Abstract
Tryptophan decarboxylase (EC 4.1.1.28) catalyzes pyridoxal 5′-phosphate (PLP)-dependent decarboxylation of tryptophan to produce tryptamine for recruitment in a myriad of biosynthetic pathways of metabolites possessing indolyl moiety. A recent report of certain indolyl metabolites in Withania species calls for a possible predominant functional role of tryptophan decarboxylase (TDC) in the genome of Withania species to facilitate production of the indolyl progenitor molecule, tryptamine. Therefore, with this metabolic prospection, we have identified and cloned a full-length cDNA sequence of TDC from aerial tissues of Withania coagulans. The functional WcTDC gene comprises of 1506 bp open reading frame (ORF) encoding a 502 amino acid protein with calculated molecular mass and pI value of 56.38 kDa and 8.35, respectively. The gene was expressed in Escherichia coli, and the recombinant enzyme was affinity-purified to homogeneity to discern its kinetics of catalysis. The enzyme (WcTDC) exhibited much higher Km value for tryptophan than for pyridoxal 5′-phosphate and was dedicated to catalyze decarboxylation of only tryptophan or, to a limited extent, of its analogue (like 5-hydroxy tryptophan). The observed optimal catalytic functionality of the enzyme on the slightly basic side of the pH scale and at slightly higher temperatures reflected adaptability of the plant to hot and arid regions, the predominant natural habitat of the herb. This pertains to be the first report on cloning and characterization of heterologously expressed recombinant enzyme from W. coagulans and forms a starting point to further understanding of withanamide biosynthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solanaceae is rich in genera having medicinal values, and Withania is valued as the most important genus among them due to possession of diverse medicinal properties by different species of Withania. Two major medicinal species of Withania are Withania somnifera and Withania coagulans (Tuli and Sangwan 2010). W. coagulans has been attended far less than W. somnifera with respect to investigation of its phyto-chemistry, metabolic and molecular biology, and modern molecular pharmacology, despite its well-descript medicinal value in traditional Indian systems of medicine (ISM). This may also be due to restricted distribution of W. coagulans and little, if any, commercial cultivation of the herb. However, recently, it has attracted tremendous attention of researchers due to its novelties including with respect to a specific set of withanolides and pharmacological activities (Gupta et al. 2012; Chaurasiya et al. 2012; Kushwaha et al. 2013a). W. coagulans is also referred to as Indian cheese maker or vegetable rennet, due to milk-coagulating properties of its fruits. It is highly valuated for its pharmaceutical properties such as anti-hyperglycemic anti-inflammatory, hepatoprotective, cardiovascular effect, antibacterial, antifungal, and anthelminthic properties (Rajurkar et al. 2001; Hemlatha et al. 2004; Jaiswal et al. 2009; Khare 2007). Fruits of the plant are used for health benefits in case of diabetes, chronic stage of asthma and heart ailments including high blood pressure, immunological weakness, and neurological disorders (Maurya and Akanksha 2010).
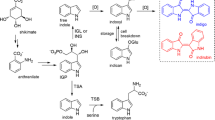
Diverse medicinal properties of Withania species are due to the presence of a variety of characteristic biologically active phytochemicals that include, among others, withanolides (the C28 steroidal lactones having an erogstane backbone) such as withanolide F, coagunolide, coagulin C, coagulin H, coagulin L, withaferin A, withanone, and withanolide A; tropane alkaloids like tropine, pseudotropine, and calistegins; and withanamides such as withanamide A, withanamide B, withanamide C, withanamide D, and withanamide E. W. coagulans produces a significant amount of many withanolides in its aerial parts. This provides a simpler option of harvesting the issue and its phytochemicals without digging/destroying the whole plant contrary to the case when the molecule is root-specific (Mishra et al. 2013). Withanamides, as reported to be present in Withania species (Jayaprakasham et al. 2010), represent a new chemical class of natural products as their structural frame and biogenic origin are quite different from other secondary metabolites of the herb. Withanamides being conjugated (sugar-serotonin-fatty acid) structures, these multi-moiety complex secondary products appear to owe their metabolic origin to multiple primary metabolic pathways including tryptophan metabolism, lipid metabolism, and carbohydrate metabolism (Fig. 1). Their biosynthesis, though not characterized at all as yet, may follow the sequence of beginning with synthesis of serotonin followed by its conjugation with a specific fatty acid on amino-side and one or more glucose moiety(ies) on hydroxyl-side. Serotonin is synthesized from tryptophan in plants as well as animals. However, it is biochemically characterized well in animals but far little in plants. Overall, the process of serotonin biogeneration involves two sequential biochemical reactions—decarboxylation and hydroxylation. Contrary to animals, decarboxylation of tryptophan to tryptamine is believed to precede hydroxylation and forms the committed step of the pathway (Kang et al. 2007). This reaction is catalyzed by tryptophan decarboxylase (TDC) using pyridoxal 5′-phosphate as a cofactor (Fig. 1).
Biochemistry and molecular biology of secondary metabolism of W. somnifera has been well established by our group during the last decade (Chaurasiya et al. 2012; Mishra et al. 2013; Sabir et al. 2012a, b; Sangwan et al. 2004, 2007, 2008; Mishra et al. 2014; Sangwan and Sangwan 2014). To catalyze the expansion of parallel knowledge base on W. coagulans with respect to its metabolic and molecular aspects of secondary metabolites, we have published an efficient in vitro regeneration and genetic transformation method for the plant (Mishra et al. 2013). In sequences, we have reported isolation and characterization of a tropinone reductase involved in tropane alkaloid metabolism in W. coagulans (Kushwaha et al. 2013a, b). On the basis of recent studies that report an occurrence of withanamides in Withania, we aimed to isolate TDC gene from W. coagulans for making a beginning in understanding the metabolic pathway of withanamide synthesis in this species through observations from cloning and characterization of a new candidate gene, tryptophan decarboxylase, from the plant. This report describes molecular details of a full-length gene of tryptophan decarboxylase from the leaves of W. coagulans, its cloning in Escherichia coli (BL21) for induced overexpression in the heterologous system, homogeneity purification of the recombinant enzyme, and catalytic and kinetic aspects of the enzyme catalyzed reaction, and their regulatory significance at the committed step of the pathway.
Materials and methods
Plant material
Multiple shoot cultures of W. coagulans were established and maintained in tissue culture conditions in our lab at Central Institute of Medicinal and Aromatic Plants, Lucknow, India, as reported earlier (Mishra et al. 2013). The tissue culture raised plants (Fig. 2) were maintained on MS media supplemented with 5 mg l−1 kinetin and 10 mg l−1 BAP.
Chemicals
All chemicals, protein molecular weight markers, DNA markers and restriction enzymes, etc. were purchased from Sigma-Aldrich (USA) and/or Thermo Scientific (Fermentas). Solvents were purchased from Merck Chemicals (Germany). Ni-NTA affinity chromatography resin was procured from Novagen. Tissue culture grade agar, sucrose, MS media components, and plant hormones used in the study were purchased from HiMedia (India). All other chemicals and reagents were of highest purity available from local vendors.
RNA isolation and cDNA synthesis
In vitro grown shoots of W. coagulans were sampled and processed for total RNA isolation using TRI reagent (Sigma-Aldrich). After checking the quality and quantity, the RNA was used for cDNA synthesis using RevertAid™ first strand cDNA synthesis kit (Fermentas) according to the manufacturers’ instructions. For 5′RACE, SMARTer™ cDNA library was synthesized using SMARTer™ cDNA synthesis kit (Clontech).
Cloning of full-length cDNA of TDC from W. coagulans
Degenerate primers, TDCNTerF and TDCNTerR (Table 1), were designed to isolate the TDC from W. coagulans. Core fragment of tryptophan decarboxylase from W. coagulans (WcTDCI) was obtained by PCR with these degenerate primers. The PCR product was analyzed by agarose gel electrophoresis and purified using Sigma GeneElute™ gel extraction kit as per protocol described in the kit. The PCR amplicon was cloned in cloning vector (pJET1.2/blunt) from Fermentas and was subjected to sequencing. After confirming the significance homology of the amplicon with other tryptophan decarboxylases from plants, gene-specific primers were designed to carry out 3′ RACE and 5′RACE. For 3′ RACE, 3′AP and gene-specific primers (WcTDCDF1 and WcTDCDF2) were used, while for 5′RACE, CDSIIA (provided in SMARTer™ cDNA synthesis kit) and gene-specific primers (WcTDCUR1 and WcTDCUR2) were used. For cloning of full-length cDNA of WcTDC, primers WcTDCFLF and WcTDCFLR flanked with restriction sites of BamHI and SacI, respectively, were used. Amplified fragment was ligated in pJET1.2, the blunt end cloning vector, and the vector with insert was used to transform E. coli (DH5α) cells. Plasmid was isolated and subjected to sequencing to for its identification based on sequence homology.
Computational analysis of the gene sequence
The basic local alignment search tool (BLAST) of National Center for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/Blast) was used for sequence similarity analysis of WcTDC with other known TDCs from plants. CLUSTALW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2) was used to align nucleotide and amino acid sequences. Open reading frame (ORF) finder graphical analysis tool of NCBI (www.ncbi.nlm.nih.gov/projects/gorf) was used to identify the coding sequence. Hosted tools at the site of ExPASy Bioinformatics resource portal of Swiss Institute of Bioinformatics (http://www.expasy.org/proteomics/) was used for deduction of amino acid sequence of the cloned WcTDC. Databases of protein motifs (ProSite, http://prosite.expasy.org/scanprosite/) and fingerprints (PRINTS, www.bioinf.man.ac.uk/dbbrowser/PRINTS/) were used to document the conserved motifs in the enzyme. To profile the sequence divergence-based evolutionary position of WcTDC, a phylogenetic tree was developed using MEGA5 software (http://www.megasoftware.net) following maximum likelihood method. Bootstrap method was followed for corroborating the consistency of each node.
A homology-based 3-D model was built for analyzing the structural aspects of WcTDC protein, and it was generated through SWISS-MODEL Workspace in an automated mode using 1js3A.pdb as template. A 3-D model of aromatic amino acid decarboxylase from Sus scrofa was used as a template. For expanding the knowledge on topological contours, the developed model of WcTDC was overlapped with a template model using UCSF-Chimera package. The Consurf server (http://consurf.tau.ac.il) was used to determine the degree of conservation. Amino acids participating in a binding site for pyridoxal 5′phosphate were determined using 3-D LigandSite server (www.sbg.bio.ic.ac.uk/~3dligandsite/).
Inducible heterologous production of the recombinant WcTDC protein in E. coli
The pJET vector having cloned full-length cDNA of WcTDC was digested with BamHI and SacI restriction enzymes, and the digested reaction mixture was checked on 1 % agarose electrophoresis gel. Restriction digestion WcTDC fragment, cleaved out from the vector, was picked as gel plug, eluted, and ligated into pET 28a vector and linearized with the appropriate restriction enzyme. The pET 28a construct having WcTDC, an insert was used to transform competent cells of E. coli BL21 (DE3). Recombinant colonies were selected on kanamycin and chloramphenicol (50 μg/ml each) supplemented Luria agar medium. Induction of the recombinant WcTDC protein was carried out by adding isopropyl β-d-1 thiogalactopyranoside (IPTG) solution up to a concentration of 0.8 mM in media and growing the culture at 18 °C for overnight. To harvest the protein, bacterial cells were pelleted down by centrifugation at 5000 rpm for 5 min and pellet was suspended in lysis solution (10 mM imidazole, 50 mM Tris, 300 mM NaCl, 10 % glycerol). The suspension was sonicated and then centrifuged at 12,000×g to collect the soluble intracellular protein fraction. Overexpression of the recombinant protein was checked by SDS-PAGE analysis of protein preparations from induced and un-induced cultures.
Western blot analysis
Induced soluble protein fraction was further subjected for Western blot analysis to confirm induction of the recombinant His6-WcTDC protein. Crude extract of induced soluble protein was separated on SDS-PAGE and recombinant protein then transferred to nitrocellulose membrane. Protein-bound membrane was screened against (His)6 tag antibodies as primary antibodies, and this membrane was washed to remove the unbound antibodies. It was again exposed to a solution of anti-mouse IgG monoclonal antibodies as secondary antibodies conjugated with alkaline phosphatase. The membrane was soaked with substrate BCIP/NBT, and recombinant protein was visualized as colored product of reaction.
Purification of recombinant protein WsTDC
Recombinant WcTDC protein was purified using Ni++-NTA-affinity column chromatography. To purify the WcTDC protein, bacterial culture was inoculated in a large volume (1 l) and the culture was induced by using 0.8 mM IPTG as above. Buffers and solutions for protein purification through the metal-affinity chromatography were used as per the manufacturer’s protocol. Crude induced bacterial protein was passed through a column of Ni++-NTA Superflow resin (Novagen). Purified protein fractions were collected, checked on SDS-PAGE for their homogeneity purification. The concentration of protein was determined by Bradford’s assay.
TDC catalytic activity assay
Catalytic activity of WcTDC was determined by using fluorometric assay developed by Sangwan et al. (1998), using a fluorescence spectrometer (Cary Eclipse, Perkin Elmer). In brief, tryptamine formed by the catalytic action of the enzyme was selectively separated from tryptophan by pH-guided liquid-liquid partitioning and measured by fluorometrically using 280 and 350 nm as the excitation and emission wavelength, respectively. Composition and conditions of the assay were changed according to the requirement of experiments for the kinetic characterization of the recombinant enzyme.
Identification of catalytic reaction product
Product was identified by using HPLC (C18 column, Waters, MA, USA). Briefly, enzymatic reaction was set in a large volume (10 ml). The reaction was terminated with addition of NaOH, and the product was extracted into ethyl acetate. Ethyl acetate phase was evaporated, and the residue was dissolved in 50 μl of methanol. Methanol and water containing 20 mM ammonium acetate were used as mobile phase with a flow rate of 1 ml/min, and PDA was used as detector (280 nm).
Results
Cloning of WcTDC
To isolate TDC gene from W. coagulans, a cDNA library was prepared from total RNA isolated from in vitro grown multiple shoot cultures of the plant (Fig. 2). The PCR reaction of cDNA with degenerate primers resulted in a 0.4-kb amplicon that was cloned and sequenced. The amplicon sequence displayed a significant sequence homology with plant TDCs. On the basis of sequence of the putative core WcTDC fragment, primers were designed to amplify the rest of the gene fragments toward 5′ and 3′ends of the WcTDC cDNA by 5′RACE and 3′RACE approach. 5′RACE and 3′RACE PCR produced 0.2 and 1.2 kb amplicons, respectively. Collation of sequences of the three PCR fragments (internal, 3′RACE, and 5′RACE fragments) resulted in assembly of full-length cDNA of WcTDC that was 1.5 kb in size (Online Resource 1A, B). Full-length cDNA of WcTDC with flanking restriction sites was PCR-amplified using appropriate full-length primers flanking, and the amplicon was cloned in cloning vector (pJET 1.2 blunt end vector) and sequenced (Online Resource 1C).
Bioinformatic analysis of WcTDC sequence
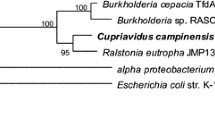
Sequence of WcTDC BLASTx analysis results revealed that WcTDC had highest similarity (86 %) with Capsicum annum TDC (accession number ACN62127.1). It also showed significant similarity with other TDCs of plant origin (Fig. 3), the levels of homology being close to 70 %, for example, 72, 72, 70, and 68 % similarity with CacTDC (Cyanea acuminata, accession number AAB39708.1), MspTDC (Mitragyna speciosa, accession number AEQ01059.1), OpuTDC (Ophiorrhiza pumila, accession number BAC415151.1), and VmiTDC (Vinca minor accession number AEY82397.1), respectively (Fig. 3). Complete ORF of the WcTDC gene cloned and sequenced represented a WcTDC protein of 502 amino acids, with calculated pI value of 8.35 and computed molecular weight of 56.38 kDa.
Sequence comparison of the tryptophan decarboxylase from the Withania coagulans (WcTDC) with other TDCs of plant origin. Signature amino acid sequences of aromatic l-amino acid decarboxylases are presented as highlighted regions. Amino acids depicted in green denote the ProSite motif PS00392 described as DDC/GAD/HDC/TyrDC pyridoxal 5′-phosphate attachment site
Heterologous expression of WcTDC and purification of the recombinant enzyme
The full-length cDNA of TDC from W. coagulans was cloned in pET28a protein expression vector, and the pET28a-WcTDC construct was mobilized in E. coli (BL21) cells through transformation of competent cells. Transformed E. coli cells were selected on kanamycin (50 mg l−1) + chloramphenicol (50 mg l−1) supplemented LA culture media plates. E. coli (BL21) cells harboring WcTDC were inoculated and induced with 0.8 mM IPTG at 18 °C. Production of induced recombinant protein (WcTDC) was checked by SDS-PAGE and then confirmed by immunoblot analysis using antibodies against His-hexamer (Fig. 4). The recombinant protein was purified by Ni2+-NTA metal-affinity chromatography to homogeneity level, as discerned by SDS-PAGE wherein single band matching to the size of WcTDC (56 kDa) was observed. The pure recombinant enzyme preparation was examined for catalytic and biochemical characteristics by HPLC-aided validation of the enzymatic reaction product and fluorescence assay of TDC developed by Sangwan et al. (1998).
Analysis of IPTG-induced overexpressed of WcTDC protein in E. coli expressing W. coagulans tryptophan decarboxylase gene (WcTDC). a Western blot analysis of crude extract from bacterial cells expressing WcTDC: lane 1—protein molecular weight marker, lane 2—soluble fraction of IPTG induced bacterial cells expressing WcTDC; b SDS-PAGE analysis of Ni++-NTA affinity chromatography purified protein fraction: lane 1—protein molecular weight marker, lane 2—catalytically active protein fractions
Catalytic activity and reaction product identification
Purified WcTDC protein possessed catalytic ability to produce tryptamine from tryptophan in the presence of pyridoxal 5′-phosphate (PLP), and the enzyme assay could be assessed and optimized for linearity with respect to the enzymatic protein concentration in the assay mixture as well as reaction time (Online Resource 2). HPLC analysis of the reaction mixture at the end of the catalytic reaction, when compared with control, revealed the presence of a significant peak corresponding to standard tryptamine (Online Resource 3) validating the catalytic ability of the recombinant enzyme to carry out PLP-dependent decarboxylation of tryptophan into tryptamine.
pH and temperature optima
The enzyme exhibited the optimum level of catalytic activity at pH 7.8 with phosphate as assay buffer (Fig. 5a). The optimum temperature for the WcTDC activity was found to be 45 °C (Fig. 5b).
Substrate saturations kinetics
The substrate saturation curve of WcTDC was simple hyperbolic for the substrate (tryptophan) as well as co-factor (pyridoxal 5′-phosphate, PLP) as shown in Fig. 6a, b. Double reciprocal (Line-Weaver-Burk) plots of the enzyme (Fig. 6a, b) led to estimates of its Km values for tryptophan and PLP as 1.49 mM and 5.77 μM, respectively (Table 2).
Substrate saturation and double reciprocal curves of Withania coagulans TDC (WcTDC) for substrate (l-tryptophan) and cofactor (pyridoxal 5′-phosphate, PLP). Standard assay mixtures were a set containing sequentially increasing substrate concentration. a Substrate saturation curve and double reciprocal plot of WsTDC for l-tryptophan. b Substrate saturation curve and double reciprocal plot of WcTDC for pyridoxal 5′-phosphate (PLP)
Thermostability
The thermostability of WcTDC was observed by incubating the purified enzyme protein preparation at different temperatures before its use in the enzyme assay. The enzyme retained almost up to 60 % of its catalytic ability on incubation at 50 °C (Fig. 7a).
Thermostability and substrate-specificity of the recombinant tryptophan decarboxylase from W. coagulans (WcTDC). a Pattern of thermostability—the enzyme preparation (WcTDC) was assessed for catalytic levels remaining when used in the assays after incubation at different temperatures for 30 min; b substrate specificity of WcTDC with respect to acceptable amino acids for decarboxylation
Substrate specificity
For PLP-dependent decarboxylation, WcTDC was highly specific for tryptophan as substrate (Fig. 7b). The enzyme could not use other aromatic amino acids like tyrosine as substrate for decarboxylation. 5-Hydroxytryptophan, an analog of tryptophan, was the only other substrate acceptable for the enzyme and that too at relatively quite low catalytic rate.
Phylogenetic position of WcTDC
A phylogenetic tree was constructed by using MEGA 5.0 software on the basis of maximum likelihood method to trace the evolutionary relationship of WcTDC with other plant amino acid decarboxylases. The phylogenetic tree showed that tryptophan decarboxylases (TDCs) formed a distinctly discrete group, separate from tyrosine decarboxylases (Fig. 8). TDCs from O. pumila and Ophiorrhiza prostrate joined together being originated from the same genus. On the other hand, the two TDCs known from C. annum were positioned relatively distant in the phylogenetic tree, CanTDC1 grouped along with WcTDC while CanTDC2 placed in group consisting PtrTDC (Populus trichocarpa), GmaTYDC (Glycine max), and CsaTYDC (Cucumis sativus). Distant from decarboxylases of dicotyledonous plant, decarboxylases of monocots formed a distinct group but an unusual pattern was observed for OsaTDC (Oryza sativa) that was found to be kept between the tyrosine decarboxylases while HvuTDC (Hordeum vulgare) showed closeness with AraTDC (Actae racemosa) which belongs to Ranunculaceae family, a primitive family of angiosperms. It indicated of a common ancestor for the origin of TDC of both monocots and dicots.
Homology-based modeling and structural analysis
A 3-D model of WcTDC protein was computed in SWISS-MODEL workspace, the protein structure of l-DOPA decarboxylase isolated from S. scrofa (1js3A.pdb) was taken as template to perform the homology modeling or template-based modeling (Fig. 9a). Superimposition of WcTDC and template model was viewed in UCSF-Chimera package (Fig. 9b). A superimposed model showed that WcTDC had a quite similar type of tertiary structure belonging to a conserved aromatic amino acid decarboxylase. Group II of decarboxylase has tightly conserved regions showing evolutionary conservancy throughout the living organisms. According to the prediction of Consurf Server tool, WcTDC also maintains a degree of conservation (Fig. 9c). The presence of a binding pocket for pyridoxal 5′-phosphate in WcTDC was also viewed by 3-D Ligand site viewer (Fig. 9d).
Homology-based molecular modeling of WcTDC protein. a A 3-D model of WcTDC, built on SWISS-MODEL workspace taking 1js3A.pdb as a template; b constructed structural model of WcTDC (yellow) superimposed on 1js3A.pdb (blue)—the superimposition was performed using UCSF-Chimera package; c conserved residues in a 3-D model of WcTDC as discerned through ConSurf server; d epiction of putative pyridoxal 5′-phosphate (PLP) binding site in 3-D structural model of WcTDC—the prediction, as performed through 3-D LigandSite server, displays PLP molecule as green highlight and amino acids that participate in the binding/catalysis in blue color
Discussion
The genus Withania comprises of a large number of species with varying distributions throughout the world. In India, W. coagulans is second ranked, next to W. somnifera (Ashwagandha), among the species of Withania commonly used for medicinal purposes. Despite that, the plant is not been far less explored as compared to W. somnifera (Mishra et al. 2013). After the discovery of serotonin-based bioactive secondary metabolite (withanamides) in the genus (Jayaprakasham et al. 2010), it is desirable to characterize their biosynthesis pathway. On the basis of documented structure of withanamides, serotonin is supposed to be a core component in the biogeneration of these indolyl molecules. In animals, serotonin is a neurotransmitter and its biosynthesis has been studied extensively while in plants, there is very scanty information available about its synthesis and role (Akula et al. 2011). Though, relatively far little known, available evidences indicate the key role of TDC in tryptamine production through pyridoxal-5′-phosphate-dependent decarboxylation of tryptophan for its diversified role including follow-up conversion to serotonin (5-hydroxy tryptamine) by an appropriate hydroxylase (Kang et al. 2007). Though, the mechanism of withanamide biosynthesis has not at all been examined as yet. Here, we report, for the first time, a putative pathway for withanamide biosynthesis in Withania species (Fig. 1) whereby the sequence of reactions involving TDC and a tryptamine hydroxylase generates hydroxyl-tryptamine that may be glycosylated as well as N-acylated to generate diverse members of this group of compounds (Fig. 1). Accordingly, identification and detailed characterization of relevant genes and enzymes of this model of metabolic pathway become important to discern the biosynthetic process and its regulation. To make a beginning of investigations in this direction, the results of this investigation with respect to molecular characteristics of the TDC gene from W. coagulans (WcTDC), catalytic attributes of the recombinant enzyme (WcTDC) and kinetics of the catalytic reaction have been discussed as comparative account with TDCs from other plants as well as in the kinetics of flux of tryptophan metabolism toward production of tryptamine and potential tryptamine-linked downstream indolyl metabolites.
The observed overall high level of amino acid sequence similarity of WcTDC with other pyridoxal 5′-phosphate (PLP)-dependent aromatic amino acid decarboxylases (AADC) owes to the mechanistic essentiality of decarboxylation through the involvement of PLP at the cofactor binding site. Although, Sandmeier et al. (1994) further grouped the PLP-dependent enzymes in four different classes, (i) group I represented by glycine decarboxylase; (ii) group II comprised of l-glutamate decarboxylase (GAD), l-histidine decarboxylase (HDC), l-3, 4-dihydroxyphenylalanine decarboxylase (DDC), l-tyrosine decarboxylase (TYDC), and l-tryptophan decarboxylase (TDC); (iii) group III membered by ornithine decarboxylases (ODCs), lysine decarboxylases (LDCs) and arginine decarboxylase (ADC) of prokaryotic origin; and (iv) group IV not only constituted by ODC and LDC of eukaryotic origin but also included the ADC and diaminopimelate decarboxylase (DPDC) of prokaryotic origin. This similarity of amino acid decarboxylases may be more meaningful in terms of their evolution and phylogeny than the catalytic functionality, as, despite clustering together, catalytic specificity with respect to ability to decarboxylate amino acid(s) is stringent despite using the same cofactor. Thus, amino acid binding site similarity is severely limited, for example, even TDC and TYDC catalyzing decarboxylation of two structurally quite close aromatic amino acids (tryptophan and tyrosine) though belonged to the same group (in terms of amino acid similarity quotient) but, in terms of catalytic ability/properties, they are as much apart from each other as from other amino acids. TDCs are unable to decarboxylate tyrosine, and TYDCs are unable to decarboxylate tryptophan (Facchini et al. 2000). The existence of a similarity in TDC gene from plants and animals reflects a trace of parallel of pattern of evolution (Facchini et al. 2000).
Functionally, TDC-aided tryptamine production is not an isolated catabolic reaction rather a commitment to a pathway for production of different classes of indolyl compounds in different plants or even plant parts, for instance, serotonin in a select set of plants across several genera/families, indole alkaloids particularly in some genera (Catharanthus, Camptotheca, Ophiorrhiza, and Rauwolfia) of Apocyanaceae, a sub-class of glucosinolates in some species of Brassicaceae, and recently reported withanamides in a genus of Solanaceae (De Luca et al. 1989; López-Meyse and Nessler 1997; Park et al. 2008; Yamazaki et al. 2003; Liu et al. 2012).
The observed conservation of Lys319 in WcTDC with the Lys319 positioned in the AADC PLP-binding signature motif (312 SLsLsphKWLlSyLDCccMWvK 333) led to the identification of co-factor (PLP) binding site in the enzyme. Generally, pH optima values reported for plant TDCs that are slightly basic in alignment with the cytosolic localization of the enzyme suggest its optimal operation under in vivo intracellular micro milieu. The observed relatively better temperature stability and higher temperature optima for WcTDC catalytic activity adapt to the usual xerophytic/arid habitat of the herb. Although, TDC isolated from rice has also been to have temperature optima of more than 40 °C (Kang et al. 2008). A lower Km value of WcTDC for PLP is an obvious essentiality as it participates in a variety of reactions of primary metabolism that is operative at far higher rates than reactions of secondary metabolism, as also it does not accumulate intracellularly at substantial concentrations. The affinity of TDC for PLP nearly competitive to that for counterparts from primary metabolism ensures pace of the reaction to be non-limited by PLP. Indeed, PLP-dependent enzymes contribute to almost 4 % of enzymes participating in cellular processes (Percudani and Peracchi 2003), and PLP serves as a versatile organic cofactor due to its ability to covalently bind with diverse substrates and acts as an electrophilic agent providing stability to carbanionic reaction intermediates (Schneider et al. 2000). Conversely, higher Km value for tryptophan may not only reflect its regulatory role to some extent, though compared to PLP, tryptophan intracellular steady state levels are usually much higher. Anaplerotic point catalytic functionality of TDC as an interlink step between primary and secondary metabolism (Murch et al. 2000) necessitates balance of flux of tryptophan across competing metabolic/uses in alignment with their relative physiological functions/priorities. Availability of tryptophan and the catalytic protein levels may be the catalytic rate limiting attributes of TDC reaction in regulating the synthesis of the biogenic amine (tryptamine). TDCs characterized from some other plants have also been shown to have higher Km for tryptophan than for PLP, for example, Km values for OpuTDC (O. pumila TDC), CroTDC (Catharanthus roseus TDC), and RvTDC (Rauwolfia verticillata TDC) have been reported to be 0.72, 1.31, and 2.89 mM, respectively (Yamazaki et al. 2003; Liu et al. 2012). Low catalytic efficiency, Kcat/Km, of WcTDC toward tryptophan (103) implicated the previously acquired data that enzymes of secondary metabolism have this value lower than primary metabolism (≥105), but the Kcat/Km of WcTDC for PLP is in range of 105, nearly close to primary metabolism (Sharma et al. 2013).
Aromatic amino acid decarboxylases, particularly tryptophan decarboxylase from plants, appear to be very peculiar for their stringency of substrate specificity in contrast to many amino acid decarboxylases from animals (Facchini et al. 2000). The observed stringent substrate specificity of WcTDC also reflected the same pattern. This specificity is due to the shape of the binding pocket of the enzyme being restrictive to recognize/bind only indole moiety. Physiologically, it facilitates amino acid specific balance with their respective amines for each amino acid. Further, for secondary metabolism, it allows generation of only the amines serving as progenitors of specific alkaloids and/or other congener secondary metabolites. For example, indole alkaloids through TDC catalysis generated tryptamine and benzylisoquinoline (BIS or morphinan) alkaloids through TYDC-catalyzed tyramine production from tyrosine. Indeed, the observed 5-hydroxy tryptophan as the only other substrate acceptable, albeit to a limited extent, for WcTDC supports the binding site specificity. Incidentally, 5-hydroxy tryptophan is toxic for plants and TDC can alleviate the toxicity to some extent.
In terms of observed relatively better thermostability of WcTDC, it joins other enzymes of secondary metabolism enzymes characterized from W. coagulans and reported to have higher thermostability. For example, Kushwaha et al. (2013) have reported that tropinone reductase I of the plant (WcTRI) retained >92 % of the activity up to 50 °C. It reflects the adaptability of the plant metabolism to operate even in hot and dry conditions of cultivation/thriving of the plant. Although primary metabolic enzymes are considered more crucial for survival, but enzymes of secondary metabolites also play very important roles in adaptation to stress conditions (Yadav et al. 2014). Genes of such enzymes may be new joiner targets for genetic engineering of plants for survival in xerophytic conditions.
Differences at amino acid level in TDCs from plants cast the changes in the gene accompanying speciation process. The phylogenetic tree also includes the TYDC from plant species as TDC and TYDC belong to the same group of amino acid decarboxylases (group II) homology model of any plant TDC is not yet submitted in PDB database; hence, by using the AADC of animal origin as a template, a 3-D model of WcTDC was constructed. The model showed resemblance with DOPA decarboxylase (Burkhard et al. 2001) with well-identified three domains. A central large domain and a domain positioned each at C-terminal and N-terminal. A large domain was comprised of seven beta sheets surrounded by eight α-helices. C-terminal end domain had four antiparallel beta sheets, embedded within three alpha helices. The third (N-terminal) domain contained two helices joined together by an extended strand. The study presents Ligand Explorer tool-aided identification of amino acid residues constituting a PLP binding site in the enzyme. In the 502 amino acid residue catalytic, these are Phe101, Thr166, Thr167, Ser168, His203, Thr205, Thr258, Gly260, Thr262, Asp287, Ala289, Tyr290, Ser316, His318, and Lys319.
Conclusions
Tryptophan-originated indole metabolism in plants for secondary metabolite production is quite diverse across different genera covering their specialized types of indolyl phytochemicals. These include, among others, indole alkaloid producer species in Apocyanaceae, indolyl glucosinolates in some Brassicaceae species, serotonin in several species across several families, and withanamides in Solanaceae species like Withania. Tryptophan decarboxylase catalyzed tryptamine production is specifically connected to these biosynthetic needs in different plants for different indolyl molecules calling for understanding of catalytic and kinetic properties of TDCs from them. This study gives an account of the 1.5-kb full-length TDC gene of W. coagulans as well as catalytic and biochemical features of the recombinant enzyme as well as their comparison with other plant TDCs. A significant sequence similarity of WcTDC with TDCs but not TYDCs from other, conserved lysine residue (K319) in PLP binding motif, higher Km for tryptophan than for pyridoxal 5′-phosphate, substrate specificity limited to tryptophan or its analog having indole structural frame, and a 3-D homology model resembling other aromatic amino acid decarboxylases are the major characteristics of the recombinant enzyme. The study prospects significant metabolic and physiological significance of the gene in generating tryptamine-linked secondary metabolites like serotonin, withanamides, etc. Accordingly, WcTDC would be a very promising gene for targeting and understanding its role in the metabolism of indolyl secondary metabolites and associated physiological roles like defense (Li et al. 2015), disease resistance, etc. This pertains to be the first report on cloning and characterization of heterologously expressed recombinant enzyme from W. coagulans and forms a starting point for further understanding of withanamide biosynthesis.
Abbreviations
- PCR:
-
Polymerase chain reaction
- RACE:
-
Rapid amplification of cDNA ends
- ORF:
-
Open reading frame
- kDa:
-
Kilo dalton
- pI:
-
Isoelectric point
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- IPTG:
-
Isopropyl β-d-1 thiogalactopyranoside
- WcTDC:
-
Tryptophan decarboxylase from Withania coagulans
References
Akula R, Giridhar P, Ravishankar GA (2011) Phytoserotonin: a review. Plant Signal Behav 6:800–809
Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN (2001) Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol 8:963--967
Chaurasiya ND, Sangwan NS, Sabir F, Misra LN, Sangwan RS (2012) Withanolide biosynthesis recruits both mevalonate and DOXP pathways of isoprenogenesis in Ashwagandha Withania somnifera L. (Dunal). Plant Cell Rep 31:1889–1897
De Luca V, Marinea C, Brisson N (1989) Molecular cloning and analysis of cDNA encoding a plant tryptophan decarboxylase: comparison with animal dopa decarboxylases. Proc Natl Acad Sci U S A 86:2582–2586
Facchini PJ, Huber-Allanach KL, Tari LW (2000) Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 54:121–138
Gupta P, Agarwal AV, Akhtar N, Sangwan RS, Singh SP, Trived PK (2012) Cloning and characterization of 2-C-methyl-d-erythritol-4-phosphate pathway genes for isoprenoid biosynthesis from Indian ginseng, Withania somnifera. Protoplasma 250:285–295
Hemlatha S, Wahi AK, Singh PN, Chansouria JPN (2004) Hypoglycemic activity of Withania coagulans Dunal in streptozotocine induced diabetic rats. J Ethnopharmacol 93:261–264
Jaiswal D, Rai PK, Watal G (2009) Antidiabetic effect of Withania coagulans in experimental rats. Indian J Clin Biochem 24:88–93
Jayaprakasham B, Padmanabhan K, Nair MG (2010) Withanamides in Withania somnifera fruit protect PC-12 cells from β-amyloid responsible for Alzheimer’s disease. Phytother Res 24:859–863
Kang S, Kang K, Lee K, Back K (2007) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227:263–272
Kang K, Kang S, Lee K, Park M (2008) Enzymatic features of serotonin biosynthetic enzyme and serotonin biosynthesis in plants. Plant Signal Behav 3:38
Khare CP (2007) Indian medicinal plants. Springer, Berlin
Kushwaha AK, Sangwan NS, Tripathi S, Sangwan RS (2013a) Molecular cloning and catalytic characterization of a recombinant tropine biosynthetic tropinone reductase from Withania coagulans leaf. Gene 516:238–247
Kushwaha AK, Sangwan NS, Trivedi PK, Mishra LN, Sangwan NS, Tripathi S, Sangwan RS (2013b) Tropine forming tropinone reductase gene from W. somnifera (Ashwagandha): biochemical characteristics of the recombinant enzyme and novel physiological overtones of tissue-wide gene expression patterns. PLoS ONE 8(9): e74777. doi:10.1371/journal.pone.0074777
Li L, Zheng M, Long H, Deng G, Ishihara A, Liu F, Liang J, Pan Z, Yu M (2015) Molecular cloning and characterization of two genes encoding tryptophan decarboxylase from Aegilops variabilis with resistance to the cereal cyst nematode (Heterodera avenae) and root-knot nematode (Meloidogyne naasi). Plant Mol Biol Rep. doi:10.1007/s11105-015-0909-3
Liu W, Chen R, Chen M, Zhang H, Peng M, Yang C, Ming X, Lan X, Liao Z (2012) Tryptophan decarboxylase plays an important role in ajmalicine biosynthesis in Rauvolfia verticillata. Planta 236:239–250
López-Meyse M, Nessler CL (1997) Tryptophan decarboxylase is encoded by two autonomously regulated genes in Camptotheca acuminate which are differentially expressed during development and stress. Plant J 11:1167–1175
Maurya R, Akanksha J (2010) Chemistry and pharmacology of Withania coagulans: an ayurvedic remedy. J Pharma Pharmacol 62:153–160
Mishra S, Sangwan RS, Bansal S, Sangwan NS (2013) Efficient transgenic plant production of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma 250:451–458
Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS (2014) Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 251:1031–1045
Murch SJ, KrishnaRaj S, Saxena PK (2000) Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep 19:698–704
Park M, Kang K, Park S, Back K (2008) Conversion of 5hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli and yeast. Biosci Biotehnol Biochem 72:2456–2458
Percudani R, Peracchi A (2003) A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4:850–854
Rajurkar SM, Thakre PN, Waddukar SG (2001) Phytochemical and pharmacological screening of Withania coagulans berries as antiinflammatory. IPC, New Delhi, Dec., Sci 53 Abst CP38: 215
Sabir F, Mishra S, Sangwan RS, Jadaun JS, Sangwan NS (2012a) Qualitative and quantitative variations in withanolides and expression of some pathway genes during different stages of morphogenesis in Withania somnifera Dunal. Protoplasma 250:539–549
Sabir F, Sangwan RS, Kumar R, Sangwan NS (2012b) Salt stress induced responses in growth and metabolism in callus cultures and differentiating in vitro shoots of Indian ginseng (Withania somnifera Dunal). J Plant Growth Regul 31:537–548
Sandmeier E, Hale TI, Christen P (1994) Multiple evolutionary origin of pyridoxal-5′ phosphate-dependent amino acid decarboxylases. Eur J Biochem 221:997–1002
Sangwan NS and Sangwan RS (2014) Secondary metabolites of traditional medical plants—a case study of Ashwagandha (Withania somnifera) L. in Applied Plant Cell Biology Cellular Tools and Approaches for Plant Biotechnology in series Plant Cell Monographs Eds Zdeněk Opatrný and Peter Nick, Springer pp. 325–367. Series ISSN 1861–1370
Sangwan RS, Mishra S, Kumar S (1998) Direct fluorometry of phase-extracted tryptamine-based fast quantitative assay of L-tryptophan decarboxylase from Catharanthus roseus leaf. Anal Biochem 255:39–46
Sangwan RS, Chaurasiya ND, Mishra LN, Lal P, Uniyal GC, Sharma R, Sangwan NS, Suri KA, Quazi GN, Tuli R (2004) Phytochemical variability in commercial herbal products and preparations. Curr Sci 86:461–465
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Uniyal GC, Tuli R, Sangwan NS (2007) Withanolide A biogeneration in in vitro shoot cultures of Ashwagandha (Withania somnifera DUNAL), a main medicinal plant in Ayurveda. Chem Pharm Bull 55:1371–1375
Sangwan RS, Chaurasiya ND, Lal P, Misra L, Tuli R, Sangwan NS (2008) Root-contained withanolide A is inherently de novo synthesized within roots in Ashwagandha (Withania somnifera). Physiol Plant 133:278–287
Schneider G, Kack H, Lindqvist Y (2000) The manifold of vitamin B6 dependent enzymes. Struct Fold Des 8:9–390
Sharma PK, Sangwan NS, Bose SK, Sangwan RS (2013) Biochemical characteristics of a novel vegetative tissue geraniol acetyltransferase from a monoterpene oil grass (Palmarosa, Cymbopogon martinii var. Motia) leaf. Plant Sci 203:63–73
Tuli R, Sangwan RS (2010) Ashwagandha (Withania somnifera)—a model Indian medicinal plant. Council of Scientific and Industrial Research (CSIR), New Delhi, ISBN no. 978-93-80235-29-5
Yadav RK, Sangwan RS, Sabir F, Srivastava AK, Sangwan NS (2014) Effect of prolonged water stress on specialized secondary metabolites, peltate glandular trichomes, and pathway gene expression in Artemisia annua L. Plant Physiol Biochem 74:70–83
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthesis genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44:395–403
Acknowledgments
RSS and NSS thank New Millenium Indian Technology Leadership Initiative (NMITLI) for NMITLI Project grant on Ashwagandha. NSS also thanks Network project BSC 0203 for the grant for research. JSJ acknowledges financial support of the University Grant Commission as research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Additional information
Handling Editor: Néstor Carrillo
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 972 kb)
Rights and permissions
About this article
Cite this article
Jadaun, J.S., Sangwan, N.S., Narnoliya, L.K. et al. Withania coagulans tryptophan decarboxylase gene cloning, heterologous expression, and catalytic characteristics of the recombinant enzyme. Protoplasma 254, 181–192 (2017). https://doi.org/10.1007/s00709-015-0929-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0929-8