Abstract
This review provides detailed insight on the effects of magnetic fields on germination, growth, development, and yield of plants focusing on ex vitro growth and development and discussing the possible physiological and biochemical responses. The MFs considered in this review range from the nanoTesla (nT) to geomagnetic levels, up to very strong MFs greater than 15 Tesla (T) and also super-weak MFs (near 0 T). The theoretical bases of the action of MFs on plant growth, which are complex, are not discussed here and thus far, there is limited mathematical background about the action of MFs on plant growth. MFs can positively influence the morphogenesis of several plants which allows them to be used in practical situations. MFs have thus far been shown to modify seed germination and affect seedling growth and development in a wide range of plants, including field, fodder, and industrial crops; cereals and pseudo-cereals; grasses; herbs and medicinal plants; horticultural crops (vegetables, fruits, ornamentals); trees; and model crops. This is important since MFs may constitute a non-residual and non-toxic stimulus. In addition to presenting and summarizing the effects of MFs on plant growth and development, we also provide possible physiological and biochemical explanations for these responses including stress-related responses of plants, explanations based on dia-, para-, and ferromagnetism, oriented movements of substances, and cellular and molecular changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Life and magnetic fields
All organisms live under the influence of the Earth’s magnetic field (MF), also termed the geoMF (GMF), (5 × 10−6 Tesla (T) with geographical variations in its intensity ranges from 25 to 65 μT, its inclination, and declination (Maus et al. 2010). The GMF is a natural component of their environment (Belyavskaya 2004) but constitutes a type of abiotic stress (Wang et al. 2006). Despite this wide influence, only relatively few studies have shown how biological systems are affected by external MFs, in strengths lower or higher than GMF (Atak et al. 2003, 2007; Belyavskaya 2004; Dhawi and Al-Khayri 2009) even though studies have been conducted since at least 1930 (Savostin 1930). Initial studies found a simple cause and effect relationship between MF treatment and plant growth (Audus 1960; Pittman 1977). In those studies, it was observed that MFs affected the metabolism and growth of different plants based on the type of magnet used; the intensity of the MF; and the polarity, orientation, and length of duration of exposure. According to Galland and Pazur (2005), four types of MF are based on the sensitivity of plants to MF: “(1) weak static homogeneous magnetic fields (0–100 μT, including GMF), (2) strong homogeneous magnetic fields (milliTesla to Tesla), (3) strong inhomogeneous magnetic fields, (4) extremely low frequency (ELF) magnetic fields of low to moderate (several hundred μT) magnetic flux densities.” According to Belyavskaya (2004), a weak magnetic field (WMF) lies between 100 nT and 0.5 mT while extremely low electromagnetic field (EMF) is <100 μT. In this review, the limits specified by Belyavskaya (2004) have been adopted.
Biophysical methods like magnetic and electromagnetic stimulation can be promising and environmentally sound methods in the future agriculture. Therefore, the aim of this review is to summarize our recent knowledge of how MFs influence seed germination, seedling growth, and yield and to overview the potential basic biological reasons for the observed growth patterns. This review limits itself to the effect of MFs on ex vitro seed-related parameters using static MFs or electromagnetic fields (EMFs). The use of magnetized water (MW) or magnetic water (Teixeira da Silva and Dobránszki 2014) and the impact of MFs on in vitro growth and development are discussed elsewhere (Teixeira da Silva and Dobránszki 2015), while the evolutionary implications are indicated in detail by Maffei (2014).
Effects of magnetic fields on seed germination, vigor, and seedling development
Seed vigor includes “those seed properties, which determine the potential for rapid, uniform emergence and development of normal seedling under a wide range of field conditions” (AOSA 1983). MFs may serve in agriculture as a physical pre-treatment for seeds, in a bid to improve germination and seedling emergence, increasing production without harmful effects on the environment (Vasilevski 2003). The bio-stimulative impact usually depends on the following factors: genotype, the frequency of alternating fields, magnetic flux density, seed exposure time, absolute exposure dose, and polarity (north or south). Earlier studies indicated that roots were more pre-disposed to being affected by MFs than shoots (Bathnagar and Deb 1977; Kavi 1983), hence increasing and improving nutrient assimilation.
Effects of super-weak (near to 0 T) magnetic field
Application of a super-weak MF (near 0 T) is important to investigate the behavior of plants under space conditions. A near-zero MF (ZMF) can be generated in the absence of the main static component of GMF for compensating GMF (i.e., ZMF condition) or by shielding (i.e., WMF) (Neamţu and Morariu 2005). The A P index (NGDC 2015) was used to represent the magnitude of geomagnetic field activity (GMA) and variations in GMA (standard deviations of daily A P index) was used to model the effect of magnetic fluctuation induced by a magnetic storm at minor, moderate and major levels. Seed germination of alfalfa (Medicago sativa L.) (GMA (A P index) 2–10), rye (Secale cereale L.) (GMA (A P index) 2–11), French marigold (Tagetes patula L.) (GMA (A P index) 2–8), pot marigold (Calendula officinalis L.) (GMA (A P index) 2–8), buckwheat (GMA (A P index) 2–8), wheat (GMA (A P index) 2–11), and cress (Lepidiumus sativum L.) (GMA (A P index) 2–7) was not different in ZMF or in GMF in a quiet GMA. However, germination of alfalfa and rye seeds was stimulated in a disturbed period of GMF activity in ZMF conditions (Neamţu and Morariu 2005) when GMA was increased to the level of a major (A P = 80) magnetic storm. After 13-h-long incubation, the germination rate of rye seeds was 133 % higher in ZMF than in GMF (control) and a moderate (A P = 53) magnetic storm caused a significant increase in the germination of alfalfa seeds (10 %) when it was applied at the beginning of the experiment. After 5 days of incubation, the early growth of seedlings depended on the species but the growth of pot marigold was not influenced; however, the root growth of rye and wheat was inhibited while the growth of cress and alfalfa was stimulated by ZMF. By applying a magnetic storm, the root growth inhibition of rye seedlings was diminished. Anatomical and ultrastructural changes were detected in soybean seedlings exposed to space conditions (Foton-M2 mission on board the Foton-M2 capsule between 31 May 2005 and 16 June 2005) during germination and seedling growth for 5 days (De Micco et al. 2008) by monitoring cellulose microfibril orientation and assembly. Cell wall development was perturbed but not prevented at an early stage of primary vessel development indicating that cell wall building and hereby seedling growth slowed in space conditions. Although no differences were detected in biomass accumulation during the vegetative growth of Arabidopsis under near-null MF, it negatively affected reproductive development and growth (Xu et al. 2013). Under near-zero MF, a delay of flowering was detected, and seed production per plant was significantly lower (by 19 %) compared to the local GMF condition (45 μT), causing a 20 % reduction in harvest index.
Effects of WMF (100 nT–0.5 mT) and ELF EMFs
Cabbage (Brassica oleracea L.) seeds and seedlings exposed to a 0.4–0.5 mT MF showed decreased growth and germination—relative to control plants—after the external MF was removed (Namba et al. 1995).
In contrast, other studies concluded that weak MFs inhibited seedling growth in one genotype (G3.27) of oak out of three genotypes studied when isolated somatic embryos were exposed to EMF (15 μT for 8 weeks; Celestino et al. 1998), in sugar beet (Beta vulgaris var. saccharifera; 20 nT–0.1 mT for ≥ 4 days; Belyavskaya 2004). However, Govoroon et al. (1992, cf. Belyavskaya 2004) observed no effect of 1.0 nT MF on seed germination in pea (Pisum sativum L.), common flax (Linum usitatissimum L.), and lentil.
Kordas (2002) surrounded potted spring wheat plantlets with a ring of permanent magnets (roots and shoots separately surrounded), and noted a slight improvement in root qualities, but a significant decrease in plant height. In that study, however, the strength of the MF or the time of exposure to MFs, as well as other important methodological parameters were not mentioned, nor was the distance between magnets and plant parts specified, making the conclusions unreliable.
When sunflower and wheat were exposed to a weak vertical MF of 16 2/3 Hz and sinusoidal 20 μT for 12 days (Fischer et al. 2004), the effect of MF depended on the species; in sunflower, a significant increase was detected in the fresh weight of both shoots and roots but germination rate and the dry weight of plants were not affected. In wheat, germination rate and root fresh and dry weights as well as total fresh weight increased significantly compared to the controls.
Electromagnetic field (EMF) is the combination of a MF produced by moving charges and an electric field produced by stationary charges and it is generated by an alternating current (AC) in electrical conductors. An experiment by Smith et al. (1993) was based on a hypothesis that the same authors had developed earlier (Smith et al. 1987) in which the movement of ions in their passage through a membrane channel was promoted if the energetic conditions were configured exactly accordingly to the channel dimensions such that when energetic conditions fitted other parameters, the EMFs either had no effect, or an inhibitory effect. In the experiments, EMFs were tuned to ion cyclotron resonance (ICR) frequencies of Ca2+ and K+ ions, respectively. Radish (Raphanus sativus L.) seeds were exposed to a 60 Hz EMF for 21 days and daily for 24 h (Smith et al. 1993). If seeds were exposed to the Ca2+-tuned frequency, germination was slower than in control seeds but the seedlings grew rapidly. However, if the frequency was tuned to K+ ions, germination occurred rapidly and seedling shoot growth was slower but root weight was higher than in the control treatment. Davies (1996) applied a continuous EMF of 60 Hz tuned to the ion cyclotron resonance frequency of Ca2+ to radish, mustard (Sinapsis alba L.) and barley (Hordeum vulgare L.) seeds for 9–21 days and found clear species-specific dependence regarding the effect of EMF. Both shoot growth (shoot dry weight, leaf dry weight, stem diameter, and plant height) and root growth (dry weight) were stimulated by EMF treatments in radish, but EMF treatments did not affect the growth of mustard plants. The growth of barley plants were also affected in two of the three experiments: fresh root weight increased but stem diameter and seed dry weight decreased compared to the control. Low frequency (50 Hz) EMF had stimulatory and inhibitory effects on the germination of wheat seeds depending on the exposure period (Aksyonov et al. 2007). A single and brief exposure (15 h) at the start of germination increased germination percentage and was able to overcome the adverse effect of osmotic pressure (10–16 atm). However, the application of EMF for 6 days continuously was harmful, with a 45–84 % decrease in germination percentage and a 17–33 % decrease in seedling length relative to the control, but the exact magnitude depended on the cultivar. Treatment with high electric field (50 Hz, 2–16 kV/cm, 1–30 s) successfully stimulated the germination of common bean (Phaseolus vulgaris L.) seeds infected with Colletotrichum lindemuthianum (Morar et al. 1999). The germination percentage of the treated seeds increased from 30 to 99 % and plant weight was also significantly higher after electrostatic treatments. Both the exact method and the used apparatus were patented. Treatment of cork oak (Quercus suber L.) acorns with EMF (50 Hz, 15 μT for 13 weeks using a Helmholtz coil system) did not affect the final germination percentage but increased the rate of sprouting and seedling growth (shoot length, axillary shoot formation, and shoot weight) (Celestino et al. 2000). EMF (50 Hz full wave rectified sinusoidal voltage, 60, 120 or 180 mT for 5, 10, or 15 min) treatments, when applied before the germination of pea seeds (pre-sowing treatments), affected the growth of pea seedlings (Iqbal et al. 2012). The length, fresh and dry mass of shoots, roots, and seedlings increased but chlorophyll (chl) content was not significantly affected. Among the MF treatments applied, two treatments were superior: 120 mT for 15 min and 180 mT for 10 min.
The effect of different EMF field strengths of 380 kV was tested on the yield of maize and winter wheat for 5 years by planting the crops at different distances (40, 14, 8, and 2 m) from the Dürnrohr-Slavetice transmission line (Soja et al. 2003). At these distances between the two cities, the electric field strength was between 0.2 and 4.0 kV/m and the MF strength varied between 0.4 and 4.5 μT. As field strength decreased, grain yield of winter wheat increased, on average for 5 years 7 % higher at the innermost plots than plots nearer to the transmission line but the degree of differences depended on the drought periods in each year. No significant effect on maize yield was detected. Hasan et al. (2011) modeled the effect of high voltage transmission lines under laboratory conditions with an MF strength of 0.084 mT coinciding with 400 kV and an MF strength of 0.045 mT. The leaf growth of maize (leaf area, dry weight of leaves) was depressed by MF, but the extent depended on its strength. The effects of transmission line were studied on the nitrogen, protein and chl content and peroxidase (POX) activity in oil palm (Elaeis guineensis var. tenera) leaves when leaves were exposed to an EMF of 275 kV for 6 months and 7 years at different distances (0, 8.8, 17.6, 26.4, 35.2, 44, 52.8, and 61.6 m) from the transmission line (Mahmood et al. 2013). Chlorophyll content (chl a, chl b, and total chl) was greatest in those leaves placed at a distance 8.8 m from the transmission line and it decreased as the distance increased. Protein content did not change significantly in the leaves when they were exposed long term (7 years), but three different protein banding patterns were detected after exposure for short term (6 months). When the strength of the EMF increased (i.e., decreasing the distance from the line) the activity of POX increased, which indicated a stress response in the leaves.
Rezaiiasl et al. (2012) exposed cucumber (Cucumis sativus L.) seeds to 20 μT AC for 30 min or to 5 μT direct current (DC) for 30 min. Most fruit and flower parameters were negatively affected by both AC and DC MFs, but the number of fruits per plant and the length of the main stem increased relative to the untreated control.
Effects of strong magnetic and electromagnetic fields (mT–T)
Some studies (Table 1) have shown that MFs and EMFs between 1.5 and 250 mT had a positive effect on seed germination and seedling growth in different plant species, enhanced the biomass and the yield (Alexander and Doijode 1995; Phirke et al. 1996; Carbonell et al. 2000; Moon and Chung 2000; Aladjadjiyan 2002; Martínez et al. 2002, 2008; Eşitken 2003; Flórez et al. 2004, 2007; Podleśny et al. 2004; de Souza et al. 2006; Rãcuciu et al. 2008; Vashisth and Nagarajan 2008; Shabrangi and Majd 2009; Subber et al. 2012; Radhakrishnan and Kumari 2012, 2013a; Bilalis et al. 2013; Krawiec et al. 2013) and had stimulatory effects on enzyme activities in seeds (Vashisth and Nagarajan 2010). Observations from experiments with lentil (Lens culinaris Med.) seeds exposed to a stationary MF of 150 mT (Aladjadjiyan 2012) slightly contradicted the results of Vashisth and Nagarajan (2010) detailed in Table 1 and also with the results of the same author on soybean (Aladjadjiyan 2003). The magnetic treatment did not significantly affect germination and early (1-week-old) seedling growth, although, after 14 days of MF treatment, a 120 and 104 % increase in shoot length and a 11 and 12 % increase in root length were observed after seeds were exposed to MF for 6 and 9 min, respectively (Aladjadjiyan 2012).
A MF of 60 or 100 mT for 7.5, 15, or 30 min at 60 Hz improved the germination of maize by a maximum of 23 and a 30 % increase in seedling dry weight when 100 mT for 7.5 min was applied, although the pole was not mentioned and the response was strongly cultivar-dependent, showing positive, neutral, or even negative effects relative to the control (Aguilar et al. 2009). In wheat, lentil, and soybean exposed to MFs of 2.1 and 17.5 mT four times over a 10-month period root growth was inhibited on a species-dependent way (Peñuelas et al. 2004). The effects of the same MF field strength and the same exposure periods were investigated on the germination and early seedling development of different plant species, such as tall fescue (Festuca arundinacea Schreb.), perennial ryegrass (Lolium perenne L.), pea (var. ‘Aravalle’) triticale and tomato (cultivar not indicated) (Carbonell et al. 2008, 2011; Martínez et al. 2009; Flórez et al. 2014). In tall fescue and perennial ryegrass, germination time decreased more than 10 % and germination percentage increased by 8–10 %. Moreover, root length increased as much as 107 % when seeds were exposed to a MF for 24 h, or continuously (Carbonell et al. 2008). Both 125 and 250 mT MFs stimulated the initial growth of pea seedlings, mainly if continuously exposed to MF, and a 99 and 97 % increase in stem length and a 67 and 58 % increase in total seedling length at 125 and 250 mT, respectively, were detected after 7 days. After 10 days, total length of seedlings was 14 and 13 % higher and total weight was 53 % higher than in the control treatment (Carbonell et al. 2011). In experiments with tomato (Martínez et al. 2009), when the exposure period exceeded 1 min, the mean germination time was also significantly reduced (from 117.6 to 110.4–113.04 h), depending on the field strength and exposure period. The time required to reach 10 % germination, the indicator of early germination, was reduced after exposure period of at least 10 min at 125 mT but it was independent of exposure period at 250 mT. Seedling length increased when MF was exposed at 250 mT and chronically. Stationary MF (125 or 250 mT), when applied for 24 h to triticale seeds, significantly decreased the mean germination rate by 12 % at both field strengths; seeds sprouted earlier and MF treatments resulted in the tallest seedlings (Flórez et al. 2014). Mean germination time of tomato seeds (cultivar was not reported) was decreased by 62 % when using continuous MF (applying 1 × 5 cm magnetic tape pieces of 3 mT at the bottom of Petri dishes) and by 30 % by using a MF of 15 mT (permanent magnet of 2.5 × 5 × 2.3 cm) for 25 min (Feizi et al. 2012). Moreover, in their study, continuous MF and an MF of 25 mT applied for 5 min increased seedling length by 33 and 25 %, respectively. In an earlier experiment with two tomato cultivars (‘Rocco’, ‘Monza’; Danilov et al. 1994), seeds (factor A), seedbeds (factor B), plots (factor C), and irrigation water (factor D) were treated by MF (3200–4800 amp/min, 4–6 mT) in three different combinations (MF treatments of all four factors; i.e., factors A+B+C+D; only seedbeds and plots were treated by MF, i.e., factors B+C or no MF-treated irrigation water was used but seeds, seedbeds, and plots were treated by MF, i.e., factors A+B+C). ‘Monza’ responded positively to all MF treatments: early yield was 51, 28 and 39 % higher than the control, respectively (i.e., A+B+C+D, B+C, A+B+C); similarly, the yield of first-class fruits was 25–40 % higher after MF treatments. ‘Monza’ plants started to flower 3–4 days earlier when plots were treated by MF. However, MF treatments were ineffective for ‘Rocco’.
The optimum MF treatment was determined in field experiments for soybean, cotton and wheat (Phirke et al. 1996) and exposure period (tested from 13 to 27 min) was more important than the strength of the MF tested (from 72 to 128 mT). The optimal MF level for seeds of all three species was 100 mT although optimal exposure period varied for each species: 25 min for cotton and soybean but 13 min for wheat. Yield increased by 46 % (soybean), 32 % (cotton), and 3 % (wheat) at optimal MF treatment. In a pre-sowing treatment, tomato (Solanum lycopersicum hybrid Noxana) seeds were pulsed with EMF (12.5-mT amplitude) for 10 or 15 min. Consequently, shoot diameter (by 5–10 %), leaf number/plant (37–47 %), fresh and dry weight (14–15 and 13 %, respectively), and the number of flowers (3–12 %) increased significantly (Efthimiadou et al. 2014). There were no significant differences in lycopene content but plants were significantly shorter when exposed to MF.
The growth and yield of butterhead lettuce (Lactuca sativa var. ‘Salina’) seeds exposed to static MF (N, S, 40–8 mT, for 24 or 72 h) were evaluated after cultivation in hydroponic culture (Poinapen et al. 2005). The effects depended both on the polarity and the exposure period of the MF. When seeds were exposed to south MF for 24 h, there was a 12.9 % increase in the dry to fresh mass ratio and an 18.8 % increase in yield.
Increasing MFs from 96–192 to 384 mT when treated greenhouse-grown strawberry (Fragaria × ananassa Duchesne ‘Camarosa’) negatively affected the leaf growth (13.6 leaves/plant) unlike the stimulating effect of 96–192 mT on leaf growth and development (18.3 and 19.4 leaves/plant, respectively; Table 1) (Eşitken 2003). The effect of higher MF (1.5 T) depended on the duration and temporal pattern of its exposure when winter wheat seeds were treated (Eskov and Darkov 2003). When the on-off time ratio of the treatment increased, early growth was stimulated and germination increased by 8.3–12.3 %. When seeds were air dried (9.2 % water content) and moistened (40.7 % water content), they responded similarly to the MF, indicating the lack of a direct effect of water in the response.
The direction of root growth and the growth rate were modified by MF when caryopses with primary roots of maize ‘Golden Cross Bantam 70’ were incubated on 0.4 % agar-solidified medium and exposed to a MF of 5000 G (Kato 1988). When the direction of root growth was parallel with the direction of the MF in line with it or opposite to it, the growth rate of roots was 27 and 22 % higher than the control treatment (MF of 10 G), respectively, depending on the direction of the north and south poles. When the direction of MF was perpendicular to the direction of root growth, MF increased the growth rate of the maize roots by 15 %. Geotaxis of the shoots and roots of cucumber seedlings was modified when germinating seedlings were treated by a non-uniform MF by applying a super-conducting magnet with 10 T MF in its center used in a horizontal position (cryogen-free type magnet; Sumitimo Heavy Industries Co., HFM 10-100VHT-1) (Hirota et al. 1999). Seeds were placed in the bore (i.e., auger-hole) and were kept in darkness to avoid the influence of phototaxis. The rate of the modifying effect of MF on geotaxis was correlated with the intensity of the MF, which varied in the horizontal direction. Shoots and roots leaned toward the field center of the magnet. The inclination of both shoots and roots depended on the resultant force of gravity and the magnetic force and therefore, the degree of the inclination correlated with the amplitude of MF. Maximum inclination (θ) was detected at or near 100 mm from the field center on both sides where the MF reached its maximum value (Suppl. Fig. 1).
MF treatment was observed to affect also the biotic and abiotic stress tolerance of different plant species. The exposure of wheat and common bean seeds to 7 mT for 7 days improved seedling growth and germination percentage, even under salt or osmotic pressure (Cakmak et al. 2010). Gubbels (1982) reported earlier germination and more vigorous seedling growth after seeds of common flax, buckwheat (Fagopyrum esculentum Moench.), sunflower, and pea were exposed to MF (300 G, horseshoe magnet) but the results from these experiments were quite inconsistent. When the first seed lot germinated at 10 °C, there was no difference between the germination of MF-treated and non-treated seeds, but in the second lot germinated at 10 °C and in both seed lots germinated at 20 °C, small significant differences were detected. Sunflower seed yield in field experiments increased significantly after MF treatment of seeds in one of the years examined, but no differences were detected in the three remaining years and in other crops, no significant change in yield was detected in field experiments. Poinapen et al. (2013) evaluated the role and contribution of different environmental factors on the seed germination of tomato (var. MST/32) and ranked the effects of relative humidity (7.0, 25.5, and 75.5 %), static MF (flux density 332.1 ± 37.8, 108.7 ± 26.9, 50.6 ± 10.5 mT; exposure times of 1, 2, and 24 h) and seed orientation (north and south polarities). Seed orientation and MF strength influenced seed imbibition more than relative humidity. Ranking the factors examined, mostly seed orientation followed by MF strength and relative humidity influenced seed germination (11 % higher in a south orientation and at a higher MF) and accumulation of seedling biomass. MF treatments (pre-sowing sinusoidal, non-uniform MF, 100 mT for 10 min or 170 mT for 3 min) also significantly delayed the appearance of symptoms of early blight (caused by Alternaria solani) and geminivirus; moreover, they reduced the infection rate of early blight (de Souza et al. 2006).
Possible physiological and biochemical explanations for the response of plants to magnetic fields
Living organisms, including plants, themselves generate and use different electrical fields (currents, signals) during their functioning, such as (trans)membrane, electric, action, or streaming potential; therefore, it is expected that MF and EMF can affect and influence the development and metabolism of plants when interacting (Goldsworthy 2006; Trontelj et al. 2006). The mechanisms of the interaction of MFs with biological systems are still not well understood. There is still very scanty and dispersed information on the effects of MFs on plant growth in the literature despite the literature stretching as far back as ~25 years. Moreover, theory is massively divided from practice, making a clear understanding of the practical effects of MFs on plant growth and development difficult to understand from the physical and mathematical theorems currently available and thus beyond the reach or comprehension of most plant scientists. Due to the complexities of the theoretical nature of MFs, the mathematical understanding of MFs on plant growth will be covered in a separate review, although some broad understanding can be obtained from Goldsworthy (2006) and Asemota (2010).
Stress-related explanation
External MFs higher or lower than GMF and external EMFs are one type of abiotic stresses in plants (Wang et al. 2006). Consequently, there are some stress-related biological explanations for their effects on biological systems. Early studies already hinted at the action of proteins and enzymes in biochemical processes involving free radicals during enhanced seed vigor (Murray 1965).
Chl is degraded in vitro by POX in the presence of some phenolic compounds. POX oxidizes phenolic compounds with hydrogen peroxide and forms a phenoxy radical, which then oxidizes chl and its derivative to a colorless low molecular weight compound (Yamauchi et al. 2004). A study by Atak et al. (2007) on the effects of MFs on POX activities of soybean shoot tip culture showed an increase in POX activity after exposure to MFs of 2.9–4.6 mT for 19.8 s. At this dose of MF treatment, chl content decreased. It was concluded that chl content decreased when POX activity increased, suggesting the existence of an abiotic stress response. Therefore, at 150 mT MF, regardless of polarity, orientation and duration of exposure, MFs might increase POX activity in other plants, thus decreasing the chl content. The activity of ascorbate peroxidase (APX), another abiotic stress indicator, increased significantly in lentil shoots and roots at 180–360 mT, although much more in shoots, but no change in superoxide dismutase (SOD) activity was detected in response to these MFs (Shabrangi and Majd 2009). There was a significant increase in SOD activity when shoot tips were exposed to 2.9–4.6 mT and a magnetic flux density 0, 1, 9, and 15-times at 2.2, 19.8, and 33 s (Büyükuslu et al. 2006). Similarly, the activities of some stress-related enzymes were also affected by MF treatment (2.9 and 4.8 mT, at 1 m/s for 2.2 and 19.8 s) in mature zygotic embryos of wheat ‘Flamura-85’ (Alikamanoglu and Sen 2011). The rate of increase in enzyme activities depended on the exposure time: 62 and 88 % increase in SOD activity after 2.2 and 19.8 s, respectively; 80 % increase in POX activity, 73 % increase in catalase (CAT) activity, and 48 % increase in APX activity after 19.8 s. The percentage of dead cells, soluble and covalently bound POX activity, and lignin content of the cell wall significantly increased and ionic POX activity decreased significantly when a suspension culture of tobacco (Nicotiana tabacum L. cv. ‘Burley 21’) cells was exposed to static MF (10 and 30 mT) for 5 days when cells were in a logarithmic phase of growth (Abdolmaleki et al. 2007). Pre-sowing magnetic treatment (static MF of 200 mT for 1 h) of maize ‘HQPM.1’ seeds caused changes in growth (78 % increase in leaf area and 40 % increase in root length), 26 % reduction in the level of superoxide radicals, reduction of antioxidant enzymes (43 % reduction in SOD; 23 % reduction in POX) and of photosynthesis (twofold higher performance index of plants) under field conditions (Shine and Guruprasad 2012).
The activities of enzymes involved in carbohydrate metabolism were altered when soybean seeds were pre-treated with pulsed MF of 1500 nT at 10 Hz for 20 days and daily for 5 h (Radhakrishnan and Kumari 2012). Activity of α-amylase decreased 50 % but that of β-amylase showed a very slight increase (2 %), indicating that starch degradation was stimulated, which is necessary for enhanced growth of seedlings. A 9 % increase in the activity of acid phosphatase and a considerable decrease in the activity of alkaline phosphatase (57 %), protease (10 %) and nitrate reductase (30 %) were also observed. The activity of enzymes related to the tolerance of plants to abiotic stresses increased drastically: 41 % increase in peroxidase (POX) activity and 95 % increase in catalase (CAT) activity.
The effect of weak permanent MFs (flux density of 185–650 μT) on the antioxidant system was reported in 5-day-old radish seedlings by Serdyukov and Novitskii (2013). Although the effect of MFs depended on its intensity, the dependence was non-linear. At 185–325 μT, SOD and CAT activity decreased by 25–35 and 60 %, respectively, relative to the control while the accumulation of malondialdehyde (MDA) increased (210 % higher than the control) in seedlings grown in the dark. However, in seedlings grown in light, SOD activity did not decrease. Increasing MF strength to 650 μT, SOD activity increased 135 % more than the control, while CAT activity increased 135 or 150 % more than control seedlings grown in the dark or light, respectively. Static MF (30 mT) increased CAT activity but reduced APX activity in parsley (Petroselium crispum L.) cells (Rajabbeigi et al. 2013).
The conditioning or stress-protecting effect of extremely low MF (50 Hz, 100 μT) was reported by Ružič and Jerman (2002) when it was applied to cress seedlings for 12 h before heat stress (41, 42, and 45 °C for 40 min). Monselise et al. (2003) reported the accumulation of alanine when axenic culture of duckweed (Spirodela oligorrhiza) was exposed to weak sinusoidal waves with varying MF (0.7 mT, 60 or 100 Hz for 24 h). Since alanine accumulated in response to several stress conditions, such as osmotic stress, heat stress, anoxia, or MF, Monselise et al. (2003) concluded that it was the universal stress signal in plants. When non-magnetized and magnetized tomato ‘StrainB’ seeds were irrigated with MW (magnetron model U.T.I., 1 in. Magnetic technologies L.C.C., Russia), the harmful effects of water stress at 60 and 40 % of field capacity were overcome, as observed from physio-anatomical characteristics of tomato plants (Selim and El-Nady 2011). Considering that out of naturally occurring minerals, magnetite is the most magnetic; its effect on plant growth was investigated by Ali et al. (2011). The protective effect of a magnetic treatment (0, 1, 2, 3, and 4 g/pot magnetite added to the pots twice during the entire plant growth phase) on the growth, yield and fruit quality of pepper (Capsicum annuum L.) occurred when seedlings were irrigated with saline water (240, 1000, 2000, 3000, and 4000 mg/l NaCl) (Ali et al. 2011). Growth and yield characters of pepper were improved by increasing the dose of magnetite with the best effect caused by a highest dose (4 g/pot). Similarly, pulsed MF was protective as it enhanced in vitro organogenesis under salt stress (Radhakrishnan and Kumari 2013b). Both shoot and root regeneration frequency from cotyledonary nodal explants of soybean were improved on medium containing 10–40 mM of NaCl when seedlings were exposed to pulsed MF (1.0 Hz). After cadmium (Cd) stress (5 μM Cd for 4 days) was applied to mung bean (Vigna radiata (L.) R. Wilczek) seedlings, the concentrations of MDA, H2O2, and O2−, and the conductivity of electrolyte leakage increased; however, the activity of nitric oxide synthase and thus the concentration of nitric oxide, as well as the net photosynthetic rate, decreased (Chen et al. 2011). When 600 mT MF (755R magnetometer, Model 2G Co., USA) was applied to seedlings before Cd stress, the toxic effects caused by Cd stress were alleviated and all growth parameters of seedlings (shoot and root length, number of lateral roots, fresh and dry weight, water concentration) were higher than in Cd-stressed seedlings although shoot and root length was not significantly different from the control.
Explanation based on diamagnetism, paramagnetism, ferromagnetism, and magnetotropism
MFs have been shown to increase the oscillation and concentration of free oxygen radicals in cells (Scaiano et al. 1995; Jajte 2000), which would increase cell stress and thus cells’ response through the production of antioxidant enzymes (Hasanuzzaman et al. 2012). Moreover, since most biological substances are proteins that contain metal ions, such as hemoglobin, cytochrome, or ferritin, they can be paramagnetic (Piruzyan et al. 1980). Vaezzadeh et al. (2006) proposed a theoretical model based on the oscillation of ferritin, the iron-storage protein in ferritin cells when exposed external MF. The paramagnetic (Fe, Co content) and diamagnetic (starch) components of lentil, soybean, and wheat differed when exposed to different static MFs and forces (176, 21 Gauss (G)) (Peñuelas et al. 2004). Peñuelas et al. stated that the effect of MF could be linked to the diamagnetic susceptibility of the plant species and depended on the magnetic force.
Although MFs have a direct effect on organisms by affecting water and solutes (Galland and Pazur 2005; Pang and Deng 2008), it is assumed that living organisms are capable of magnetoreception, i.e., they can perceive the Earth’s MF or GMF (Binhi 2001; Galland and Pazur 2005). A weak static homogeneous MF (<100 μT), which has a low energy content (Galland and Pazur 2005), is directly connected to magnetotropism in plants (Galland and Pazur 2005, based on seven studies in the literature). This low energy content is not enough for breaking chemical bonds, therefore, Galland and Pazur (2005) discussed three other possible mechanisms based on physical mechanism for the effect of magnets on plant growth beside ferrimagnetism, which is already well proved for bacterial magnetotaxis (Blackmore 1982) and ferrimagnetic crystals, such as magnetite or hematite, was also detected in plants (McClean et al. 2001): ion cyclotron resonance (ICR), quantum coherence, and the radical pair models. The ICR model is based on the path of ions in a WMF. Charged particles (ions) moving perpendicularly to a MF are kept on a circular path according to Lorentzian forces (Griffiths 1999). The force, which acts on charged particles moving in an EMF, has two components, a magnetic and an electric component. Charged particles have a Lamor frequency (Griffiths 1999) and can interfere with the altering EMF and therefore, the equilibria of biochemical processes can be altered by application of a MF. This model is further developed in a quantum coherence model based on wave-particle dualism and on quasi Schrödinger boxes (Schrödinger 1935). The radical pair model is based on the competition between spin dynamics (singlet-triplet conversion rates of radical pairs) and hereby radical separation during biochemical reactions of a living organism (Galland and Pazur 2005). In biochemical reactions, during homolysis, single radicals generated with anti-parallel spins are immediately transformed into a singlet state. This radical pair can intercovert to a triplet state having radicals with parallel spins via intersystem crossing (ISC) (Galland and Pazur 2005). According to Pauli’s exclusion principle (Galland and Pazur 2005), the triplet radical pair is no longer able to recombine to the parent molecule. By application of a MF, the rate of ISC can be modified and thus also the biological responses via product formation of the biochemical reaction involving radical pair intermediates (Galland and Pazur 2005).
Related to radical pair model, cryptochromes have recently been reported to supposedly participate in the magnetoreception of plants, serving as sensors since they can form radical pairs (Xu et al. 2014; reviewed in Maffei 2014). Xu et al. (2014) described that blue light-dependent phosphorylation and dark dephosphorylation of two cryptochromes (CRY1 and CRY2) were influenced by the field strength of the MF indicating that MFs differing from GMF affected the active/inactive states of cryptochromes. Their light-dependent phosphorylations were enhanced by a MF of 500 μT but light-dependent phosphorylation of CRY2 was decreased by MF of near-null T, while their dephosphorylations were inversely affected by different MF strengths. This finding was in good agreement with earlier results of Xu et al. (2012, 2013) when near 0 T MF suppressed biomass accumulation at the transition of vegetative growth to reproductive growth (Xu et al. 2013) and delayed flowering in Arabidopsis thaliana since flowering regulating function of cryptochromes was influenced by a MF of near 0 T and its effects were suggested to be cryptochrome-related (Xu et al. 2012).
Explanation based on oriented movements of substances
There is a theory, the “MF effect,” in photosynthesis, which could partially explain the interaction of MFs with intermediate ionic pairs. One possible explanation (since the theory has not in fact yet been tested) is that the increase in chl content of plants exposed to MFs might be related to the properties of MW and the oriented movement of paramagnetic substances under external MFs (Yaycılı and Alikamanoğlu 2005; Atak et al. 2007; Çelik et al. 2008; Dhawi and Al-Khayri 2009; Yan et al. 2009), although no new studies in the past 4 years have fortified this hypothesis. Chloroplasts contain Mn2+, which plays an essential role in photosynthesis and is a paramagnetic substance. When an external MF (i.e., 0.1–0.2 T) is applied, Mn2+, which does not move in the same direction as water, is oriented in the same direction as the applied field and tends to move into the MF. This interaction absorbs energy which could therefore affect chloroplasts, disturb pigment synthesis, and thus affect photosynthesis and hence biomass production (Commoner et al. 1956; Theg and Sayre 1979; Dhawi and Al-Khayri 2009). When seedlings of date palm (Phoenix dactylifera L.) were treated with a static magnetic field (100 mT, 360 min of exposure) their chl a and b, carotenoid. and total pigment contents increased significantly. An alternating MF of 1.5 T caused the increase in the pigment content after short exposure (1 and 5 min) but decrease in it after long exposure (10 and 15 min). Chl a and carotenoids were more sensitive to the magnetic treatment than chl b (Dhawi and Al-Khayri 2009). A pre-sowing magnetic field treatment (tested from 0 to 300 mT in steps of 50 mT for 30, 60. and 90 min) of soybean ‘JS-335’ seeds accelerated the germination by up to 42 %, increased the fresh weight and length of seedlings (up to 53 and 73 %, respectively) when a MF of 150 or 200 mT was applied for 60 min (Shine et al. 2011). A twofold increase in leaf area and leaf fresh weight was detected, photosynthetic efficiency increased and the intensities of bands belonging to both the larger (53 kDa) and smaller (14 kDa) subunits of RUBISCO also increased.
MFs also affect ions in the humid environments of plants and allow those ions to absorb MF energy and mobilization; increasing ion mobility and ion uptake leads to better photostimulation (Galland and Pazur 2005). These two theories would be reasonable candidates for explaining higher chl contents in the Van et al. (2011b) study on in vitro Phalaenopsis plantlets.
Moreover, MFs have the ability to change the properties of water; MW increases chl content in leaves (Pang and Deng 2008; Dhawi and Al-Khayri 2009), also observed for in vitro grown Cymbidium and Spathiphyllum (Van et al. 2012). It is difficult, however, to interpret how such a mechanism might be at play when MFs alleviate salt and heat stress (Xi et al. 1994; Ružič and Jerman 2002) and reduce senescence (Piacentini et al. 2001). A magnetic nanoparticle supply as magnetite (Fe3O4) and cobalt ferrite (CoFe2O4), tested between 20 and 100 μl/l, affected the chl levels in sunflower seedlings (Ursache-Oprisan et al. 2011). Photosynthetic pigment content (chl a and b, carotenoids) was negatively affected by magnetite nanoparticles, decreasing by 50 %; however, they affected only slightly and non-significantly the chl a/chl b ratio. Cobalt ferrite treatments (when Fe is partially substituted by Co) buffered the decrease in chl content, it was only 28 % and thus there was only a slight reduction in chl biosynthesis compared to the Fe3O4 nanoparticles but a higher decrease in the chl a/chl b ratio when they were applied between 20 and 60 μl/l. These effects were proposed by the authors to be related to both the impact of metal ions (Fe and Co) and the magnetic effect of nanoparticles. If MF treatment (magnet pieces with a dimension of 3 × 1 cm and a strength of 10 mT) was applied in combination with silver nanoparticles (40 g/ha colloidal nanosilver in the irrigation water), the yield and quality of fodder maize improved: a 35 % increase in the fresh yield and 41.3 % increase in ear percentage which was higher than 32.4 % of the control (Berahmand et al. 2012). Similarly, Li et al. (2013), using magnetic iron oxide nanoparticles, proved that nano-Fe2O3 at an optimal of 20 mg/l increased both the germination and seedling growth of watermelon (Citrullus lanatus var. lanatus) and changed also the activities of SOD, POD, and CAT enzymes. The effects of nanoparticles of magnetite (γ-Fe2O3) 24 nm in size and modified nanoparticles (Fe2O3–NH2, Fe2O3–OH) were studied in a tobacco BY-2 cell suspension culture (Krystofova et al. 2013), specifically the effect on the growth and biochemical parameters of cells. γ-Fe2O3 had no effect on cell growth and viability, but modified nanoparticles (Fe2O3–NH2, Fe2O3–OH) decreased the growth of cells; applied at 1 ng/ml, cell viability was reduced by 62.5 and 75 %, respectively, and by 45 and 60 %, respectively, when applied at 100 ng/ml. Modified nanoparticles also increased the protein content of cells by 102–178 % when they were applied at 10–100 ng/ml. Fe2O3–NH2 at 100 ng/ml reduced the thiol content (by 56 %) of control cells and the antioxidant content (by 53.9 %).
Explanation at cellular and molecular levels
Reina et al. (2001) observed that an increase in the germination rate of lettuce seeds treated with stationary MF (0–10 mT) was consistent with the rate of the absorbed water of the seeds. Reina and Pascual (2001) hypothesized that MF affects germination by altering water relations due to changes in the ionic current through the cellular membrane in the treated seeds. After bean seeds were germinated, seedlings were grown at different concentrations of CaCl2 (0.1–10 mM) after seeds were exposed to a local GMF (DC) and sinusoidal time-varying extremely low frequency MF (AC) (28.3 Hz, 20 μT; tuned to cyclotron resonance of Ca2+) (Sakhnini 2007). AC field enhanced the germination of seeds independent of CaCl2 concentration. At 10 mM CaCl2, the length of radicals increased significantly indicating a change in the calcium efflux due to the MF.
Goldsworthy (2006) described the (non-specific) effects of non-polar and polar DC fields, and alternating EMF. According to his explanations, the growth-affecting effects of non-polar effects of DC fields can be due to changes in the membrane potential and membrane permeability of the cells to Ca2+ ions. The increase in the intercellular Ca2+ results in changes to metabolism by activation of a second messenger system. The polar effect of DC fields includes changes in electric control of cell polarity. He hypothesized that the biological effects of weak time-varying EMF are based fundamentally also on changing cell membrane permeability by selective removing Ca2+ from the membrane and replacing it with other cations (mainly by K+), but this depends on the frequency of the EMF. EMFs at 16 Hz (resonant frequency of potassium) can increase, but EMFs at 32 Hz (resonant frequency of calcium) can reduce the permeability of membranes. Pulsed and amplitude modulated waves have different effects.
When pea seeds were grown under a low MF (0.5–2 nT; syn. WMF) for 3 days, Belyavskaya (2001) observed some disturbances at the cellular level in the root tips of seedlings. Besides ultrastructural changes, such as the accumulation of lipid bodies, development of the lytic compartment and reduction of phytoferritin in plastids, larger mitochondria with an electron-transparent matrix and reduced cristae were observed; moreover, the Ca2+ balance of the cell was disrupted and the localization of Ca2+ changed. Belyavskaya (2001) concluded that Ca2+ was the potential sensible component of the effect of low MF, assuming that Ca2+ ions connected to Ca2+-binding sites of proteins were the link in processes triggered by low MF and confirming “the parametric resonance of ions” theory in magnetobiological effects (in Binhi 2001), a theory that tries to describe how the intensity of ionic quantum transitions can be affected by MF.
Extremely low frequency MF treatments (0 Hz (DC) at 5 mT; 50, 60, and 75 Hz each at 1.5 mT) applied for 3 days to broad bean (Vicia faba L.) seedlings increased the length of the prophase in the meristem cells of root tips (Rapley et al. 1998). Belyavskaya (2004) indicated that MFs affected the G2 phase of the cell cycle in lentil and flax, causing G2 to become longer and decreasing cell division.
Weak horizontal extremely low frequency MF treatments (50 Hz, 500 μT) affected lipid metabolism of 5-day-old radish seedlings (Novitskii et al. 2014). When exposed to MF at 20–22 °C in light, lipid synthesis was stimulated, production of polar lipids reached a threshold, glycolipid production increased 4-fold and phospholipid production increased 2.5-fold compared to the control.
Promotional effects of EMF (10 kHz for 4 days, each day for 5 h) on membrane integrity, such as an increase in CAT activity and proline content and a reduction in POX activity and electrolyte leakage of membranes, were reported also in wheat (cv. ‘Kavir’) seedlings (Payez et al. 2013).
Using transgenic Arabidopsis thaliana (L.) Heynh. with a GUS (β-glucuronidase) gene driven by a stress-inducible alcohol dehydrogenase (Adh) promoter, Paul et al. (2006) proved that high MF (from a threshold of 15 T) induced the expression of the Adh/GUS transgene in leaves and roots, suggesting the perturbing effect of a high MF on the genome by modifying gene expression. This is at least partly due to the perturbation of conformation dynamics of macromolecules involved in gene regulation. In order to accomplish that much, and to be able to link the theory of MFs in plant research with current practice, a three-dimensional coordinate system was used to analyze the effects of time-varying electric and MFs effects in plants (Griffiths 1999). It is possible to have electric charges in plants because of the likely presence of electrolytes in the conductive paths and it is the presence of these charges in plants that make them electromagnetic (Griffiths 1999). Furthermore, the different types of interactions, orientations, and manifestations between and among these charges, make them magnetic, confounding a plant’s behavior in the presence of other MFs (Ramo et al. 2004).
Examining the electrophoretic pattern between 9 and 85 kDa of the wheat cultivars in the experiments of Almaghrabi and Elbeshehy (2012) when seeds were exposed to 0.3 T for 30 min, the number of protein bands increased in all seven cultivars affected positively by MF treatment. In cultivars in which the MF treatment decreased the germination percentage, the number of protein bands either decreased from 17 to 9 (‘Sakha 93’) or was not affected (‘Masr 1’) compared to the control treatment. Similar changes was detected in the protein content and protein profile of 8-day-old seedlings of soybean cultivar ‘CO-3’ when seeds were treated by a pulsed MF of 1500 nT at 10 Hz for 20 days and daily for 5 h (Radhakrishnan and Kumari 2012).
Diamagnetic levitation is a technique to stimulate ground-based low gravity (reviewed in Qian et al. 2013). A super-conducting solenoid magnet (MF in the geometric center of the solenoid was 16.5 T) was used for diamagnetic levitation of transgenic Arabidopsis thaliana ecotype Columbia seedlings containing the CycB1-GUS proliferation marker and the DR5-GUS auxin-mediated growth marker, respectively (Manzano et al. 2013). Seedlings and imbibed seeds levitated 80 mm above the solenoid center. In the control, strong MF (16.5 T) was used without levitation and seedlings were exposed to strong MF and hypergravity (2 g). In microgravity, cell growth decreased but cells proliferated presumably due to the disruption of meristematic competence. In the root meristem of seedlings exposed to high MF, auxin signaling delocalized in the root tips and the distribution of auxin changed, indicating partial inhibition of its polar transport. In addition, the size of the nucleolus after 4 days of growth decreased while the proliferation of meristematic cells was decoupled from ribosome biogenesis.
The effects of EMF with different voltages (high tension wires of 132, 220, and 500 kV) on the meiosis and pollen viability of 33 species of Mimosaceae, Molluginaceae, Nyctaginaceae, and Papilionaceae were studied by Zaidi et al. (2013). Different abnormalities were observed at different stages of meiosis, such as pairing disturbances, stickiness, precocious chromosomes, multipolar divisions, and abnormal meiotic products, such as dyads and hypertetrads (in three species from Papilionaceae). Meiotic abnormalities increased as the voltage increased and also depended on the species; most meiotic abnormalities were observed in species from Papilionaceae and Mimosaceae. Similarly, pollen sterility increased as the EMF force increased and it was highest (41 %) in Indigofera oblongifolia, a species of the Papilionaceae.
The loss of calcium in membranes, as a result of varying and alternating EMFs and eddy currents, make holes that cause leakage and consequently weaken plant tissues, through more tears, slower repair capacities and more overall solute leakage (Goldsworthy 2007; Asemota 2010). 16 Hz is the ICR frequency for potassium ions in the earth’s MF, and when exposed to EMF at this frequency, plants absorb the field’s energy and convert it to energy of motion, increasing their ability to replace calcium ions in cell membranes (Goldsworthy 2007). In contrast, the extra energy gained by each K+ may be small. As there are about 10,000 K+ ions competing with just one Ca2+ for each place on the membrane implies that a slight increase in their energies due to resonance is enough to overwhelm those calcium ions, as their sympathetic and synergistic support for each other can easily produce more than twice the work function (that is the energy required to dislodge Ca2+ from the surface of the cell membrane) to replace and substitute K+ for Ca2+, which is a binding or cementing agent for cell membranes and thereby undermine the overall quality and integrity of such plants or crops (Goldsworthy 2006, 2007; Naidu and Kamaraju 2007; Asemota 2010). This push-and-pull mechanism of valuable Ca2+ as a binder and symbol of membrane integrity is essentially MF dependent. These studies support the concept that the motion of ions in plants depends on magnetic field strength and its frequency (Lednev 1991). Applying a sigmoidal versus a pulsed MF of 60 Hz to mung bean seeds, Huang and Wang (2007) showed that the former caused 20–30 % more mortality to seeds than the pulsed MF, although it also produced sprouts of greater diameter, suggesting that resonance within cells or tissues/organs, could induce cell death if the excitation of ions (such as Ca2+) exceeds the natural state of control, non-MF-induced plants. Contradictory growth data, however, indicates that the mechanism is not as simple as it seems, suggesting that there is an overlap with or an over-ride of endogenous rhythms by MFs.
Conclusions and future objectives
The focus of this review is to synthesize how MFs have influenced ex vitro plant growth and development (including seed germination, seedling growth and yield) and to seek some basic biological reasons for the observed growth patterns. The MFs used to date represent an extremely wide range of force, from nanoTesla (nT) through to GT. In addition to these forces, geomagnetic and electromagnetic forces also influence plant responses. This influencing effect can be a selective advantage when plants living under dry conditions can perceive the electric fields of thunderstorms (as a signal) and hereby are able to prepare themselves by using the water to convert metabolic processes (Goldsworthy 1996, 2006).
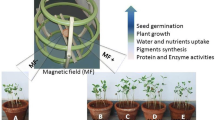
The positive impact of MFs on initial stages of growth, mainly on roots of seed-derived plantlets such as lentil, wheat, and rice, suggests that MFs could be used to extent root growth to take advantage of a greater surface area of soil, for example (Pietruszewski 1993; Martínez et al. 2002; Flórez et al. 2004; Shabrangi and Majd 2009; Fig. 1). The ability of safflower (Carthamus tinctorius L.) seeds exposed to 72 mT for 10 min, in combination with hydropriming and exposure to gibberellic acid, to increase oil yield fourfold more than the control (Faqenabi et al. 2009), suggesting that MFs could be used in a very practical way to increase the horticultural and agronomic yield of economically important crops, as was shown for Phalaenopsis in vitro (Van et al. 2011b). However, the costs need to be carefully considered. On the other hand, the plant itself can be used as an antenna for perceiving and sensing the changes in EMF or MF. One of the practical applications is the use of plants, as biosensors, for forecasting earthquakes in time, place, or magnitude (Volkov 2012).
A possible, as yet uncreated or untested experimental design in which the effect of magnetic fields (MFs) could be used to test the impact on plantlet growth, either in a soil-based, in vitro gel-based, ex vitro hydroponic-based or in vitro liquid-based culture system. a–c Dotted lines magnets. Thick black line glass or plastic slabs that would border either side of the experimental units. a Plantlets, shoots, or root systems (whichever is required by the experimental design) can be aligned at a regular spacing inside one of the four experimental systems. Such a spacing would save space and allow for easy visualization of the growth and development over time. b In such a design, one side of the experimental unit could be aligned with north, the opposite side with south, all plants being exposed to the same magnetic force (Tesla, T). c In this experimental design, individual magnetic slabs of different strengths (T) can be stacked to form a single treatment, e.g., 0.1 T. This would allow separate treatments to be aligned alongside each other. Design C would not take into account the effect of allelopathy, medium volume, or plantlet density, all of which would most likely have to be optimized prior to implementation of the MFs
Most likely, due to the size of current MF-generating equipment, most experiments have been conducted in vitro in which small explants have been exposed to MFs or to MW, and then allowed to grow with a residual effect of the MFs affecting growth. It is difficult to see large-scale MFs being applied to field conditions, due to excessive costs (as much as US$100 for a single stationary magnet of approximately 50 cm2 used by Tanaka et al. (2010) and Van et al. (2011a, b, 2012)) and the laborious nature of such an experimental set-up. Consequently, in vitro experiments and trials will likely remain the choice scale of MF research, including for space-related research, because of space constraints and scale-up experiments, although useful for elucidating mechanisms of action, would require a detailed cost analysis before practical application.
The ability of MFs to allow the growth of wheat and common bean seedlings to continue under salt or osmotic stress after exposure for 7 days at 7 mT (Cakmak et al. 2010), its stress-protecting effect under water, salt, heavy-metal or heat stress (Xi et al. 1994; Ružič and Jerman 2002; Chen et al. 2011; Selim and El-Nady 2011) and to enhance organogenesis of soybean under salt stress (Radhakrishnan and Kumari 2013b) suggests that the use of MFs could be useful in the alleviation of abiotic stress in vitro or in the field.
As can be appreciated from above, although there are some studies on the effects of MFs on plant growth, a plausible explanation has not yet been clearly stated, nor has any evidence accrued to support any hypotheses. Our results provide further evidence, nonetheless, of the effects (positive and negative) of permanent MFs on horticultural and other plant growth. However, no clear explanation about the mechanism is yet available, although it adds to a growing body of evidence that abiotic factors strongly influence morphogenesis in ornamental plants (Teixeira da Silva et al. 2006).
Based on advancements and promising effects achieved to date, many researchers envisioned the twentieth century to be the age of biophysical applications in agriculture and horticulture (Vasilevski 2003). Magnetic and electromagnetic stimulation is one of the most promising fields of biophysical methods in plant research and production. They can be one of the most efficient environmentally sound methods for modern agriculture by comprehensive extending interdisciplinary research (Vasilevski 2003; Bilalis et al. 2013). However, close examination of results indicates that in fact results were not as positive as they were initially perceived to be. For example, in the Moon and Chung (2000) study, in fact, under all treatments, after a maximum of 9 days after germination, percentage germination reached 100 %, the same as in controls; however, a spot analysis in time, for example, after 6 days, indicates that 70 % of seed germinated when 12 kV/cm was applied for 15 s. This indicates that timing of sampling can affect the interpretation by authors and readers (Teixeira da Silva and Dobránszki 2013). Timing is also of great importance from the view point of the timing of application. As Aksyonov et al. (2001) demonstrated, the effect of an EMF (30, 50 Hz, 30 mT) was different if it was applied at different stages of germination of wheat seeds. If EMF was applied at the stage of esterase activation, leakage of products from the esterase reaction increased relative to both the untreated and earlier treated seeds. If seeds (50 % germinability) were treated at the stage of root formation, the number of seeds with roots and also the shoot length of seedlings increased compared to non-treated or later treated seeds. Finally, it was clear from results thus far that the effect of MF on plant growth and development is species- and genotype-specific. Furthermore, systematic cytogenetic and molecular analyses are needed to study both the irreversibility of changes caused by MF and EMF treatments and their aftereffects.
Therefore, better understanding of a plant’s behavior to the effects of MFs, especially from a theoretical viewpoint, would help to strengthen, sharpen, and further the current practice in being able to identify which organs or cells, or at the molecular level, could produce better yields and results using MF-related techniques.
Abbreviations
- AC:
-
Alternating current
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- chl:
-
Chlorophyll
- DC:
-
Direct current
- EMF:
-
Electromagnetic field
- G:
-
Gauss
- GMF:
-
Geomagnetic field
- ICR:
-
Ion cyclotron resonance
- MDA:
-
Malondialdehyde
- MF:
-
Magnetic field
- MW:
-
Magnetized water
- nT:
-
nanoTesla
- POX:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- T:
-
Tesla
- WMF:
-
Weak magnetic field
References
Abdolmaleki P, Ghanati F, Sahebjamei H, Sarvestani AS (2007) Peroxidase activity, lignification and promotion of cell death in tobacco cells exposed to static magnetic field. Environmentalist 27:435–440
Aguilar CH, Dominguez-Pacheco A, Carballo AC, Cruz-Orea A, Ivanov R, Bonilla JLL, Montañez JPV (2009) Alternating magnetic field irradiation effects on the three genotype maize seed field performance. Acta Agrophysica 14(1):7–17
Aksyonov SJ, Bulychev AA, Grunina TY, Goryachev SN, Turovetsky VB (2001) Effects of ELF-EMF treatment on wheat seeds at different stages of germination and possible mechanism of their origin. Electromagn Biol Med 20:231–253
Aksyonov SI, Grunina TY, Goryachev SN (2007) On the mechanism of stimulation and inhibition of wheat seed germination by low-frequency magnetic field. Biophysics 52:233–236
Aladjadjiyan A (2002) Study of the influence of magnetic field on some biological characteristics of Zea mais. J Cent Eur Agric 3(2):89–94
Aladjadjiyan A (2003) Use of physical factors as an alternative to chemical amelioration. J Environ Prot Ecol 4(3):662–667
Aladjadjiyan A (2012) Physical factors for plant growth stimulation improve food quality. In: Aladjadjiyan A (ed) Food production—approaches, challenges and tasks. InTech, Croatia, pp 145–168
Alexander MP, Doijode SD (1995) Electromagnetic field, a novel tool to increase germination and seedling vigour of conserved onion (Allium cepa L.) and rice (Oryza sativa L.) seed with low viability. Plant Genet Res Newslett 104:1–5
Ali TB, Khalil SE, Khalil AM (2011) Magnetic treatments of Capsicum annuum L. grown under saline irrigation conditions. J Appl Sci Res 7:1558–1568
Alikamanoglu S, Sen A (2011) Stimulation of growth and some biochemical parameters by magnetic field in wheat (Triticum aestivum L.) tissue culture. Afr J Biotechnol 10:10957–10963
Almaghrabi OA, Elbeshehy EKF (2012) Effect of weak electro magnetic field on grain germination and seedling growth of different wheat (Triticum aestivum L.) cultivars. Life Sci J 9:1615–1622
Asemota GNO (2010) Alternating electromagnetic fields in plantains. Afr J Plant Sci Biotechnol 4(Special Issue 1):59–75
Association of Official Seed Analysts (AOSA) (1983) Seed vigor testing handbook. AOSA Handbook 32
Atak Ç, Emiroğlu O, Alikamanoğlu S, Rzakoulieva A (2003) Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J Cell Mol Biol 2:113–119
Atak Ç, Çelik O, Olgum A, Alikamanoğlu S, Rzakoulieva A (2007) Effect of magnetic field on peroxidase activities of soybean tissue culture. Biotechnol Biotechnol Equip 21:166–171
Audus LJ (1960) Magnetotropism: A new plant growth response. Nature 185:132–134
Bathnagar D, Deb A (1977) Some aspects of pregermination exposure of wheat seeds to magnetic field. I. Germination and early growth. Seed Res 5:129–137
Belyavskaya NA (2001) Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetic fields. Adv Space Res 28:645–650
Belyavskaya NA (2004) Biological effects due to weak magnetic field on plants. Adv Space Res 34:1566–1574
Berahmand AA, Panahi AG, Sahabi H, Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A, Karimpour H, Gallehgir O (2012) Effects silver nanoparticles and magnetic field on growth of fodder maize (Zea mays L.). Biol Trace Elem Res 149:419–424
Bilalis DJ, Katsenios N, Efthimiadou A, Karkanis A, Khah EM, Mitsis T (2013) Magnetic field pre-sowing treatment as an organic friendly technique to promote plant growth and chemical elements accumulation in early stages of cotton. Aust J Crop Sci 7:46–50
Binhi VN (2001) Theoretical concepts in magnetobiology. Electro-Magnetobiol 20:43–58
Blackmore RP (1982) Magnetotactic bacteria. Annu Rev Microbiol 36:217–238
Büyükuslu N, Çelik Ö, Atak Ç (2006) The effect of magnetic field on the activity of superoxide dismutase. J Cell Mol Biol 5:57–62
Cakmak T, Dumlupinar R, Erdal S (2010) Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 31:120–129
Carbonell MV, Martínez E, Amaya JM (2000) Stimulation of germination in rice (Oryza sativa L.) by a static magnetic field. Electro-Magnetobiol 19:121–128
Carbonell MV, Martínez E, Flórez M, Maqueda R, Pintor-López A, Amaya JM (2008) Magnetic field treatments improve germination and seedling growth in Festuca arundinaceae Screb. and Lolium perenne L. Seed Sci Technol 36:31–37
Carbonell MV, Flórez M, Martínez E, Maqueda R, Pintor-López A, Amaya JM (2011) Study of stationary magnetic fields on initial growth of pea (Pisum sativum L.) seeds. Seed Sci Technol 39:673–679
Celestino C, Picazo ML, Toribio M, Alvarez-Ude JA, Bardasano JL (1998) Influence of 50 Hz electromagnetic fields on recurrent embryogenesis and germination of cork oak somatic embryos. Plant Cell Tiss Org Cult 54:65–69
Celestino C, Picazo ML, Toribio M (2000) Influence of chronic exposure to an electromagnetic field on germination and early growth of Quercus suber seeds preliminary study. Electro-Magnetobiol 19:115–120
Çelik O, Atak Ç, Rzakulieva A (2008) Stimulation of rapid regeneration by a magnetic field in Paulownia node cultures. J Cent Eur Agric 9:297–304
Chen Y, Li R, He J (2011) Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 20:760–769
Commoner B, Heise JJ, Townsend J (1956) Light-induced paramagnetism in chloroplasts. Proc Natl Acad Sci U S A 42(10):710–718
Danilov V, Bas T, Eltez M, Rzakoulieva A (1994) Artificial magnetic field effect on yield and quality of tomatoes. Acta Horticult 366:279–285
Davies MS (1996) Effects of 60 Hz electromagnetic fields on early growth in three plant species and a replication of previous results. Bioelectromagnetics 17:154–161
De Micco V, Aronne G, Joseleau J, Ruel K (2008) Xylem development and cell wall changes of soybean seedlings grown in space. Ann Bot 101:661–669
De Souza A, Garcia D, Sueiro L, Gilart F, Porras E, Licea L (2006) Pre-sowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 27:247–257
Dhawi F, Al-Khayri JM (2009) Magnetic fields induce changes in photosynthetic pigments content in date palm (Phoenix dactylifera L.) seedlings. Open Agric J 3:1–5
Efthimiadou A, Katsenios N, Karkanis A, Papastylianou P, Triantafyllidis V, Travlos I, Bilalis DJ (2014) Effects of presowing pulsed electromagnetic treatment of tomato seed on growth, yield, and lycopene content. Sci World J Article ID 369745, 6 pp
Eşitken A (2003) Effect of magnetic fields on yield and growth in strawberry ‘Camarosa’. J Hortic Sci Biotechnol 78(2):145–147
Eskov EK, Darkov AV (2003) Consequences of high-intensity magnetic effects on the early growth processes in plant seeds and the development of honeybees. Biol Bull 30:512–516
Faqenabi F, Tajbakhsh M, Bernoosi I, Saber-Rezaii M, Tahri F, Parvizi S, Izadkhah M, Gorttapeh AH, Sedqi H (2009) The effect of magnetic field on growth, development and yield of safflower and its comparison with other treatments. Res J Biol Sci 4(2):174–178
Feizi H, Sahabi H, Moghaddam PR, Shahtahmassebi N, Gallehgir O, Amirmoradi S (2012) Impact of intensity and exposure duration of magnetic field on seed germination of tomato (Lycopersicon esculentum L.). Not Sci Biol 4(1):116–120
Fischer G, Tausz M, Köck M, Grill D (2004) Effects of weak 16 Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics 25:638–641
Flórez M, Carbonell MV, Martínez E (2004) Early sprouting and first stages of growth of rice seeds exposed to a magnetic field. Electro Magnetobiol Med 23:167–176
Flórez M, Carbonell MV, Martínez E (2007) Exposure of maize seeds to stationary magnetic fields: effects on germination and early growth. Environ Exp Bot 59:68–75
Flórez M, Martínez E, Carbonell MV, Álvarez J, Campos A (2014) Germination and initial growth of triticale seeds under stationary magnetic treatment. J Adv Agric 2(2):72–79
Galland P, Pazur A (2005) Magnetoreception in plants. J Plant Res 118:371–389
Goldsworthy A (1996) Electrostimulation of cells by weak electric currents. In: Lynch PT, Davey NR (eds) Electrical manipulation of cells. Chapman and Hall, New York, pp 249–272
Goldsworthy A (2006) Effects of electrical and electromagnetic fields in plants and related topics. In: Volkov AG (ed) Plant electrophysiology-theory and methods, 1st edn. Springer, Berlin, pp 247–267
Goldsworthy A (2007) The biological effects of weak electromagnetic fields. Available online: http://www.radiationresearch.org/pdf/goldsworthy_bio_weak_em_07/pdf. Accessed 5 May 2015
Govoroon RD, Danilov VI, Fomicheva VM, Belyavskaya NA, Zinchenko SY (1992) Effects of fluctuations of a geomagnetic field and its screening on early phases in development of higher plants. Biofizika 37:738–743. (in Russian)
Griffiths DJ (1999) In: Ghosh AK (ed) Introduction to electrodynamics, 2nd edn. Prentice-Hall of India Pvt. Ltd, New Delhi, pp 55–507
Gubbels GH (1982) Seedling growth and yield response of flax, buckwheat, sunflower and field pea after preceding magnetic treatment. Can J Plant Sci 62:61–64
Hasan GT, Ali KJ, Ahmed MA (2011) Investigation the influence of magnetic field emitted by high voltage transmission lines on plant growth. Eur J Sci Res 56:272–278
Hasanuzzaman M, Hossain MA, Teixeira da Silva JA, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, The Netherlands, pp 261–315
Hirota N, Nakagawa J, Kitazawa K (1999) Effects of a magnetic field on the germination of plants. J Appl Phys 85(8):5717–5719
Huang HH, Wang SR (2007) The effects of 60 Hz magnetic fields on plant growth. Nat Sci 5(1):60–68
Iqbal M, Haq ZU, Jamil Y, Ahmad MR (2012) Effect of presowing magnetic treatment on properties of pea. Int Agrophys 26:25–31
Jajte JM (2000) Programmed cell death as a biological function of electromagnetic fields at a frequency of (50/60 Hz). Med Pr 51:383–389
Kato R (1988) Effects of a magnetic field on the growth of primary roots of Zea mays. Plant Cell Physiol 29:1215–1219
Kavi PS (1983) The effect of non-homogeneous gradient magnetic field susceptibility values in situ ragi seed material. Mysore J Agric Sci 17:121–123
Kordas L (2002) The effect of magnetic field on growth, development and the yield of spring wheat. Pol J Environ Stud 11(5):527–530
Krawiec M, Komarzynski K, Palonka S, Kaplan M, Baryla P, Kiczorowski P (2013) Does the magnetic field improve the quality of radish seeds? Acta Sci Pol Hortorum Cultus 12(6):93–102
Krystofova O, Sochor J, Zitka O, Babula P, Kudrie V, Adam V, Kizek R (2013) Effect of magnetic nanoparticles on tobacco BY-2 cell suspension culture. Int J Environ Res Public Health 10(1):47–71
Lednev VV (1991) Possible mechanism for the influence of weak magnetic fields on biological systems. Bioelectromagnetics 12:71–75
Li J, Chang PR, Huang J, Wang Y, Yuan H, Ren H (2013) Physiological effects of magnetic iron oxide nanoparticles towards watermelon. J Nanosci Nanotechnol 13(8):5561–5567
Maffei ME (2014) Magnetic field effects on plant growth, development and evolution. Front Plant Sci 5:445
Mahmood M, Bee OB, Mohamed MTM, Subramaniam S (2013) Effects of electromagnetic field on the nitrogen, protein and chlorophyll content and peroxidase enzyme activity in oil palm (Elaeis guineensis Jacq.) leaves. Emir J Food Agric 25(6):471–482
Manzano AI, Larkin OJ, Dijkstra CE, Anthony P, Davey MR, Eaves L, Hill RJA, Medina FJ (2013) Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant Biol 13:124
Martínez E, Carbonell MV, Flórez M (2002) Magnetic biostimulation of initial growth stages of wheat (Triticum aestivum L.). Electromagn Biol Med 21(1):43–53
Martínez E, Carbonell MV, Flórez M, Amaya JM (2008) Effect of static magnetic field exposure of salvia seeds on germination characteristics (Salvia officinalis L.). In: Technical and Technological Progress in Agriculture, 13th International Conference, Institute of Agricultural Engineering LUA, Raudondvaris, 25–26 September 2008, pp. 226–232
Martínez E, Carbonell MV, Flórez M, Amaya JM, Maqueda R (2009) Germination of tomato seeds (Lycopersicon esculentum L.) under magnetic field. Int Agrophys 23:45–49
Maus S, Macmillan S, McLean S, Hamilton B, Thomson A, Nair M, Rollins C (2010) The US/UK world magnetic model for 2010–2015. http://www.ngdc.noaa.gov/geomag/WMM/data/WMM2010/WMM2010_Report.pdf. Accessed 5 May 2015
McClean RG, Schofield MA, Kean WF, Sommer CV, Robertson DP, Toth D, Gajdardziska-Josifovska M (2001) Botanical iron minerals: correlation between nanocrystal structure and modes of biological self-assembly. Eur J Mineral 13:1235–1242
Monselise EB-I, Parola AH, Kost D (2003) Low-frequency electromagnetic fields induce a stress effect upon higher plants, as evident by the universal stress signal, alanine. Biochem Biophys Res Commun 302:427–434
Moon JD, Chung HS (2000) Acceleration of germination of tomato seed by applying AC electric and magnetic fields. J Electrost 48:103–114
Morar R, Munteanu R, Simion E, Muteanu I, Dascalescu L (1999) Electrostatic treatment of bean seeds. IEEE Trans Ind Appl 35(1):208–212
Murray LE (1965) Plant growth response in electrostatic field. Nature 207:1177–1178
Naidu MS, Kamaraju V (2007) High voltage engineering, 3rd edn. Tata McGraw-Hill Publishing Co., New Delhi, pp 1–366
Namba K, Sasao A, Hibusawa S (1995) Effect of magnetic field on germination and plant growth. Acta Horticult 399:143–148
Neamţu S, Morariu VV (2005) Plant growth in experimental space flight magnetic field conditions. Rom J Biophys 1:41–46
NGDC (National Geophysical Data Center, USA) (2015) http://sec.noaa.gov/Data/index.html#indices. Accessed 5 May 2015
Novitskii YI, Novitskaya GV, Serdyukov YA (2014) Lipid utilization in radish seedlings as affected by weak horizontal extremely low frequency magnetic field. Bioelectromagnetics 35(2):91–99
Pang XF, Deng B (2008) Investigation of changes in properties of water under the action of a magnetic field. Sci China Ser G Phys Mech Astro 51:1621–1632
Paul AL, Ferl RJ, Meisel MW (2006) High magnetic field induced changes of gene expression in Arabidopsis. Biomagn Res Technol 4:1–10
Payez A, Ghanati F, Behmanesh M, Abdolmaleki P, Hajnorouzi A, Rajabbeigi E (2013) Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and a 10-KHz electromagnetic field. Electromagn Biol Med 32(4):417–429
Peñuelas J, Llusia J, Martínez B, Fontcuberta J (2004) Diamagnetic susceptibility and root growth response to magnetic fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromagn Biol Med 23:97–112
Phirke PS, Patil MN, Umbarkar SP, Dudhe YH (1996) The application of magnetic treatment to seeds: methods and responses. Seed Sci Technol 24:365–373
Piacentini MP, Fraternale D, Piatti E (2001) Senescence delay and change of antioxidant enzyme levels in Cucumis sativus L. etiolated seedlings by ELF magnetic fields. Plant Sci 161:45–53
Pietruszewski S (1993) Effects of magnetic seed treatment on yields of wheat. Seed Sci Technol 21:621–626
Piruzyan LA, Kuznetsov AA, Chikov VM (1980) About the magnetic heterogeneity of biological systems. Izv Acad Sci USSR Ser Biol 5:645–653
Pittman UJ (1977) Effects of magnetic seed treatment on yields of barley, wheat and oats on Southern Alberta. Can J Plant Sci 57:37–45
Podleśny J, Pietruszewski S, Podleśna A (2004) Efficiency of the magnetic treatment of broad bean seeds cultivated under experimental plot conditions. Int Agrophys 18:65–71
Poinapen D, Beeharry GK, Bahorun T, Bunwaree M, Préfumo S (2005) Effect of static magnetic fields on the growth and yield of butterhead lettuce seeds (Lactuca sativa var. Salina). MAS 2005 Food and Agricultural Research Council, Proceedings 7th Meeting of Agricultural Scientists. Réduit, Mauritius. Lalouette, JA, Bheenick, K J, Nundalallee, C (Eds), p. 207–216. http://www.gov.mu/portal/sites/ncb/moa/farc/amas2005/pdf/MAS20051stDraft.pdf. Accessed 5 May 2015
Poinapen D, Brown DCW, Beeharry GK (2013) Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform static magnetic fields. J Plant Physiol 170:1251–1258
Qian AR, Yin DC, Yang PF, Lv Y, Tian ZC, Shang P (2013) Application of diamagnetic levitation technology in biological sciences research. IEEE Trans Appl Supercond 23(1):3600305
Rãcuciu M, Creangǎ D, Horga I (2008) Plant growth under static magnetic field influence. Rom J Phys 53:353–359
Radhakrishnan R, Kumari BDR (2012) Pulsed magnetic field: a contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol Biochem 51:139–144
Radhakrishnan R, Kumari BDR (2013a) Influence of pulsed magnetic field on soybean (Glycine max L.) seed germination, seedling growth and soil microbial population. Indian J Biochem Biophys 50:312–317
Radhakrishnan R, Kumari BDR (2013b) Protective role of pulsed magnetic field against salt stress effects in soybean organ culture. Plant Biosyst 147(1):135–140
Rajabbeigi E, Ghanati F, Abdolmaleki P, Payez A (2013) Antioxidant capacity of parsley cells (Petroselium crispum L.) in relation to iron-induced ferritin levels and static magnetic field. Electromagn Biol Med 32(4):430–441
Ramo S, Whinery JR, Van Duzer T (2004) Fields and waves in communication electronics, 3rd edn. Wiley, Kundli, pp 114–299
Rapley BI, Rowland RE, Page WH, Podd JV (1998) Influence of extremely low frequency magnetic fields on chromosomes and mitotic cycle in the broad bean (Vicia faba L.). Bioelectromagnetics 19:152–161
Reina FG, Pascual LA (2001) Influence of a stationary magnetic field on water relations in lettuce seeds. Part 1: theoretical considerations. Bioelectromagnetics 22:589–595
Reina FG, Pascual LA, Fundora IA (2001) Influence of a stationary magnetic field on water relations in lettuce seeds. Part 2: experimental results. Bioelectromagnetics 22:596–602
Rezaiiasl A, Ghasemnezhad A, Shahabi S (2012) Study the response of cucumber plant to different magnetic fields. J Adv Lab Res Biol 3(1):42–46
Ružič R, Jerman I (2002) Weak magnetic field decreases heat stress in cress seedlings. Electromagn Biol Med 21:69–80
Sakhnini L (2007) Influence of Ca2+ in biological stimulating effects of AC magnetic fields on germination of bean seeds. J Magn Magn Mater 310:e1032–e1034
Savostin PW (1930) Magnetic growth relations in plants. Planta 12:327
Scaiano JC, Cozens FL, Mohtat N (1995) Development of a model and application of the radical pair mechanism to radicals in micelles. Photochem Photobiol 62:818–829
Schrödinger E (1935) Probability relations between seperated systems. Camb Phil Soc Proc 31:555–563
Selim AH, El-Nady MF (2011) Physio-anatomical responses of drought stressed tomato plants to magnetic fields. Acta Astronaut 69:387–396
Serdyukov YA, Novitskii YI (2013) Impact of weak permanent magnetic field on antioxydant enzyme activities in radish seedlings. Russ J Plant Physiol 60(1):69–76
Shabrangi A, Majd A (2009) Effect of magnetic fields on growth and antioxidant systems in agricultural plants. PIERS Proceedings, Beijing, China, March 23–27, 2009, pp. 1142–1147
Shine MB, Guruprasad KN (2012) Impact of pre-sowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol Plant 34:255–265
Shine MB, Guruprasad KN, Anand A (2011) Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 32:474–484
Teixeira da Silva JA, Dobránszki J (2015) How do magnetic fields affect plants in vitro? In Vitro Cell Dev Biol Plant. doi:10.1007/s11627-015-9675-z
Smith SD, McLeod BR, Liboff AR, Cooksey K (1987) Calcium cyclotron resonance and diatom mobility. Bioelectromagnetics 8(3):215–227
Smith SD, McLeod BR, Liboff AR (1993) Effects of CR-tuned 60 Hz magnetic fields on sprouting and early growth of Raphanus sativus. Biochem Bioenerg 32:67–76
Soja G, Kunsch B, Gerzabek M, Relchenauer T, Soja AM, Rippar G, Bolhar-Nordenkampf HR (2003) Growth and yield of winter wheat (Triticum aestivum L.) and corn (Zea mays L.) near a high voltage transmission line. Bioelectromagnetics 24:91–102
Subber ARH, Hail RCA, Jabail WA, Hussein HF (2012) Effects of magnetic field on the growth development of Zea mays seeds. J Nat Prod Plant Resour 2(3):456–459
Tanaka M, Van PT, Teixeira da Silva JA, Ham LH (2010) Novel magnetic field system: application to micropropagation of horticultural plants. Biotechnol Biotechnol Equip 24(4):2160–2163
Teixeira da Silva JA, Dobránszki J (2013) How timing of sampling can affect the outcome of the quantitative assessment of plant organogenesis. Sci Hortic 159:59–66
Teixeira da Silva JA, Dobránszki J (2014) Impact of magnetic water on plant growth. Environ Exp Biol 12(4):137–142
Teixeira da Silva JA, Singh N, Tanaka M (2006) Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tiss Org Cult 109:368–378
Theg SM, Sayre RT (1979) Characterization of chloroplast manganese by electron magnetic resonance spectroscopy. Plant Sci Lett 16:319–326
Trontelj Z, Thiel G, Jazbinsek V (2006) Magnetic measurements in plant electrophysiology. In: Volkov AG (ed) Plant electrophysiology—theory and methods. Springer, Berlin, pp 187–218
Ursache-Oprisan M, Focanici E, Creanga D, Caltun O (2011) Sunflower chlorophyll levels after magnetic nanoparticles supply. Afr J Biotechnol 10:7092–7098
Vaezzadeh M, Noruzifar E, Faezeh G, Salehkotahi M, Mehdian R (2006) Excitation of plant growth in dormant temperature by steady magnetic field. J Magn Magn Mater 302:105–108
Van PT, Teixeira da Silva JA, Ham LH, Tanaka M (2011a) Effects of permanent magnetic fields on the proliferation of Phalaenopsis protocorm-like bodies using liquid medium. Sci Hortic 128:479–484
Van PT, Teixeira da Silva JA, Ham LH, Tanaka M (2011b) The effects of permanent magnetic fields on Phalaenopsis plantlet development. J Hortic Sci Biotechnol 86(5):473–478
Van PT, Teixeira da Silva JA, Ham LH, Tanaka M (2012) Effects of permanent magnetic fields on growth of Cymbidium and Spathiphyllum. In Vitro Cell Dev Biol Plant 48(2):225–232
Vashisth A, Nagarajan S (2008) Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectromagnetics 29:571–578
Vashisth A, Nagarajan S (2010) Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J Plant Physiol 167:149–156
Vasilevski G (2003) Perspectives of the application of biophysical methods in sustainable agriculture. Bulgarian J Plant Physiol Special Issue:179–186
Volkov AG (2012) Plant Biosensor and method. Patent US 8,205,502. https://drive.google.com/viewerng/viewer?url=patentimages.storage.googleapis.com/pdfs/US8205502.pdf. Accessed 5 May 2015
Wang BC, Zhou J, Wang YC, Zhu LC, Teixeira da Silva JA (2006) Physical stress and plant growth. In: Teixeira da Silva JA (ed) Floriculture, ornamental and plant biotechnology: advances and topical issues, vol 3, 1st edn. Global Science Books Ltd, Isleworth, pp 68–85
Xi G, Fu ZD, Ling J (1994) Change of peroxidase activity in wheat seedlings induced by magnetic field and its response under dehydration condition. Acta Bot Sin 36:113–118
Xu CX, Yin X, Lv Y, Wu CZ, Zhang YX, Song T (2012) A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis. Adv Space Res 49:834–840
Xu C, Wei S, Lu Y, Zhang Y, Chen C, Song T (2013) Removal of the local geomagnetic field affects reproductive growth in Arabidopsis. Bioelectromagnetics 34(6):437–442
Xu C, Lv Y, Chen C, Zhang Y, Wei S (2014) Blue light-dependent phosphorylations of cryptochromes are affected by magnetic fields in Arabidopsis. Adv Space Res 53:1118–1124
Yamauchi N, Funamoto Y, Shigyo M (2004) Peroxidase-mediated chlorophyll degradation in horticultural crops. Phytochem Rev 3:221–228
Yan D, Guo Y, Zai X, Qin P (2009) Effects of electromagnetic fields exposure on rapid micropropagation of beach plum (Prunus martima). Ecol Eng 35:597–601
Yaycılı O, Alikamanoğlu S (2005) The effect of magnetic field on Paulownia tissue cultures. Plant Cell Tiss Org Cult 83:109–114
Zaidi S, Khatoon S, Imran M, Zohair S (2013) Effects of electromagnetic fields (created by high tension lines) on some species of family Mimosaceae, Molluginaceae, Nyctaginaceae and Papilionaceae from Pakistan – V. Pak J Bot 45(6):1857–1864
Acknowledgments
The authors thank Dr. Pham Thanh Van (Vietnam Academy of Agricultural Sciences, Institute of Agricultural Genetics, Hanoi, Vietnam) for initial feedback on the first version of the draft. The authors also thank Prof. Alexander G. Volkov (Department of Chemistry, Oakwood University, AL, USA), Dr. Marcela Krawiec (Department of Seed Production and Nurseries, University of Life Sciences, Lublin, Poland), and Prof. Elvira Martínez Ramírez (Dpto. Ingeniería Agroforestal, E.T.S.I. Agrónomos (UPM), Madrid, Spain) for constructive comments on the final version of the manuscript in a pre-publication peer review.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Teixeira da Silva, J.A., Dobránszki, J. Magnetic fields: how is plant growth and development impacted?. Protoplasma 253, 231–248 (2016). https://doi.org/10.1007/s00709-015-0820-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0820-7