Abstract
Confocal laser scanning microscopy for the first time is used to examine the structure of the tobacco microsporocytes involved in the intercellular migration of nuclei (cytomixis). As is observed, the cytomictic channels are distributed over the surface of tobacco microsporocytes in a non-random manner and their number depends on the meiotic stage. Analysis of non-squash cells demonstrates the differences in cytological patterns of cytomixis in a normal meiosis of control tobacco plants (SR1 line) and the abnormal meiosis of polyploids. As a rule, two to three adjacent cells are involved in cytomixis during meiosis of control tobacco plants; after cytomixis, several micronuclei are formed in recipient cells; cytoplasts (enucleated cells) are rare; and polyads are undetectable. In the meiosis of polyploids, cytomixis is massive, with a larger number of cells (sometimes, over ten) involved in nuclear migration simultaneously; recipient cells on completion of cytomixis develop tens of micronuclei; cytoplasts and polyads are frequently detectable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cytomixis, the migration of nuclei between plant cells, has been so far discovered in microsporogenesis of hundreds of plant species (for review, see Lone and Lone 2013; Mursalimov et al. 2013b). Cytomixis has been for many years in the focus of attention; however, the unanswered questions on the mechanisms underlying the migration of nuclei between cells as well as on the causes and consequences of this unusual phenomenon are still numerous. As what is now known, cytomixis is most frequently observed during the first meiotic prophase (Barton et al. 2014; Liu et al. 2012; Mursalimov and Deineko 2012; Negron-Ortiz 2007). Before the nuclei start their migration, cytomictic channels—specific intercellular channels, significantly exceeding plasmodesmata in their size—are formed in the cell wall of microsporocytes (Wang et al. 2002, 2004; Yu et al. 2004). All the nuclear components, including the chromatin, nucleolus, and nuclear matrix, migrate between cells through the cytomictic channels being enclosed into the nuclear membrane displaying no signs of damage (Mursalimov and Deineko 2011). In cytomixis, a whole nucleus can pass into another cell, thereby forming a binucleate microsporocyte, and a cell completely lacking the chromatin (cytoplast) or only part of the nucleus can migrate through a cytomictic channel, which buds off in the recipient cell forming micronuclei (Mursalimov and Deineko 2012, 2015; Sidorchuk et al. 2016). The latter situation, with micronuclei formed in the recipient cell, is much more frequent in tobacco meiosis (Mursalimov et al. 2015b; Mursalimov and Deineko 2015). The cytomictic chromatin carries normal markers of euchromatin and lacks the markers of additional DNA heterochromatization and damage (Mursalimov et al. 2015a). In addition, not only nuclei but also other DNA-containing organelles—mitochondria and plastids—can migrate between cells through cytomictic channels (Fuentes et al. 2014; Mursalimov et al. 2010; Thyssen et al. 2012; Wang et al. 2006).
Since genetic material migrates between the cells producing pollen and then gametes, cytomixis attracts attention not only as a special kind of communication between higher plant cells but also as a possible mechanism changing the karyotype and having an evolutionary significance.
So far, the major part of the data on cytomixis has been obtained with the help of transmission electron microscopy and routine light microscopy on squash preparations. These methods impose serious constraints on analysis of a native 3D structure of the studied cells, resulting from the specific features of the technique used for preparing the material for analysis. Examination of the squash preparations implies that the cells, although being fixed, undergo strong mechanical impacts. On the other hand, cell examination using transmission electron microscopy is confined to the thickness of ultrathin sections.
Thus, examination of a 3D structure of cytomictic cells using state-of-the-art confocal laser scanning microscopy (CLSM) is a topical issue. CLSM makes it possible to analyze a 3D structure without preparing cell sections or squashing cells as well as to examine individual subcellular structures specifically stained with fluorescent dyes.
This paper reports the results of the first 3D analysis of the tobacco microsporocytes with cytomixis by CLSM. After fixation, the cells were stained with fluorescent dyes specific for the cell wall and chromatin, placed on a glass slide, and examined, preserving their integrity. We have studied the distribution of cytomictic channels over the surface of tobacco microsporocytes and analyzed a 3D cytological pattern of cytomixis in a normal meiosis of control tobacco plans and an abnormal meiosis of polyploids.
Materials and methods
Plant material
Tobacco line SR1 (Nicotiana tabacum L. cv. Petit Havana SR1, 2n = 4x = 48) was used in the work. Polyploid plants (2n = 96) were produced by treating SR1 seeds with 0.5 % (w/v) colchicine for 48 h. All plants were grown in a hydroponic greenhouse with a photoperiod of 16/8 h (day/night) at a temperature of 22/18 °C (day/night).
Confocal laser scanning microscopy
The anthers (2–4 mm) were fixed with freshly prepared 4 % paraformaldehyde in PBS (pH 7.3) for 30 min and washed with PBS three times for 15 min. Then, microsporocytes were extracted from anthers. The cell suspension (0.5 ml in PBS) was stained with a drop of calcofluor white (1 mg/ml, Fluka) and a drop of propidium iodide (10 mg/ml, BioVision) for 15 min, marking the cell wall and chromatin, respectively. The cells were then placed onto a glass slide into a chamber formed of a cover glass attached to the slide with double-sided stick tape and filled with ProLong Gold antifade reagent (Life Technologies). The sample thus prepared is guaranteed from deformation and allows for microscopic examination of 3D cell structure (Kokhanenko et al. 2013, 2014). The preparations were examined with an LSM 780 NLO (Carl Zeiss, Germany) laser scanning confocal microscope. At least 200 cells were used for the analysis from both control and polyploid plants in the first meiotic prophase and at least 100 cells in the second meiotic division.
Results
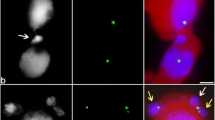
CLSM has allowed us for the first time to study the distribution of cytomictic channels in the cell wall of tobacco microsporocytes (Fig. 1). As has been shown, the cytomictic channels are non-randomly distributed in the cell wall but rather form local clusters (5–10 channels). As a rule, two clusters of cytomictic channels localized to the opposite cell poles are distinguished in the first meiotic prophase, when the rate of cytomixis is maximum (Fig. 1a, b, arrows). Such an arrangement of the cytomictic channels in some cases leads to formation of a chain of cells successively joined by cytomictic channels (Fig. 1c).
At later meiotic stages, the arrangement and number of cytomictic channels change. On completion of the first meiotic prophase, cytomictic channels reduce in their number, are located without any visible regularity (Fig. 2), and, as a rule, are not involved in the migration of nuclei.
Not all the cytomictic channels formed within the cell wall are concurrently involved in the nuclear migration during prophase I (Fig. 3). In the simplest case, cytomixis involves two cells and the nucleus passes between these cells through a single cytomictic channel (Fig. 3a, d, yellow arrow). In other cases, several cells may be simultaneously involved in this process and the nuclei from different donor cells migrate into one recipient cell (Fig. 3b, arrows). Another variant is when nuclei migrate between cells concurrently through several cytomictic channels (Fig. 3c, arrow).
Cytomixis in tobacco line SR1 microsporogenesis in pachytene (CLSM). The chromatin is colored red and the cell wall, blue-violet; yellow arrow denotes the nucleus passing to another cell through a cytomictic channel and turquoise arrows, the micronuclei formed as a result of cytomixis. Scale bars 10 μm
In the tobacco microsporogenesis, the nucleus passes into another cell as a whole only in a small number of cases (Mursalimov and Deineko 2015). The most frequent variant is when the migrated nuclear fragments after passing through cytomictic channel bud off in the recipient cell to form there one or several micronuclei (Fig. 3d, turquoise arrows).
The described cytological pattern of cytomixis is characteristic of the tobacco SR1 line. We have also compared the cytological pattern of cytomixis in the microsporogenesis of tobacco SR1 line plants (2n = 48) with the analogous processes in the male meiosis of polyploid tobacco plants (2n = 96) constructed using the SR1 line.
The conducted analysis has demonstrated that cytomixis is of a mass character in the microsporogenesis of polyploid tobacco plants as compared with the tobacco SR1 line (Fig. 4), i.e., a large number of cells (in some cases, even all cells of an anther) are involved in the process of nuclear migration. This results in formation of aberrant meiotic products. The enucleated cells (cytoplasts) appear when the entire nucleus migrates from a microsporocyte to another cell or several adjacent cells (Fig. 4a, asterisk). Such enucleated cells further stay off the meiotic division and are frequently detectable in the same state at the stage of tetrads (Fig. 4b, asterisk). Because of a mass migration of nuclei during cytomixis in polyploids, the recipient cells develop a large number (20–50) of micronuclei (Fig. 4c, arrows), which is one of the causes for abnormal tetrads in meiotic products of these plants (Fig. 4b, turquoise arrow, d).
Cytomixis and its consequences in the microsporogenesis of polyploid tobacco plants (CLSM). a, c Pachytene; b, d stage of tetrads of microspores; a, b formation of enucleated cells (asterisk) as a result of cytomixis (yellow arrow denotes cytomictic micronuclei and turquoise arrow, an abnormal cell at the tetrad stage); c formation of a large number of micronuclei (arrows) as a result of cytomixis; and d polyads. The chromatin is colored red and the cell wall, blue-violet. Scale bars 10 μm
Discussion
Examination of cytomixis with the help of electron microscopy has allowed for description of different ways how cytomictic channels are formed at early and late meiotic stages (Mursalimov et al. 2010, 2013a; Wang et al. 2002, 2004; Yu et al. 2004). Our CLSM data confirm the earlier observations. A large number of cytomictic channels actively involved in intercellular migration of nuclei are observed at early meiotic stages. After the callose wall is formed around the microsporocytes, a larger part of the cytomictic channels disappears so that rather few channels remain detectable in the second meiotic division and, as a rule, are not involved in cytomixis. The presence of a small number of cytomictic channels in the second meiotic division are explainable by that they either were not stopped by callose or were de novo formed after the callose wall appeared for certain functions unconnected with cytomixis.
The discovery of a non-random distribution of the cytomictic channels over the surface of the cell wall of tobacco microsporocytes in the first meiotic prophase is one of the important results in CLSM examination of cytomixis. The fact that the cytomictic channels are localized to the opposite cell poles makes it possible to explain the earlier described phenomenon, namely, the chains of cells united by migrating nuclei (Mursalimov and Deineko 2011; Sidorchuk et al. 2007). Interestingly, the nuclei in such chains of cytomictic cells always migrate in one direction; we have never met a case of the nuclei migrating toward one another. It is unknown what prevents the nuclei from migrating toward one another; presumably, this is determined by certain changes in the cytoskeletal structure of the corresponding cells. No differences in the formation of cytomictic channels in the microsporogenesis of tobacco SR1 line and polyploids have been detected. It is important to take into account that the specific features in location of cytomictic channels in the cell wall of microsporocytes may well be species-specific (Whelan et al. 1974).
CLSM has allowed the differences in the cytomictic patterns characteristic of SR1 line and polyploid plants to be detected. As has been shown, cytomixis is massive in the meiosis of polyploids. The nuclei actively migrate frequently involving more than three cells and give rise to a large number of micronuclei in recipient cells as well as cytoplasts. On the average, about 25 % of the cells in an anther are involved in cytomixis in polyploid plants; in some cases, this rate attains 100 % (Sidorchuk et al. 2007). As for the SR1 line plants, typically two to three cells are involved in cytomixis and only part of the nucleus migrate to recipient cell to form several micronuclei; the rate of this phenomenon in SR1 microsporogenesis rarely exceeds 5 % (Mursalimov et al. 2015c; Sidorchuk et al. 2007).
The result of active nuclear migration in the microsporogenesis of polyploids in combination with numerous abnormalities in the spindle assembly and cytokinesis (Sidorchuk and Deineko 2014; Sidorchuk et al. 2007) is abnormal meiotic products (polyads, cytoplasts, etc.). On the other hand, no abnormal cells are detectable among the meiotic products of the initial tobacco SR1 line. Although the rate of cytomixis in microsporogenesis of the initial SR1 line is several fold lower as compared with polyploids, the absolute number of microsporocytes involved in cytomixis and carrying cytomictic micronuclei is rather high and they are detectable in the first meiotic prophase to metaphase–anaphase I. In the second meiotic division, it is unfeasible to unambiguously identify the chromatin that has migrated from other cells. Such cytomictic chromatin at that stage is undetectable as micronuclei located separately from the main cell nucleus. No polyads or small sterile pollen grains originating from the cytomictic process are observable among the meiotic products of SR1 plants.
Thus, two possible explanations for the disappearance of the cytomictic micronuclei from tobacco SR1 microsporocytes are possible. On the one hand, the explanation may be an active elimination/death of the cells carrying micronuclei or elimination of micronuclei alone. However, the cells with cytomictic micronuclei in this case should display characteristic markers of cell death, namely, certain alterations in the posttranslational histone modification, TUNEL-positive reaction, and so on, which are undetectable in the cells during migration of nuclei and on completion of cytomixis and formation of micronuclei (Mursalimov et al. 2015a).
On the other hand, there is a certain probability that the chromatin of the cytomictic micronuclei remains in the recipient cell and incorporates into its nucleus. Several mechanisms could potentially provide the integration of cytomictic chromatin with the recipient cell nucleus. In the microsporogenesis of tobacco at an ultrastructural level, we have repeatedly observed a direct fusion of the nuclear membrane surrounding the migrating chromatin with the nuclear membrane of recipient cell nucleus (Mursalimov and Deineko 2011, 2015). In addition, certain data suggest that chromatin in cytomixis migrates between cells as whole bivalents with a functional centromere, which allows for their possible contact with the spindle and incorporation of the migrated bivalents into the metaphase plate of recipient cell with their subsequent segregation to the poles and changing in the karyotype of the future pollen (Mursalimov et al. 2015a, b; Mursalimov and Deineko 2015).
However, the cytomictic chromatin is currently undetectable in the cells during the second meiotic division and later, at the stage of tetrads/pollen/gametes independently of whether the migrated chromatin has or has not integrated with the recipient cell nucleus. The reason for this lies in that any specific markers for cytomictic chromatin have not been so far discovered, since the cytomictic chromatin in all the studied characteristics does not differ from the chromatin of intact cells (Mursalimov et al. 2015a). Thus, it is impossible to identify the cytomictic chromatin in the tobacco microsporogenesis after metaphase–anaphase I and, correspondingly, to prove or reject the hypothesis that the cells that have incorporated the migrated chromatin continue their meiotic division and produce viable pollen.
On the other hand, a drastic increase in the rate of cytomixis in microsporogenesis of polyploid plants cannot help but attract attention. This pattern has been observed not only in the tobacco but also in tens of other plant species (Guan et al. 2012; Kumar et al. 2011; Negron-Ortiz 2007). As has been hypothesized, an increase in the rate of cytomixis in the microsporogenesis of polyploids and hybrids is the mechanism allowing the excess chromatin to be eliminated and the newly constructed genome to be stabilized (Baptista-Giacomelli et al. 2000; Kalinka et al. 2010; Zhou 2003). However, this hypothesis yet lacks any direct evidences. In addition, a high rate of cytomixis in the microsporogenesis of polyploids is as a rule associated with an increase in other meiotic abnormalities, first and foremost, spindle function and cell cytokinesis (Sidorchuk et al. 2008; Sidorchuk and Deineko 2014).
It is known that in the case of hybridization or polyploidization, a considerable level of chromosome instability is observed in meiosis of the newly formed plants, which mainly appears as multiple meiotic abnormalities and a decrease in pollen fertility. This process is frequently non-random and is implemented as elimination of individual chromosomes or even complete genomes (Li et al. 2015). It is hypothesized that cytomixis may be involved in this process (Baptista-Giacomelli et al. 2000; Kalinka et al. 2010; Zhou 2003). However, this implies that the migrating chromatin should have some markers of degradation, which is not the case according to molecular data (Mursalimov et al. 2015a).
Anyway, there is still no unambiguous opinion on the role of cytomixis in normal and abnormal plant meiosis, and the described cytological results in no way pretend to prove the potential evolutionary significance of cytomixis. However, our data serve to propose a new approach to studying this most interesting aspect of communication between higher plant cells and prove its natural character.
Among other issues, an important conclusion of this work is the confirmation of the data on cytomixis earlier obtained using microscopic examination of squash preparations and transmission electron microscopy. CLSM has allowed for the first time to perform a 3D analysis of the cytological pattern of cytomixis in unsquashed cells, not subject to any mechanical injuries. The obtained CLSM data not only confirm the adequacy of results obtained earlier by other microscopic methods, but also provide one more piece of evidence that cytomixis is not a consequence of a mechanical impact on cells.
References
Baptista-Giacomelli FR, Pagliarini MS, Almeida JL (2000) Elimination of micronuclei from microspores in a Brazilian oat (Avena sativa L.) variety. Genet Mol Biol 23:681–684
Barton DA, Cantrill LC, Law AMK et al (2014) Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ 37:2781–2794
Fuentes I, Stegemann S, Golczyk H et al (2014) Horizontal genome transfer as an asexual path to the formation of new species. Nature 511:232–235
Guan JZ, Wang JJ, Cheng ZH et al (2012) Cytomixis and meiotic abnormalities during microsporogenesis are responsible for male sterility and chromosome variations in Houttuynia cordata. Genet Mol Res 11:121–130
Kalinka A, Achrem M, Rogalska SM (2010) Cytomixis-like chromosomes/chromatin elimination from pollen mother cells (PMCs) in wheat-rye allopolyploids. Nucleus 53:69–83
Kokhanenko A a, Anan’ina TV, Stegniy VN (2013) The changes in chromosome 6 spatial organization during chromatin polytenization in the Calliphora erythrocephala Mg. (Diptera: Calliphoridae) nurse cells. Protoplasma 250:141–149
Kokhanenko A, Anan’ina T, Stegniy V (2014) Localization of rRNA genes in the nuclear space of Calliphora erythrocephala Mg. nurse cells during polytenization. Protoplasma 251:93–101
Kumar P, Singhal VK, Rana PK et al (2011) Cytology of Ranunculus laetus Wall. ex Royle from cold desert regions and adjoining hills of North-West Himalayas (India). Caryologia 64:25–32
Li H, Guo X, Wang C, Ji W (2015) Spontaneous and divergent hexaploid triticales derived from common wheat × rye by complete elimination of D-genome chromosomes. PLoS One 10, e0120421
Liu Y, Hui RK, Deng RN et al (2012) Abnormal male meiosis explains pollen sterility in the polyploid medicinal plant Pinellia ternata (Araceae). Genet Mol Res 11:112–120
Lone A, Lone S (2013) Cytomixis—a well known but less understood phenomenon in plants. Int J Recent Sci Res 4:347–352
Mursalimov SR, Deineko EV (2011) An ultrastructural study of cytomixis in tobacco pollen mother cells. Protoplasma 248:717–724
Mursalimov SR, Deineko EV (2012) An ultrastructural study of microsporogenesis in tobacco line SR1. Biologia 67:369–376
Mursalimov SR, Deineko EV (2015) How cytomixis can form unreduced gametes in tobacco. Plant Syst Evol 301:1293–1297
Mursalimov SR, Baiborodin SI, Sidorchuk YV et al (2010) Characteristics of the cytomictic channel formation in Nicotiana tabacum L. pollen mother cells. Cytol Genet 44:14–18
Mursalimov S, Sidorchuk Y, Deineko E (2013a) The role of spherosome-like vesicles in formation of cytomictic channels between tobacco microsporocytes. Biol Plant 57:291–297
Mursalimov SR, Sidorchuk YV, Deineko EV (2013b) New insights into cytomixis: specific cellular features and prevalence in higher plants. Planta 238:415–423
Mursalimov S, Permyakova N, Deineko E et al (2015a) Cytomixis doesn’t induce obvious changes in chromatin modifications and programmed cell death in tobacco male meiocytes. Front Plant Sci 6:1–13
Mursalimov S, Sidorchuk Y, Baiborodin S, Deineko E (2015b) Distribution of telomeres in the tobacco meiotic nuclei during cytomixis. Cell Biol Int 39:491–495
Mursalimov S, Sidorchuk Y, Demidov D et al (2015c) A rise of ploidy level influences the rate of cytomixis in tobacco male meiosis. Protoplasma. doi:10.1007/s00709-015-0907-1
Negron-Ortiz V (2007) Chromosome numbers, nuclear dna content, and polyploidy in Consolea (Cactaceae), an endemic cactus of the Caribbean Islands. Am J Bot 94:1360–1370
Sidorchuk YV, Deineko EV (2014) Deformation of nuclei and abnormal spindles assembly in the second male meiosis of polyploid tobacco plants. Cell Biol Int 38:472–479
Sidorchuk YV, Deineko EV, Shumny VK (2007) Peculiarities of cytomixis in pollen mother cells of transgenic tobacco plants (Nicotiana tabacum L.) with mutant phenotype. Cell Tissue Biol 1:570–576
Sidorchuk Y, Dorogova N, Deĭneko E, Shumnyi V (2008) Premature cytokinesis in pollen mother cells of transgenic tobacco plants (Nicotiana tabacum L.). Cell Tissue Biol 2:337–341
Sidorchuk Y, Novikovskaya A, Deineko E (2016) Cytomixis in the cereal (Gramineae) microsporogenesis. Protoplasma 253:291–298
Thyssen G, Svab Z, Maliga P (2012) Cell-to-cell movement of plastids in plants. Proc Natl Acad Sci U S A 109:2439–2443
Wang XY, Nie XW, Guo GQ et al (2002) Ultrastructural characterization of the cytoplasmic channel formation between pollen mother cells of David lily. Caryologia 55:161–169
Wang XY, Yu CH, Li X et al (2004) Ultrastructural aspects and possible origin of cytoplasmic channels providing intercellular connection in vegetative tissues of anthers. Russ J Plant Physiol 51:110–120
Wang CY, Li X, Wu QF, Wang X (2006) Cytoplasmic channels and their association with plastids in male meiocytes of tobacco, onion and lily. Cell Biol Int 30:406–411
Whelan EDP, Haggis GH, Ford EJ (1974) Scanning electron microscopy of the callose wall and intermeiocyte connections in angiosperms. Can J Bot 52:1215–1218
Yu CH, Guo GQ, Nie XW, Zheng GC (2004) Cytochemical localization of pectinase activity in pollen mother cells of tobacco during meiotic prophase I and its relation to the formation of secondary plasmodesmata and cytoplasmic channels. Acta Bot Sin 46:1443–1453
Zhou SQ (2003) Viewing the difference between the diploid and the polyploid in the light of the upland cotton aneuploid. Hereditas 138:65–72
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (grant no. 16-34-60007 mol_a_dk) and Siberian Branch of the Russian Academy of Science under the program “Fundamental Bases of Biotechnology Creating Therapies and Diagnosis of Diseases,” theme VI.62.1.5 (0324-2014-0017). The microscopy was conducted at the Joint Access Center for Microscopy of Biological Objects with the Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Benedikt Kost
Rights and permissions
About this article
Cite this article
Mursalimov, S., Sidorchuk, Y. & Deineko, E. Analysis of cytomixis in tobacco microsporocytes with confocal laser scanning microscopy. Protoplasma 254, 539–545 (2017). https://doi.org/10.1007/s00709-016-0973-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0973-z