Abstract
The aim of this work was to investigate the influence of the magnetic field on seed germination percentage and wheat seedlings, such as root and stem length, water content, photosynthetic pigments, antioxidants, phenols, flavonoids, and gene tests. Triticum turgidum L. ssp. durum Desf. seeds were exposed to a magnetic field with an induction of 12.5 and 25 mT for 6 days with 15 and 30 min exposure times per day. The magnetic field effect increased the water, chlorophyll, and carotenoid contents while reducing the germination percentage and root length. Significant associations were found between gene expression and related parameters, especially under 25 mT magnetic induction for 30 min. Further research could contribute to explaining the relationship between the influence of the applied magnetic field and the qRT-PCR genes (EF1, PhAL, Rubisco, CBP4) and changes occurring in wheat seedling growth. The variability in wheat genotypes could be caused by the applied magnetic field with different magnetic induction and exposure times. In magnetic field induction of 25 mT for a 30 min exposure time, changes were observed in all genes compared to the control group. The findings have different and unexpected implications that germination can be regulated by gene expression and related enzymes, associated with induction, exposure method, and duration of the magnetic field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic field (MF) is a natural component and an unavoidable environmental factor for all organisms on the Earth, and thus all plants have to experience this force. Interactions between MF and plants are becoming increasingly popular as new evidence has emerged for the ability of plants to perceive and respond quickly to varying MF by regulating their metabolism and altering gene expression and phenotype (Maffei 2014). The studies on plant development and magnetic field interactions argue that the magnetic fields affect the membrane structure of plant cells, allowing the plants to absorb more water and nutrients. Naturally, this situation triggers other physiological and biochemical processes. Furthermore, the great majority of biological molecules, such as hemoglobin, cytochrome, and ferritin, include metal ions that can be paramagnetic (Hozayn and Qados 2010). Also, it was proposed that magnetic fields influence the development characteristics and function of the mRNA regulation process, protein biosynthesis, and enzyme activities, resulting in changes in various organ and tissue activities (Atak et al. 2003). Although a number of theories had been proposed to explain this phenomenon, there is no complete conclusion on how these fields achieve such a change in the plants.
Previous research on the effects of MFs on organisms contains many conflicting reports. Because of the lack of understanding of the biological effects and mechanism of MFs, most of the studies are not systematic, lack testable theoretical predictions, and the results are not convincing (Harris et al. 2009). The effect of MF was described in a number of studies with different and conflicting results. In a previous study, wheat seeds were embedded in water overnight before being exposed to a 30mT static magnetic field (SMF) and 10 kHz EMF, 4 day and 5 h per day. Exposure to both MFs was found to increase the germination rate and results suggested that EMFs promote the membrane integrity and development properties of wheat seedlings (Payez et al. 2013). However, when compared to control groups, the mean germination time of rice (Oryza sativa) seeds exposed to one of two MF inductions (125 or 250 mT) for varied periods (1 min, 10 min, 20 min, 1 h, 24 h) was dramatically reduced. The results show that this form of magnetic treatment clearly influences rice germination and seedling growth (Florez et al. 2004).
Increased nutrient uptake is responsible for the improvement in germination and growth metrics after imbibition (Hussain et al. 2020). Indeed, application of MF has been shown to affect seed membranes, causing membrane damage due to reduced electron leakage upon absorption of water. However, this does not always mean that germination will be positively affected. The result of the effect differs according to the applied magnetic field and the plant species. The most important thing is which metabolic or molecular structures will be affected by the application of the magnetic field. Current study will try to understand the effectiveness of the two different magnetic field intensities with different exposure times on wheat (Triticum turgidum L.) germination and seedling growth, at physiologic, biochemical, and molecular levels. Finally, the aim of this study is to: I) determine how wheat seeds and seedlings react after being exposed to varied magnetic fields and II) comprehend the underlying process of MF-induced growth modification.
Materials and methods
Plant materials and growth conditions
The research material was the seeds of durum wheat (Triticum turgidum L.) cultivar Kızıltan-91 (Field Crops Central Research Institute, Ankara). Uniform and undamaged seeds from the previous harvest season were chosen and sterilized for 5 min in 2.5% sodium hypochlorite. The experiment was performed on glass jars in climatic chambers. Two layers of sterile filter paper were placed on the bottom of each sterile glass jar (8 × 5 × 5.5 cm) and three replications with 12 seeds per replication were sown in each jar. Seeds were soaked in 6 ml of Hoagland’s solution (Hoagland and Arnon, 1950) and placed in a temperature-controlled chamber at 24 °C with a 16/8 day/night photoperiod, 2500 lx light intensity, and 70% relative humidity. Wheat seeds were exposed to different durations and inductions of magnetic field.
Magnetic field application
The experimental factors were composed of two levels of magnetic field induction and exposure time. Two different Helmholtz coils (30 cm diameter/1200 turns and 40 cm diameter/1500 turns) and three sources of direct current (DC) power supply were used for the generation of the magnetic field of 12.5 and 25 mT, respectively, according to preliminary experiment experience. By adjusting the current in the Helmholtz coils, the requisite magnetic field induction was obtained (Fig. 1).
The generated magnetic field was measured regularly by a tesla meter (HT 20 Digital Gaussmeter, SMA, China) during the exposure period. Seeds placed in glass jars were exposed adjusted magnetic field for the same period in a day during the germination period. The experimental group had two different magnetic field induction and durations (Table 1).
Seed germination and growth parameters
At the end of the magnetic field application (6 days after imbibition), the germination ratios were calculated and expressed as a percentage in all groups. Root and stem lengths (cm) were manually measured. Also, fresh and dry weights were determined using a precision scale ± 0.01 mg. Then relative water content of the seedlings was calculated according to the formula based on three measured values (dry, fresh weights, and turgor) following Hu et al. (2010).
Pigment contents
Chlorophyll a, b and carotenoids were determined by using a spectrophotometer (FLUOstar Omega Microplate Reader with UV/Vis Spectrometer, BMG LABTECH, Germany). 0.1 g healthy leaf samples were homogenized in 80% acetone to determine chlorophyll concentration. The chlorophyll and carotenoid contents were determined by measuring absorbance at 480, 645, and 663 nm. Arnon (1949). Lichtenthaler and Wellburn (1983) method was used to calculate the chlorophyll and carotenoid contents.
Preparation of seedling extract
100 mg of seedling material (ground in liquid nitrogen) was mixed thoroughly with 10 ml of ethanol (80%) and shaken on a magnetic stirrer for 5 min at room temperature. The solutions were then sonicated at 37 °C for 40 min and incubated at 4 °C for 24 h and then centrifuged at 10,000 RPM. Supernatants were taken and stored at − 20 °C until further analysis.
Antioxidant capacities
The antioxidant activities were carried out by using the extract samples obtained from the seedlings. The DPPH radical scavenging activity was measured according to Blois (2002) at 520 nm. The measurements of DPPH radical scavenging activities of samples that were exposed to the magnetic field application and control groups were calculated by using the following formula: Inhibition % = (AC − AS)/AC × 100 (AC: absorbance of control, AS: absorbance of the sample) (Moraes-de-Souza et al. 2008).
Total reducing capacity (TPTZ technique) was measured with a spectrophotometer at 595 nm for the FRAP test. Based on an iron sulfate standard (FeSO4) curve against a blank control, the extracts’ reducing ability was represented as mol of iron (Fe2+) per gram of dry weight (mol Fe2+ /gDW) (Sudha et al. 2012).
Phytochemical content analysis
The total phenolic content was measured at 600 nm using the Folin–Ciocalteu method. According to gallic acid standard curve and a blank control, the results were calculated as mg of total phenolic content (gallic acid equivalent) per gram dry weight (mg GAE/gDW). At 510 nm wavelength, the total flavonoid content was determined. The extracts were tested in triplicate and the results were represented as milligrams of total flavonoid content (rutin equivalent, RE) per gram of dry weight of the ground powder (mg RE/gDW), based on the rutin standard curve. The analyses were carried out in triplicate (Dalar and Konczak 2013).
cDNA synthesis and qRT-PCR
Seven-day-old seedlings were harvested to perform q-PCR analysis and ground in liquid nitrogen immediately. Powdered tissue was poured into 2 ml tubes and used for total RNA extraction. For the RNA extraction process, a commercial Rneasy kit (QIAGEN Rneasy Mini Kit 250) was used according to the manufacturers' instructions, and DNase I (Fermentas) kit was used to remove DNA residues in RNA. PolyA-RNA cDNA synthesis was carried out utilizing reverse transcriptase and oligodT18 primers to generate the cDNA. Table 2 shows the gene-specific primers used in the qRT-PCR tests using complementary DNAs as templates.
The spectrophotometric quantification of RNA was additionally validated using a NanoDrop (Thermo Fisher Scientific, Inc). Following the manufacturer's instructions, real-time PCR was done with the cDNA using the q-PCR SYBR Green master mix. The Ct-method was used to compile and evaluate data from three independent experiments. Fold changes were calculated by the 2−ΔΔCt method referenced to actine (Livak and Schmittgen 2001).
Statistical analysis
All experiments were performed as three replications. The results were presented as the mean value ± standard deviation. The obtained results were analyzed using Minitab software (v. 19.1.0, State College, PA: Minitab, Inc.), by applying one-way analysis of variance (ANOVA) at a significance level of p ≤ 0.05.
Results
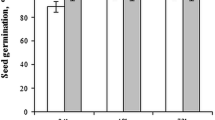
The effects of the magnetic field on the germination of the seeds were examined, and it was interestingly determined that the germination percentage of all seeds decreased compared to the control group. It was noticed that there was a significant interaction between germination and magnetic field induction and duration, especially at the time point of 30 min. So it was found that the magnetic field applications significantly reduced the germination percentage at both 12.5 and 25 mT induction (Fig. 2a).
According to the data obtained in the study, an inverse symmetrical situation was observed between germination and relative water content. It was found that the relative water content in the seedlings with a low germination percentage was found to be significantly higher than that in the others (Fig. 2b). There was no significant difference between the root and stem lengths of the seedlings exposed to the magnetic field; however, it was determined that 25 mT and 30 min of exposure significantly reduced both root and stem lengths (Fig. 2c).
Chlorophyll pigment contents were evaluated, magnetic field applications caused contrasting results. According to results, 25 mT and 30 min magnetic field applications caused an increase in chlorophyll pigments, while other applications caused negative (decrease) or nonsignificant changes in chlorophyll pigments. The effects of the magnetic field on chlorophyll a and b varied according to time and induction ratios. Short exposure of 25 mT for 15 min was effective to decrease photosynthetic pigments; however, prolonged exposure time for 30 min at the same induction increased the level of the pigments significantly (Table 3).
The effect of MF treatments on total phenolic content was observed only at 25 mT/30 min. Moreover, the flavonoid content decreased in all magnetic field groups compared to the control. The antioxidant activities of DPPH and FRAP decreased compared to the control. The exception was the insignificant increase at 12.5 mT induction for both exposure times (DPPH). The 25 mT magnetic field treatment with a duration of 15 and 30 min significantly reduced the FRAP content by 25 and 28%, respectively, compared to the control group (Table 4).
Figure 3 indicates the graphs comparing the gene expression patterns of seedlings exposed to 12.5 and 25 mT magnetic fields for 15 and 30 min. Within each treatment, values are shown as fold induction or repression relative to the control.
The expression levels of all studied genes were strongly affected by magnetic field applications. In general, a proportional increase in the expression of all genes was determined, depending on the increase in magnetic field induction and duration. The qRT-PCR results indicate that representative genes show more than a twofold difference in expression between the exposure time and intensities especially in Rubisco and CBP4 genes (Fig. 3) Fig. 2c.
Discussion
In the current study, a significant effect was observed for both magnetic field and exposure times, which had a negative effect on the wheat seeds’ germination percentage compared to the control. However, the duration of exposure time is thought to be more dominant on the negative effect, because of the prominent effect of 30 min than 15 min. The effect of magnetic field application, is dependent not only on its intensity, but also on plant genotype and duration.
The stimulatory effect of the application of different magnetic field levels and durations on the germination is reported in several species including rice (Florez et al. 2004), wheat and barley (Martinez et al. 2000), Pisum (Iqbal et al. 2012), sugar beet (Szajsner et al. 2017), and chickpea (Mridha and Nagarajan 2014). The researchers associated their results with hydrolysis or the expression of hormones (indole, gibberellins, or zeatin) or amylolytic enzymes (α-β amylase). However, there is no complete and uniform theory explaining the function or effects of magnetic fields. Changes in hormone concentrations, enzyme activities or ion transport across the cell membrane and also changes in DNA synthesis or transmission can all be influenced by variable magnetic fields (Strasak et al. 2002). In our study, depending on the type of wheat seed and the duration/intensity, magnetic field treatment may act on imbibition of water, abscisic acid pathway, or hydrolytic enzyme,s so germination inhibition may have occurred depending on the type of magnetic field treatment at 25 mT and 30 min. The short duration of the magnetic field is reported to cause a negative effect on most of the indicators characterizing sunflower seed germination and growth (Matwijczuk et al 2012). In a study, results showed that lower strengths (≤ 125 mT) of MF treatment affects the germination of barley seeds. Confocal microscopy examinations demonstrated MF-induced cell membrane disruption in roots, which could affect tissue elemental composition (Ercan et al. 2022). Similar effects were detected on root and shoot length. Germination delay caused a significant decrease in the lengths, especially in 25 mT—30 min application.
One of the most interesting results of our study was that the relative water content (RWC) was quite high in the groups that were more affected by the magnetic field and had germination inhibition. RWC percentage of all applications was significantly higher than that of control. The results indicated that there is a contrasting effect between germination inhibition and RWC. Magnetically treated water, acting on calcium ions, may improve the permeability of cell membranes in seeds. This may alter ion transport via cell membranes, causing an imbalance in ion concentration in the cell and variations in intracellular pH. It is possible to explain the response of magnetic field on variations in water characteristics and impurities (Matwijczuk et al. 2012). Another reason for the high RWC may be the effect of the magnetic field on aquaporin in the membranes.
Magnetic field has effects on leaf gas exchange performance as well as photosystem II (PSII) efficiency, photosynthetic pigments (chlorophyll a and b), and the performance index (Yano et al. 2004; Rochalska 2005; Baghel et al. 2018). In our study, especially chl_b contents indicate significant differences caused by magnetic field application. In addition, different values were obtained in 25 mT/15 min compared to other applications, particularly in carotenoids. Magnetic field influences pigment content (carotenoids, chlorophyll a, b, and total pigments), with carotenoids and chlorophyll a being affected more than chlorophyll b. Shine et al. 2011. In our result, the chlorophyll and carotenoid contents were decreased during 25mT and 15 min application. MF exposure reduced the level of photosynthetic pigments in seedlings of Zea mays L. and Robinia pseudoacacia L. This effect could be related to the influence of MF on the reduction in plastids (Taia et al. 2007) or a decrease in carotenoids explained by a decrease in pigment content. In this context carotenoids are used in radical scavenging processes (Strzalka et al. 2003).
Unpaired electrons on metal ions may orient in the same direction as the applied magnetic field, gaining more energy in the enzymes. This energy may be transmitted to other molecules, causing additional radicals to develop and affect antioxidant capacity (Çelik et al. 2009). In many plant species, phenolic compounds and flavonoids act as antioxidants, and a significantly positive association between total phenols and antioxidant activity has been reported. (Rainha et al. 2011). According to the results obtained in our study, it was determined that there was a decrease in the antioxidant activities of DPPH and FRAP due to the increased phenolic content, especially in 25 mT/30 min application. Metabolic and biochemical activities improved because of magnetic treatments and changed the biochemical properties (Zareei et al.2019).
Varying intensity of low-frequency MF affects several parameters, including CO2 assimilation, pigment content, and activity of photosynthetic reactions (Sukhova et al. 2021), by the generation of electric potential (electromagnetic field). The results of the study demonstrated that low-frequency MF influence electric and signal reactions in wheat plants. This impact is achieved by the extremely low-frequency MF acting on the signaling systems that balance the intracellular concentration of Ca2+ and the activity of H+- ATPase. Because a plant cell conducts various critical tasks, MF-induced variations in the electric potential can have a specific value for its functioning. Magnetic fields cause an increase in Ca2+ concentration due to the activation of the H+/Ca2+ antiporter, which was suggested as a key mechanism of electromagnetism (Grinberg et al. 2022).
The presence of a magnetic field may trigger signal transduction and gene regulation. Also in our study, q-PCR results indicate that magnetic fields have far-reaching effects on the genome of wheat seedlings. In particular, in all four different gene regions studied, the expression levels of 25 mT/30 min were higher than both control and other treatments. In general, researchers hypothesized that magnetic fields were powerful enough to disrupt the complex conformational dynamics involved in elements of gene regulation, resulting in the varied expression of a wide range of genes in plants (Paul et al. 2006).
A magnetic field can affect the local chemical structure of any of the multiple signaling pathways, which are linked to the differential expression of any number of genes involved in various aspects of plant growth (Weaver et al. 2000). However, further research is needed to fully comprehend the findings.
Finally, it should not be thought that the application of magnetic field will always have a positive effect on germination. The responses are quite variable and complex and depend on the pathway to be triggered on metabolism.
Conclusion
The effects of the magnetic field on living organisms are evaluated differently because of its effects on various pathways. While some consider the magnetic field as an environmental stress condition for the growth of plants, others suggest the magnetic field as a promising technique for agricultural improvements. Now, the efficiency of magnetic field parameters (such as exposure time/intensity, combination with other stresses, or effects on nerves and treatments) on organisms appear to be species specific, but it remains a mystery and needs much more work.
Author contribution statement
MEE contributed to the study conception and design. Material preparation, data collection and analysis were performed by MÖ. The first draft of the manuscript was written by MEE and MÖ commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
Arnon DI (1949) Copper enzymes in isolatedchloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1–15
Atak C, Emiroglu O, Alikamanoglu S, Rzakoulieva A (2003) Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J Cell Mol Biol 2:113–119
Baghel L, Kataria S, Guruprasad K (2018) Effect of static magnetic field pretreatment on growth, photosynthetic performance and yield of soybean under water stress. Photosynthetica 56:718–730
Çelik Ö, Büyükuslu N, Atak Ç, Rzakoulieva A (2009) Effects of magnetic field on activity of superoxide dismutase and catalase in Glycine max (L.) Merr. Roots 18(2):175–182
Dalar A, Konczak I (2013) Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind Crops Prod 44:383–390
Ercan I, Tombuloglu H, Alqahtani N, Alotaibi B, Bamhrez M, Alshumrani R, Ozcelik S, Kayed TS (2022) Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Physiol Biochem 170:36–48
Florez M, Carbonell MV, Martinez E (2004) Early sprouting and first stages of growth of rice seeds exposed to a magnetic field. Electromagn Biol Med 23:157–166
Grinberg M, Mudrilov M, Kozlova E, Sukhov V, Sarafanov F, Evtushenko A, Ilin N, Vodeneev V, Price C, Mareev (2022) Effect of extremely low-frequency magnetic fields on light-induced electric reactions in wheat. Plant Signal Behav. https://doi.org/10.1080/15592324.2021.2021664
Harris SR, Henbest KB, Maeda K, Pannell JR, Timmel CR, Hore PJ (2009) Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J R Soc Interface 6:1193–1205
Hozayn MA, Qados MSA (2010) Magnetic water application for improving wheat (Triticum aestivum L.) crop production”. Agric Biol J N Am 4(1):677–682
Hu L, Wang Z, Du H, Huang B (2010) Differential accumulation of dehydrins in response to water stress for hybrid and common bermudagrass genotypes differing in drought tolerance. J Plant Physiol 167:103–109
Hussain MS, Dastgeer G, Afzal AM, Hussain S, Kanwar RR (2020) Eco-friendly magnetic field treatment to enhance wheat yield and seed germination growth. Environ Nanotechnol Monit Manag 14:100299
Iqbal M, Haq Z, Jamil Y, Ahmad MR (2012) Effect of presowing magnetic treatment on properties of pea. Int Agrophys 26:25–31
Lichtenthaler H, Wellburn A (1983) Determinations of total carotenoids, chlorophylls a, and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Livak JK, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Maffei ME (2014) Magnetic field effects on plant growth, development, and evolution. Front Plant Sci 5:445–460
Martinez E, Carbonell MV, Amaya JM (2000) A static magnetic field of 125 mT stimulates the initial growth stages of barley (Hordeum vulgare L.). Electromagn Magnetobiol 19:271–277
Matwijczuk A, Kornarzyñski K, Pietruszewski S (2012) Effect of magnetic field on seed germination and seedling growth of sunflower. Int Agrophys 26:271–278
Moraes-de-Souza RA, Oldoni TLC, Regitano-d’Arce MAB, Alencar SM (2008) Antioxidant activity and phenolic composition of herbal infusions consumed in Brazil. Cienc Tecnol Aliment 6:41–47
Mridha N, Nagarajan S (2014) Effect of pre-sowing static magnetic seed treatment on germination and root characters in chickpea (Cicer arietinum L.). J Agric Phys 14(1):22–29
Paul AL, Ferl RJ, Meisel MW (2006) High magnetic field induced changes of gene expression in Arabidopsis. Biomagn Res Technol 4(1):1–10
Payez A, Ghanati F, Behmanesh M, Abdolmaleki P, Hajnorouzi A, Rajabbeigi E (2013) Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and 10-KHz electromagnetic field. Electromagn Biol Med 32:417–429
Rainha N, Lima E, Baptista J (2011) Comparison of the endemic Azorean Hypericum foliosum with other Hypericum species. Antioxidant activity and phenolic profile. Nat Prod Res 25(2):123–135
Rochalska M (2005) Influence of frequent magnetic field on chlorophyll content in leaves of sugar beet plants. Nukleonika 50:25–28
Shine M, Guruprasad K, Anand A (2011) Enhancement of germination, growth, and photosynthesis in soybean by pre-treatment of seeds with magnetic field. Bioelectromagnetics 32:474–484
Strasak L, Vetterl V, Smarda J (2002) Effects of low-frequency magnetic fields on bacteria Escherichia coli. Bioelectrochemistry 55:161–164
Strzalka KA, Kostecka G, Latowsk D (2003) Carotenoids and environmental stress in plant: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physiol 50:168–173
Sudha G, Vadivukkarasi S, Indhu Shree RB, Lakshmanan P (2012) Antioxidant activity of various extracts from an edible mushroom Pleurotus eous. Food Sci Biotechnol 21:661–668
Sukhova E, Gromova E, Yudina L, Kior A, Vetrova Y, Ilin N, Mareev E, Vodeneev V, Sukhov V (2021) Change in H+ transport across Thylakoid membrane as potential mechanism of 14.3 Hz magnetic field impact on photosynthetic light reactions in seedlings of wheat (Triticum aestivum L.). Plants 10(10):2207
Szajsner H, Prośba-Białczyk U, Sacała E, Koszelnik-Leszek A, Szubzda B (2017) The effect of pre-sowing seed stimulation on the germination and pigment content in sugar beet (Beta vulgaris L.) seedlings leaves. Pol J Nat Sci 32(2):207–222
Taia WK, Kotbi AM, AlZahrani HS (2007) The effect of static magnetic forces on water contents and photosynthetic pigments in sweet basil Ocimum basilicum L. (Lamiaceae). Saudi J Biol Sci 14:103–107
Weaver JC, Vaughan TE, Astumian RD (2000) Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature 405:707–709
Yano A, Ohashi Y, Hirasaki T, Fujiwara K (2004) Effects of a 60 Hz magnetic field on photosynthetic CO2 uptake and early growth of radish seedlings. Bioelectromagnetics 25:572–581
Zareei E, Fariborz Z, Shahin O, Jafar H (2019) Effects of magnetic solutions on some biochemical properties and production of some phenolic compounds in grapevine (Vitis vinifera L.). Sci Hortic 253:1–3
Acknowledgements
The authors are grateful to the Scientific Research Projects Coordination Unit of Van Yuzuncu Yil University for supporting our project (FYL-2020-8769).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Rybka.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erez, M.E., Özbek, M. Magnetic field effects on the physiologic and molecular pathway of wheat (Triticum turgidum L.) germination and seedling growth. Acta Physiol Plant 46, 5 (2024). https://doi.org/10.1007/s11738-023-03631-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-023-03631-7