doi.org/10.1007/s12038-008-0057-0
Singhal VK, Rana PK, Kumar P, Kaur D (2011) Persistent occurrence of meiotic abnormalities in a new hexaploid cytotype of Thalictrum foetidum from Indian cold deserts. Biologia 66(3):458–464. https://doi.org/10.2478/s11756-011-0033-2
Stegemann S, Bock R (2009) Exchange of genetic material between cells in plant tissue grafts. Science (80- ) 324:649–651. doi: https://doi.org/10.1126/science.1170397
Stegemann S, Keuthe M, Greiner S, Bock R (2012) Horizontal transfer of chloroplast genomes between plant species. PNAS 109:2434–2438. doi: DOI https://doi.org/10.1073/pnas.1114076109
Tarkowska J (1965) Experimental analysis of the mechanism of cytomixis. I. Cytomixis in vegetative tissues. Acta Soc Bot Pol 34:27–44
Tarkowska J (1966) Experimental analysis of the mechanism of cytomixis. II Cytomixis in the pollen mother cells of the lily—Lilium candidum L. Acta Soc Bot Pol 35:25–40
Tarkowska J (1973) The nature of cytomixis. Caryologia 25(sup1):151–157. https://doi.org/10.1080/00087114.1973.10797120
Thyssen G, Svab Z, Maliga P (2012) Cell-to-cell movement of plastids in plants. PNAS 109(7):2439–2443. https://doi.org/10.1073/pnas.1114297109
Tsvetova M, Elkonin L (2013) Cytological investigation of pollen development in Sorghum line with male sterility induced by sodium ascorbate in tissue culture. Am J Plant Sci 4(07):11–18. https://doi.org/10.4236/ajps.2013.47A1002
Wang CY, Li X, QF W, Wang X (2006) Cytoplasmic channels and their association with plastids in male meiocytes of tobacco, onion and lily. Cell Biol Int 30(5):406–411. https://doi.org/10.1016/j.cellbi.2006.01.003
Wang XY, Nie XW, Guo GQ, Pan YF, Zheng GC (Kuo Chang (2002) Ultrastructural characterization of the cytoplasmic channel formation between pollen mother cells of David lily. Caryologia 55(2):161–169. https://doi.org/10.1080/00087114.2002.10589272
Wang XY, Yu CH, Li X, et al (2004) Ultrastructural aspects and possible origin of cytoplasmic channels providing intercellular connection in vegetative tissues of anthers Russ J Plant Physiol 51:110–120. doi: 1021–4437/04/5101–0097
Whelan EDP (1974) Discontinuities in the callose wall, intermeiocyte connections, and cytomixis in angiosperm meiocytes. Can J Bot 52(6):1219–1224. https://doi.org/10.1139/b74-157
Wozny M, Schattat M, Mathur N et al (2012) Color recovery after photoconversion of H2B::mEosFP allows detection of increased nuclear DNA content in developing plant cells. Plant Physiol 158(1):95–106. https://doi.org/10.1104/pp.111.187062
Xiao A, Li H, Shechter D, Ahn SH, Fabrizio l, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P, Hofmann K, Patel DJ, Elledge SJ, Allis CD (2009) WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457(7225):57–62. https://doi.org/10.1038/nature07668
Xue Z, Li Y, Zhang L, Shi W, Zhang C, Feng M, Zhang F, Tang D, Yu H, Gu M, Cheng Z (2016) OsMTOPVIB promotes meiotic DNA double-strand break formation in rice. Mol Plant 9(11):1535–1538. https://doi.org/10.1016/j.molp.2016.07.005
Yu CH, Guo GQ, Nie XW, Zheng GC (2004) Cytochemical localization of pectinase activity in pollen mother cells of tobacco during meiotic prophase and its relation to the formation of secondary plasmodesmata and cytoplasmic channels. Acta Bot Sin 46:1443–1453
Zani BG, Edelman ER (2010) Cellular bridges: routes for intercellular communication and cell migration. Comm Integr Biol 3(3):215–220. https://doi.org/10.4161/cib.3.3.11659
Zhang WC, Yan WM, Lou CH (1990) Intercellular movement of protoplasm in vivo in developing endosperm of wheat caryopses. Protoplasma 153(3):193–203. https://doi.org/10.1007/BF01354004
Zhou SQ (2003) Viewing the difference between the diploid and the polyploid in the light of the upland cotton aneuploid. Hereditas 138(1):65–72. https://doi.org/10.1034/j.1601-5223.2003.01689.x
Acknowledgments
The work was supported by the Russian Foundation for Basic Research [16-34-60007 mol_a_dk] and Siberian Branch of the Russian Academy of Science under the program “Molecular genetic bases of regulation of genes expression, morphology, differentiation and cell reprogramming” [0324-2016-0003].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Jaideep Mathur
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Mursalimov, S., Deineko, E. Cytomixis in plants: facts and doubts. Protoplasma 255, 719–731 (2018). https://doi.org/10.1007/s00709-017-1188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1188-7