Abstract
Podophyllum hexandrum Royle known as Indian mayapple is an important medicinal plant found only in higher altitudes (2,700 to 4,200 m) of the Himalayas. The highly valued anticancer drug Podophyllotoxin is obtained from the roots of this plant. Due to over exploitation, this endemic plant species is on the verge of extinction. In vitro culture for efficient regeneration and the production of podophyllotoxin is an important research priority for this plant. Hence, in the present study, an efficient plant regeneration system for mass multiplication through somatic embryogenesis was developed. We have screened P. hexandrum seeds collected from three different regions in the Himalayas to find their regenerative potentials. These variants showed variation in germination percentage as well as somatic embryogenic frequency. The seeds collected from the Milam area of Pithoragarh district showed better germination response (99.3 %) on Murashige and Skoog (MS) medium fortified with Gibberellic acid (GA3 [5 mg/l]) and higher direct somatic embryogenic frequency (89.6 %). Maximum production of embryogenic callus (1.2 g fresh weight [FW]) was obtained when cotyledons containing the direct somatic embryo clusters were cultured in MS medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D [1.5 mg/l]) after 4 week of culture in complete darkness. In the present investigation, somatic embryogenesis was accomplished either by direct organogenesis or callus mediated pathways. The latter method resulted in a higher frequency of somatic embryo induction in hormone-free MS medium yielding 47.7 embryos/50 mg of embryogenic callus and subsequent germination in MS medium supplemented with GA3 (5 mg/l). Seventy-nine percent of embryos attained complete maturity and germinated into normal plants with well-developed roots. Systematic histological analysis revealed the origin of somatic embryo and their ontogenesis. The higher level of podophyllotoxin (1.8 mg/g dry weight [DW]) was recorded in germinated somatic embryos when compared to field grown plants. The present system can be widely used for mass propagation, transgenic recovery, and podophyllotoxin production for commercial utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the population increases in the world, cancer is expected to be one of the leading causes of death. Visiongain, a leading British research company, while analyzing business markets, estimated that the world market in cancer-fighting pharmaceuticals will reach $75 billion in 2012 and precisely reports that the overall revenue for anticancer treatment will increase strongly from 2012–2022. Two species of Podophyllum (Berberidaceae) i.e., P. peltatum and P. hexandrum are widely utilized to treat several tumor diseases (Bedir et al. 2001). P. hexandrum Royle commonly known as “Indian mayapple” is a popular medicinal plant used in Indian traditional medicinal system for many years. This plant contains four times more podophyllotoxin (PTOX) than its American counterpart, P. peltatum (Uden et al. 1989). The leaves, roots, and rhizomes of this plant are rich sources of PTOX and served as a starting material for the semi-synthesis of the anticancer drugs etoposide, teniposide, and etopophos (Moraes-Cerdeira et al. 2002). Due to the presence of high-valued medicinal components, it has been sold by many pharmaceutical industries for commercial production of PTOX. Hence, many pharmaceutical companies have devoted their efforts in developing new routes to the total synthesis of PTOX. However, this is a low-yielding process due to complicated steps involved in it (Bush and Jones 1995).
The supply of Podophyllum plant materials is rather limited, as the plant needs a growth period of at least 5–7 years and can only grow in the restricted regions of the Himalayas. Because of intensive collection of the plant from wild and unorganized cultivation, P. hexandrum has reached the status of extinction. Owing to steady decline in its natural populations, it has been declared as a critically endangered species as per IUCN criteria (Samant et al. 1998). Propagation of Podophyllum plant is difficult because of low seed-set, prolonged dormancy, and laborious harvesting procedure (De kroon et al. 1991). Due to its long life span and low reproductive efficiency, conventional breeding of this plant species is very difficult and time consuming. It is therefore important to initiate steps for its large-scale multiplication to develop cultivation packages (Pandey et al. 2007). Under these ruthless situations, it is, therefore, vital to evolve large-scale multiplication strategies for its conservation as well as to sustain production of PTOX. To overcome the increasing demand with the supply of quality material, many research companies resorted into alternative sources/strategies for PTOX production. Under these circumstances, biotechnological approaches would help to mitigate secondary metabolites production through cell/organ cultures to meet the market demands (Sivanandhan et al. 2012a, b). Plant tissue culture offers a viable alternative method for plant propagation, in which somatic embryogenesis is considered as a potential system for large-scale propagation, conservation, plant genetic improvement, biochemical studies, and for the bioactive compound productions (Sajc et al. 2000; Paek et al. 2005). During the last decades a few attempts have been made to produce/improve PTOX content in cell/organ cultures (Majumder and Jha 2009) but the product yield has not been ample from an industrial point of view. None of the reports documented PTOX production in somatic embryogenesis of P. hexandrum as this species is considered as an important candidate in the pharmaceutical industry.
Although, earlier reports on the somatic embryogenic potentials of P. hexandrum were available (Arumugam and Bhojwani 1990; Nadeem et al. 2000), the efficiency is low and required long culture duration and more subcultures hinders its application. Arumugam and Bhojwani (1990) observed somatic embryogenesis in 50 % of cultures with an average of 12 dicotyledonous embryos per calli in MS medium supplemented with 2.5 μM NAA, whereas Nadeem et al. (2000) have not defined different stages of embryo developments; interactions of auxins with cytokinin or vice versa; multiplication of embryogenic calli; and frequency of response in embryo induction, development, and germination. Hence, there is an urgent need to develop the efficient in vitro regeneration method to improve efficiency of somatic embryogenesis in P. hexandrum for commercial/economical utility and transgenic recovery (Sultan et al. 2006; Kim et al. 2007). Establishing embryogenic culture has become an integral part of plant biotechnology as regeneration of transgenic plants in most important crops is dependent on formation of somatic embryos (Vasil 2008). The present study also documents the histological features of somatic embryo development. The increasing market demand compels us to find ways for PTOX production. Hence, the present research was aimed to improve somatic embryo production via establishment of embryogenic cell lines and scale up PTOX synthesis in somatic embryogenesis in P. hexandrum. In vitro regeneration system developed in this investigation provides a controlled and sustainable mass propagation of this species and PTOX production.
Materials and methods
Plant material and surface sterilization
Mature fruits of P. hexandrum were collected from three different locations, namely, (1) Parkachi (3,000 m) (Zanskar valley), Kargil, Jammu and Kashmir; (2) Dhumreda (2,590 m), Shimla, Himachal Pradesh; (3) Milam region (2,200 m), Pithoragarh, Uttarakhand, during Sep.–Oct. 2010. Mature seeds of all these varieties were separated from the ripened fruits, dried under shade, and stored at 4 ºC for further use. The seeds were washed in running tap water, followed by rinsing in Teepol® (Reckitt Benckiser Ltd. India) solution for 10 min. Then, the seeds were surface sterilized with 70 % (v/v) alcohol (Himedia, India) for 1 min, and followed by 10 min with 0.1 % (w/v) mercuric chloride (Himedia, India). Finally, the seeds were rinsed several times with sterile double distilled water to remove the sterilants. The seeds were allowed to imbibe for 24 h in sterile distilled water and placed on orbital shaker (Orbitek, India) at 120 rpm. The zygotic embryos were carefully excised from the imbibed seeds without any damage and used as explants.
Effect of GA3 on zygotic embryo germination
To evaluate the effect of GA3 on zygotic embryo germination, the excised zygotic embryos (1–2 mm) from the three varieties were cultured onto germination medium containing MS mineral salts and vitamins, 3 % (w/v) sucrose, and 0.2 % (w/v) gelrite with various concentrations (0–6 mg/l) of GA3 (Himedia, India). The pH of the medium was adjusted to 5.8 prior to autoclaving (20 min at 121 °C; 1.4 × 104 kgm−2). All the cultures were incubated under total darkness for a period of 5 days, and the cultures were then transferred to 16 h light photoperiod (50 μmol m−2 s−1), at 25 ± 2 ºC. The percentage of germination was recorded after 5 days, and PTOX content was recorded after 2 weeks.
Influence of auxins on direct somatic embryo induction
The zygotic embryos of three varieties were inoculated separately into culture tubes (2.5 × 1.5 mm, Borosil, India) containing MS medium supplemented with various auxins (2,4-D, NAA, Picloram, IAA and IBA; Himedia, India) individually at different concentrations (0.5–2.5 mg/l) to investigate their effect on somatic embryo induction. The percentage of somatic embryo induction and number of somatic embryos were recorded after 4 weeks of culture. All the cultures were incubated in complete darkness at 25 ± 2 ºC.
Effect of auxins on embryogenic callus induction
A best responding variety “Milam” in direct somatic embryo induction system has been used for further experiment. After 4 weeks, the cotyledonary segments with direct somatic embryos of the Milam variety were transferred to MS medium fortified with either one of the auxins (2,4-D, NAA, Picloram, IBA and IAA) at different concentrations ranging from 0.5–2.5 mg/l to test their efficiency on embryogenic callus induction. The percentage of embryogenic callus and non-embryogenic callus and fresh weight were recorded and tabulated after 4 weeks of culture. The maintenance of culture was followed as described earlier.
Influence of auxins and ABA on indirect somatic embryo development
To study the potential of embryogenic callus on induction and development of somatic embryos, 4-week-old yellowish white friable callus (50 mg FW) grown on MS medium fortified with 2,4-D (1.5 mg/l) were aseptically transferred onto either MS basal medium or MS medium augmented with different concentrations of 2,4-D, NAA, and Picloram in the range of 0.5–2.5 mg/l or ABA (0.5–2.5 mg/l). For ABA treatment, embryogenic callus was cultured in MS medium containing ABA for 2 weeks then transferred into MS basal medium. The cultures were incubated for 4 weeks at 25 ± 2 °C with 16-h photoperiod (50 μmol m−2 s−1) provided by cool-white fluorescent lamp. The percentage of somatic embryo induction, number of normal somatic embryos, and number of abnormal somatic embryos were recorded and tabulated after 4 weeks of culture.
Germination and transplantation
The cotyledonary staged somatic embryos were separated from the clumps and transferred to MS basal medium or MS medium supplemented with either GA3 (5 mg/l) or ABA (2 mg/l) to induce germination. After 2 weeks, germinated embryos were transferred to plastic cups containing mixture of sterilized perlite, peatmoss, and vermiculate (1:1:1 v/v/v) and covered with polythene bags to maintain high humidity and further hardening was carried out in the plant growth chamber (Sanyo, Japan) under controlled conditions. The percentage of germination and time taken for germination were recorded and tabulated at 2 weeks of culture.
Histological analysis
Histological analysis was performed by following the method of Berlin and Miksche (1976). The samples were positioned in a fixing solution of formalin acetic acid alcohol containing 5 ml of 96 % ethanol, 10 ml of 37 % formaldehyde, and 5 ml acetic acid and adjusted to a 100 ml of volume with distilled water and stored for 48 h at 10 °C. After fixing, the samples were dehydrated by successive changes in ethanol at different concentrations (30–100 %; v/v). Subsequently, after infiltration, the samples were embedded in plastic resin. Serial sections (5 μm) were cut in a rotary microtome, mounted on cytological glass slides, and stained with 0.05 % toluidine blue (4 min).
Photomicrography
Different developmental stages of somatic embryos were observed and photographed using Nikon Stereo microscope and light microscope equipped with Nikon color digital camera system DS-Fi1-U2 (100–240 V) (Nikon, Japan) consisting of NIS elements software package.
PTOX extraction and HPLC analysis
The plant materials (germinated somatic embryos from GA3 (5 mg/l), control [germinated somatic embryos without GA3] and germinated zygotic embryo from Milam variety were dried and ground into fine powder (1 g DW). Extraction was performed as described by Uden et al. (1989) with some modification. PTOX quantification was done using a Waters system (Waters HPLC, Vienna, Austria) equipped with a PDA detector and a reverse-phase Luna® column C18 (5 μm; 30 × 4.6 mm). Twenty microliters of syringe-filtered (0.22 μm) samples were injected into the column and eluted isocratically with HPLC-grade methanol and water (65:35 v/v) [Himedia, Mumbai, India] at a flow rate of 1.0 ml/min. PTOX were detected with a PDA detector at a wavelength of 250 nm. The relative amounts of PTOX were calculated by comparing their peak areas with standard curve generated using different amounts of external standards. PTOX data were expressed as milligram per gram dry weight. Each sample was run in triplicate manner to check the consistency over the previous results. Standard sample of PTOX (RT 7.0 min) was obtained from Sigma-Aldrich (Laguna Hills, CA, USA).
Statistical analysis
A completely randomized design was used for all treatments. All the experiments were repeated thrice with three replicates for each treatment. Data were statistically analyzed using analysis of variance (ANOVA). Data were presented as the mean ± standard error (SE). The mean separations were carried out using Duncan’s multiple range test, and significance was determined at 5 % level (SPSS 11.5).
Results and discussion
Plant material
Selection of appropriate explant is a critical step in in vitro culture in general and induction of somatic embryogenesis in particular (Krishnaraj and Vasil 1995). In the present study, zygotic embryos excised from imbibed seeds were used as a starting material for culture initiation and somatic embryo induction. We have tested the efficiency of cotyledon, leaf, and root, which are failed to respond to somatic embryogenesis (Data not shown). Earlier workers also used zygotic embryos for somatic embryogenesis (Arumugam and Bhojwani 1990; Nadeem et al. 2000). In high altitude alpine plants, seed germination and its conservation is dreadfully difficult. In this case, excised zygotic embryo culture is one of the promising approaches for mass propagation. The utilization of immature zygotic embryo as an initial explant is reasonable in the sense that they have diverse physiological prominence (Eudes et al. 2003), and possesses high degree of physiological consistency, regularity, and also free from contaminants (Scherwinski-Pereira et al. 2012). In addition, zygotic embryos are considered as more suitable explants for comprehensive study of various factors involved in culture establishment and embryogenic callus induction. Several treatments can be tested simultaneously, and more accurate results can be obtained from zygotic embryos (Balzon et al. 2013). During zygotic embryo development, cells differentiate early and rapidly, which is accompanied by a loss of mitotic and morphogenetic ability (Krishnaraj and Vasil 1995). Moreover, levels of endogenous hormones which differ among organs, tissues, and cells may also influence regeneration responses (Brown and Thorpe 1995). Hence, in the present study, zygotic embryos were selected for in vitro culture of P.hexandrum.
Effect of GA3 on zygotic embryos germination
As suggested by Badhwar and Sharma (1963), P. hexandrum seeds exhibit dormancy, an adaptation strategy to overcome the extreme climatic conditions prevailing at higher altitudes. The seeds are generally reported to be dormant for about 10 months under natural conditions. This major problem accentuated us to explore an alternative method to improve germination frequency. The zygotic embryos obtained from seeds collected from three different regions [Milam (Fig.1a), Dhumreda (Fig.1b), and Parkachi (Fig.1c)] varied with germination frequency as well as days required for germination. The seeds collected from the Milam region (Fig. 1a) showed better performance in germination (99.3 %) within 5 days. GA3 not only elevated the percentage of germination but also speed up the process and reduced the days required for germination when compared to control (Table 1; Fig.1g). Among the different concentrations of GA3 tested, 5 mg/l was found to be the most effective concentration and produced significantly higher germination response (99.3 %) within 5 days of culture, whereas medium lacking GA3 showed reduction in germination frequency (56.3 %) in the Milam variant. The lowest germination response was recorded in the other two variants (45.7 % in the Parkachi variant; 32.3 % in the Dhumreda variant). Besides improving percentage of germination, GA3 also improved the elongation of germinating embryo in all the three variants tested. The positive effects of GA3 on both the percentage of response and rapidity of zygotic embryo germination suggests that there is a hormonal imbalance in the embryos, probably related to embryo immaturation or dormancy, a phenomenon known to be frequent in many plants (Orozco-Segovia et al. 2003). Moreover, in the present study, it has been noted that zygotic embryos should be excised without any damage; the injured explants failed to germinate (Data not shown). Hence, the excision of zygotic embryos plays a crucial role in in vitro culture establishment of P. hexandrum. Arumugam and Bhojwani (1990) and Nadeem et al. (2000) noticed that seeds required 7 days for germination in MS basal medium. In our study, addition of GA3 (5 mg/l) enhanced the germination frequency (99.3 %) and also advances the duration of germination in all varieties tested (Table 1). This is the first report where appropriate concentration of GA3 improved germination of excised embryo in P. hexandrum. This observation was corroborated by Sadowska et al. (1997) who used MS medium with GA3 (1 mg/l) for generative propagation of P. peltatum. Similarly, Bhatt et al. (2005) observed improvement in germination percentage (96 %) of Swertia augustifolia, a high altitude medicinal plant, in the presence of GA3. Chandra et al. (2006) also observed improvement of germination in Picrorhiza kurrooa, Aconitum balfourii, and Aconitum heterophyllum by inclusion of GA3 in the medium. GA3 has been used to overcome the dormancy of seeds in many species which may either reduce the inhibitors or activate the GA3 synthesis or both (Bhatt et al. 2005). In the present study, PTOX level of the three varieties showed significant quantitative variations. The highest content of PTOX (1.3 mg/g DW) was detected in the 2-week-old Milam variety. The present result suggests that genetic makeup and in vitro culture conditions enormously influence the PTOX biosynthesis.
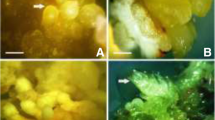
P. hexandrum seeds collected from three different locations in the Himalayas and germination of excised zygotic embryos. a Seeds collected from the Milam region, Pithoragarh district, Uttaranchal. b Seeds collected from Dhumreda, Shimla district, Himachal Pradesh. c Seeds collected from the Parkachi region, Kargil district. d Excised zygotic embryo germinated in GA3 (5 mg/l) after 2 weeks of culture from Dhumreda, Shimla district, Himachal Pradesh. e Excised zygotic embryo germinated in GA3 (5 mg/l) after 2 weeks of culture Parkachi region, Kargil district. f) Excised zygotic embryo germinated in GA3 (5 mg/l) after 2 weeks of culture from the Milam region, Pithoragarh district Uttaranchal. g Photograph showing high percentage of germination in MS medium supplemented with GA3 (5 mg/l) from seeds collected in the Milam region, Pithoragarh district Uttaranchal after 2 weeks of culture. All bars 1 cm (a–c), 0.2 cm (d–f), and 0.5 cm (g)
Influence of auxins on direct somatic embryo induction
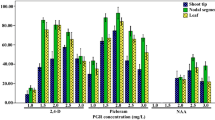
Table 2 demonstrates the influence of auxin and their concentrations on direct somatic embryo induction from zygotic embryos of the three varieties of P. hexandrum. Each variety displayed differential effect on somatic embryo induction, which was fabulously varied depending upon auxin type and concentration. Medium devoid of auxin (control) favored germination of zygotic embryos (Table 2). The highest response in the induction of somatic embryos (89.6 %) and the number of somatic embryos (14.9 embryos/explant) were obtained in the Milam variety when the zygotic embryos were exposed to MS +1.5 mg/l 2,4-D (Table 2; Fig. 2 g, h). NAA and picloram showed moderate response. Initiation of somatic embryos was noticed after 2 weeks of culture in the induction medium (Fig. 2 e, f). The explant initially bulges and initiate somatic embryogenesis on the adaxial side, which is evident from appearance of yellow color. The present results revealed that 2,4-D is more efficient in influencing somatic embryo induction irrespective of varieties examined as compared to other auxins (Table 2). These annotations implicated that an endogenous auxin pulse may be one of the first signals leading to somatic embryogenesis and increase in endogenous auxin on the induction phase of somatic embryogenesis which was triggered by exogenously added 2,4-D (Feher et al. 2003). Auxins are also considered as a major factor involved in the acquisition of embryogenic competence in somatic tissues, and it has recently been used to induce somatic embryos in more than 80 % of protocols (Feher et al. 2003). Jiménez (2005) reported that exogenously applied growth regulator interact with endogenous tissue-specific one and thus, the level of endogenous hormones in the cultured explants is considered to be the most important factor which determines the tissue-specific embryogenic potential. Such divergent hormonal requirements for somatic embryo suggest that sensitivity of the cultured tissue to embryogenesis-inducing signals, including hormonal, can be more decisive for induction of the embryogenic pathway (Fehr 2006). At the molecular level, 2,4-D-induced growth and development of somatic embryos contains changes in gene expression and leads to rapid accumulation of numerous mRNA. Due to this auxin action on somatic embryogenesis, heat shock protein genes namely Dchsp-1 and Dcarg-1 exhibit constant expression for the initiation and proliferation of somatic embryos (Chugh and Khurana 2002). Somatic embryogenesis in many species is highly genotype dependent and often efficient somatic embryo induction and development have been restricted only to a few or even one genotype (Gaj 2001). In the present study, there was a significant difference in the induction of direct somatic embryos thereby indicating presence of genotypic specificity (Table 2). This may be due to the differences in the altitudes, prevailing climatic conditions, and age of the plant. The differentiation, development, and maturation of somatic embryos occurred only when transferred to 2,4-D-free medium. This is in accordance with the observations of Choi et al. (1999) where somatic embryo maturation took place in 2,4-D-free medium in Eleutherococcus senticosus. Somatic embryos produced directly from the explants are less prone to somaclonal variation (Kim et al. 2003). However, in P. hexandrum, fewer somatic embryos are produced directly from an explant without intervening callus. When zygotic embryos are used as explant source to induce DSEs, we can get an average of 14 DSEs per seed. Because of nonavailability of seeds, continuous micropropagation through this protocol may not be feasible. Therefore, it is essential to find the possibilities to produce large number of somatic embryos within short duration. Since zygotic embryos from the Milam variety showed maximum response in in vitro germination and direct somatic embryo induction, it is used for further experiment (embryogenic callus induction, indirect somatic embryo induction and germination).
Somatic embryogenesis in P.hexandrum. a Excised zygotic embryo (bar 1 cm). b Enlarged view of zygotic embryo (bar 1 mm). c, d V-shaped cotyledons after 1 week of culture in MS medium supplemented with 2,4-D (1.5 mg/l) [bar 1 mm]. e, f Induction of direct somatic embryo from the apical region of the cotyledon portion after 3 weeks of culture [bar 1 mm]. g, h A close-up view showing many batches of somatic embryo proliferation after 5 weeks of culture [bar 1 mm]. i The cotyledonary portion completely covered by newly formed embryo-transitory callus after 6 weeks of culture [bar 1 mm]. j Induction of embryogenic callus from cotyledons having direct somatic embryo after 4 weeks of culture [bar 1 cm]. k Enlarged view of embryogenic callus [bar 1 mm]. l, m Induction of indirect somatic embryo from embryogenic callus in MS medium after 6 weeks of culture [bars 0.2 cm and 1 cm, respectively]. n Development of indirect somatic embryo [bar 1 mm]. o Globular stage [bar 1 mm]. p Heart stage [bar 1 mm]. q Torpedo stage [bar 1 mm]. r Cotyledonary stage [bar 1 mm]. s, t Maturation of somatic embryo [bars 1 mm and 0.2 cm, respectively]. u, v Germination of somatic embryo [bar 1 cm]. w Hardened plant [bar 1 cm]
Effects of auxins on embryogenic callus induction
In the present investigation, direct somatic embryos underwent callusing when it was cultured for more than 4 weeks in auxin-supplemented medium. It was interesting to note that when the direct somatic embryos transferred into auxin-supplemented medium, embryogenic callus or non-embryogenic callus was formed which was differed depending upon the auxin types. Among the various concentrations of auxins tested, maximum embryogenic callusing response (91.4 %) was recorded in MS medium supplemented with 2,4-D (1.5 mg/l) after 4 weeks of culture. Other than 2,4-D, NAA and picloram were also favored for embryogenic callus development, however with low response (Table 3). Conversely, other auxins such as IAA and IBA induced non-embryogenic callus in culture which showed non-embryogenic response (Table 3). The nature of callus i.e., either embryogenic or non-embryogenic is decided by the nature of auxin present in the medium. Auxin and cytokinin are the two most commonly employed plant growth regulators for the activation and regulation of cellular division and differentiation (Feher et al. 2003). Within these two categories of PGRs, the exogenous addition of auxin (2,4-D) is well documented to induce the transition of somatic cells into embryogenic cells in many plants (Feher et al. 2003). Feher et al. (2003) postulated that addition of 2,4-D at an appropriate concentration might directly evoke auxin effect, besides triggering the production of endogenous IAA and even act as a stress stimulus. Previously, Arumugam and Bhojwani (1990) had induced embryogenic callus in MS + BAP and IAA amended medium after four subcultures (4 weeks each). These calli differentiates into somatic embryos after 4 weeks when transferred to 2,4-D (2.2 mg/l) + BAP (0.2 mg/l) amended MS medium. In the present study, subculture of embryogenic callus in MS medium supplemented with 2,4-D (1.5 mg/l) at 4-week interval favored continuous proliferation of embryogenic callus (Fig. 2j, k). In E. senticocus, the embryogenic calli were induced only by culturing somatic embryos in 2,4-D medium (Choi et al. 1999). For continuous production of somatic embryos, multiplication of embryogenic callus without disturbing its embryogenic potential is an essential prerequisite. In the present study, higher multiplication rate of embryogenic callus was achieved in 2,4-D (1.5 mg/l) resulting in an average of 1.2 g FW after 4 weeks of culture (Table 3).
Influence of auxins and ABA on indirect somatic embryo induction
The effect of PGR’s on somatic embryogenesis from the embryogenic callus was displayed in Table 4 and (Fig. 2l–t). Continuous presence of 2,4-D in the culture medium did not favor further development and maturation. The embryos were irregular and did not reach full maturation. Further development could be achieved only when the cultures were transferred to 2,4-D-free medium. In E.senticocus, withdrawal of 2,4-D from the media was essential for the induction and development of somatic embryos (Choi et al. 1999). In the present study, somatic embryo initiation was obtained when 2,4-D had been withdrawn from the MS medium. The exposure of tissues to 2,4-D for a prolonged period may increase the incidence of abnormalities in the somatic embryos, and it has emphasized the importance of withdrawing the auxin from the MS medium after the callus induction phase in order to promote cellular polarization (Fitch 1993). From the present investigation, it has been confirmed that higher concentration of 2,4-D is essential for the first stage of somatic embryogenesis, while during the second stage, a very low or withdrawal of 2,4-D is needed in order to allow the differentiation of somatic embryos from embryogenic calli. According to Fischer-Iglesias et al. (2001), there is a well-defined pattern to highly responsive species and genotypes, in which the auxin (2,4-D) plays a positive role for induction, while withdrawal of this compound triggers expression, allowing development of somatic embryos. Once the stimulus for the development of somatic embryos is given by the reduction or elimination of 2,4-D from the culture medium, the endogenous levels of IAA will be reduced to allow the establishment of a polar gradient of auxin. The continued presence of 2,4-D in the culture medium prevents the reduction of endogenous auxin levels resulting in inhibition of somatic embryos development. Choi et al. (1999) reported that withdrawal of 2,4-D from the culture medium increased somatic embryo induction from embryogenic callus in Eleutherococcus species. Choi et al. (1999) reported that auxin is essential for embryogenic callus proliferation but it is usually inhibitory for further development and maturation of somatic embryos. In the present study, it was observed that the embryogenic callus developed in 2,4-D-amended medium when transferred to MS + ABA (2 mg/l) for 2 weeks and then to hormone-free MS medium improved the induction and differentiation of normal somatic embryos (Table 4). The differentiated embryos continued maturation in the same medium. Short exposure of embryogenic cells to ABA causes more stress which triggered normal somatic embryo development but longer exposure may cause browning of cells and death. This stress response may be the reason for the induction of indirect somatic embryos. Kim et al. (2007) also recorded similar observations in P. peltatum.
Arumugam and Bhojwani (1990) obtained a total number of 12.20 dicotyledonous embryos per culture from friable callus induced in IAA + BAP-amended medium when transferred to medium containing 2,4-D (2 mg/l) + BAP (0.1mg/l). Subsequent transfer into MS + NAA (0.46 mg/l) leads to maturation of somatic embryos. In the present study, 47.7 ± 0.25 mean number of normal somatic embryos was obtained from 50 mg of embryogenic callus (Table 4). In P.hexandrum, removal of 2,4-D and inclusion of ABA treatment is essential for stimulating normal development of somatic embryos. ABA was reported to induce direct and secondary embryo formation in Daucus carota (Nishiwaki et al. 2000). The present results are in agreement with the report of Kim et al. (2007) who observed that 21.6 μM NAA was suitable for direct somatic embryo induction from 2-week-old cotyledon explants from the germinated zygotic embryos and 2,4-D treatment was suitable for secondary embryo formation. They also proved that ABA pretreatment induced normal somatic embryos (14/embryogenic calli) in P. peltatum. In our study, 47 normal somatic embryos were produced from 50 mg of embryogenic callus which showed higher percentage (82 %) of somatic embryo development. In contrast, addition of ABA suppressed the production of multiple embryo clusters in Carum carvi (Ammirato 1974). In addition to inducing normal development, ABA affects the accumulation of storage proteins in mature embryos (Merkle et al. 1990). In the present study, higher frequency of direct embryos, embryogenic callus, and induction of somatic embryos from embryogenic callus from zygotic embryo was achieved. The same trend was documented in E. senticoccus by Choi et al. (1999). The present system has advantages over direct embryogenesis such as high multiplication rate of embryogenic callus, production of somatic embryos, and independent of explant source since the availability of P.hexandrum seeds are rare and terrain for the collection is difficult in higher altitudes of the Himalayas.
Histological analysis
Different phases/stages of somatic embryogenesis (direct and indirect) were illustrated in Fig. 3. In direct embryogenesis, the histological examination demonstrated that the initial cell divisions takes place in the inner cells (subepidermal cells) of the zygotic embryos. The basal portion of the embryo (explants) did not show any change. Contrastingly, the apical portion .i.e., the region looks like cotyledons showed the development of multicellular embryos (Fig. 3b, c). Primary embryos emerged from the outer region of proliferating apical zone. The “V”-shaped cotyledon-like structure is surrounded by newly formed primary embryos (Fig. 3c). The cells in the inner mass have a prominent nucleus, dense cytoplasm, and thin cell walls dividing on various planes, distinguishing the cells with embryogenic proficiency from non-embryogenic cells. The frequency of cell division is high and contained starch grains with lower cytoplasm density. The same trend was exactly documented by Scherwinski-Pereira et al. (2012) in Euterpe oleracea somatic embryogenesis. The succeeding cell divisions primarily produced a protuberance containing one or two mersitematic cells which is progressed into numerous multicellular nodular calli. These calli differentiated into somatic proembryos in E. oleracea (Scherwinski-Pereira et al. 2012). In our examination, the development of direct somatic embryos showed plentiful spherically arranged cells which latter differentiates into somatic embryos. Heart shaped embryo which attaches to the tissues of the cotyledons was shown in Fig. 3e. In the present study, besides the embryos of multicellular origin, somatic embryos of unicellular origin was also recorded. Embryogenic callus was formed from the clouds of primary embryos. During indirect embryogenesis, embryogenic callus undergo successive divisions prior to differentiation of somatic embryos. In the histological study, isodiametric small cells with dense cytoplasm and a voluminous nucleus was viewed in the proliferating embryogenic tissues (Fig. 3f, g). The embryogenic cells had prominent nucleus which divides rapidly and passed the stages (2 cell stage, 4 cell stage, 8 cell stage). Continuous division leads to the formation of globular embryos (Fig. 3h) thereby suggesting unicellular origin. As recorded by Balzon et al. (2013) in Elaeis guineensis somatic embryogenesis, the accumulation of starch either in the embryogenic cells or in adjacent cells of the explants appears to be a phenomenon related to the acquisition of embryogenic competence. We have also recorded similar observation. Kanchanapoom and Domyoas (1999) postulated that starch grains in the calli and bipolar embryoids have improved the somatic embryogenesis in E. guineensis. Moreover, starch grains in somatic embryos provides an energy source for intense cellular divisions and development of the embryos, as suggested by Mikuła et al. (2004) in Gentiana punctata. In P.hexandrum, multicellular origin appears to produce embryos fused with the parent tissue over a broad area of the cotyledon region, whereas unicellular embryos appears to be from a friable clump of embryogenic cells (Fig. 3g), the “proembryonal complex”, from which one to many embryos develop. Proembryogenic cell clumps have been reported to arise from single cells which undergo a redetermination event followed by quantal segmentation in Trifolium repens (Williams and Maheswaran 1986).
Ontogenesis of somatic embryogenesis in P.hexandrum. a L.S view of zygotic embryo shows the bifergated apical portion with two cotyledons (arrows). b The L.S of zygotic embryo explant showing direct induction of somatic embryo (arrow) in the cotyledonary portion. c L.S of cotyledonary tip portion shows many embryos (small arrow heads) and intact cotyledonary explant (big arrow). d L.S showing newly formed embryos each one was distinguished with boundary cells (small arrow heads). The meristematic cells delimited by epidermis-like boundary cells. e L.S showing heart-shaped embryo in apical portion of the cotyledons (arrow). f T.S showing a group of rapidly dividing embryogenic cells (small arrow heads) leading to the formation of proembryos. g Globular somatic embryo during differentiation. h Heart shape somatic embryo showing plumular pole (meristem) (arrow) and root pole (arrow). i T.S showing active cell divisions (arrow mark) in plumular meristematic segment (intensively dividing meristematic cells). j, k Formation of shoot apex and leaf primordium (arrow) from the somatic embryo (All bars represents 40 μm)
Germination and transplantation
When the cotyledonary stage embryos were removed from the clumps and transferred to the MS basal medium, the embryos turned green and continued their development. The first sign of germination was the emergence of a single leaf at the plumular region and a root from the radicle region. Simultaneous growth of leaves and root along with the gradual elongation of the hypocotyls were noticed. The expansion and greening of cotyledons occurred within a week. Fully developed cotyledonary stage embryos developed into complete plant (Fig. 2s–u). By the end of the 2nd week, 79.5 % of embryos germinated on MS medium were supplemented with GA3 (5 mg/l) (Table 5). Only 43.2 % of somatic embryos germinated in the MS basal medium which is devoid of GA3. ABA treatment on germination of somatic embryos resulted in nil response (Table 5). It was clearly documented in the present investigation that GA3 played an important role in the maturation and germination of somatic embryos. Positive influence of GA3 on germination of somatic embryos was also observed in a related species i.e., P. peltatum (Kim et al. 2007). The germinated plants were acclimatized in perlite, peat moss, and vermiculate mixture in the ratio 1:1:1 in plant growth chamber with 38 % survivability.
PTOX production and quantification
The PTOX content was estimated by HPLC. The methanolic extracts of germinated somatic embryos from the MS medium supplemented with GA3 (5 mg/l), control (germinated somatic embryo without GA3), and germinated zygotic embryos from the Milam variety were analyzed. Table 6 depicts the PTOX levels of the germinated somatic embryos and germinated zygotic embryos. Higher PTOX content (1.8 mg/g DW) was observed in somatic embryos obtained from MS medium supplemented with GA3 (5 mg/l) when compared to germinated zygotic embryo (1.3 mg/g DW) and control (1.2 mg/g DW). Anbazhagan et al. (2008) reported that 0.08 mg/g DW PTOX content was recorded in embryogenic calli of P. peltatum, whereas PTOX content (0.36 mg/g DW) was significantly enhanced in embryogenic calli when it was treated with MeJ elicitation. In our present study, under controlled-culture conditions, higher level of PTOX biosynthesis was noticed (germinated somatic embryos). It has been reported earlier that types and concentrations of PGRs, media compositions, and culture conditions (physical and chemical) trigger the secondary metabolites production in in vitro cell/organ cultures due to its physiological and metabolic regulation in plants (Sivanandhan et al. 2011, 2012b). Sakakibara et al. (2006) postulated that plant growth regulates significantly transporters of some macronutrients such as nitrate, ammonium, sulfate, and phosphate on one hand, while nitrate on the other hand regulates the expression of genes involved in secondary metabolite biosynthetic pathway. It might be a possible reason for the enhancement of PTOX level in in vitro cultured plants. According to Pasternak et al. (2002), the somatic embryo is associated with the increase in endogenous IAA levels due to the presence of 2,4-D, suggesting that this synthetic compound acts upon and alters the secondary metabolites production. This is the first report on the PTOX production in germinated somatic embryo of P. hexandrum.
Conclusion
In the present study, the protocol for induction of somatic embryos directly from zygotic embryos followed by second cycle of embryogenesis and effective regeneration for P. hexandrum was established. Somatic embryogenesis is a promising method for the production of transgenic plants as well as multiplication of rare plant species. The present investigation imparts the substantiations that in vitro plant propagation by somatic embryogenesis can elevate the PTOX levels of the plant. The method depicted here will be useful in conserving elite germplasm of this important medicinal plant. This protocol can also be used for large-scale propagation of plants and PTOX production utilizing bioreactor design in a laboratory in the future.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- ABA:
-
Abscisic acid
- DSE:
-
Direct somatic embryo
- GA3 :
-
Gibberellic acid
- HPLC:
-
High performance liquid chromatography
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
Naphthaleneacetic acid
- PGR:
-
Plant growth regulators
- PTOX:
-
Podophyllotoxin
- RT:
-
Retention time
References
Ammirato PV (1974) The effects of abscisic acid on the development of somatic embryos from cells of caraway (Carum carvi L.). Bot Gaz 135:328–337
Anbazhagan VR, Ahn CH, Harada E, Kim YS, Choi YE (2008) Podophyllotoxin production via cell and adventitious root cultures of Podophyllum peltatum. In Vitro Cell Dev Biol Plant 44:494–501
Arumugam N, Bhojwani SS (1990) Somatic embryogenesis in tissue cultures of Podophyllum hexandrum. Can J Bot 68:487–491
Badhwar RL, Sharma BK (1963) A note on the germination of Podophyllum seeds. Indian For 89:445–447
Balzon TA, Luis ZG, Scherwinski-Pereira JE (2013) New approaches to improve the efficiency of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) from mature zygotic embryos. In Vitro Cell Dev Biol Plant 49:41–50
Bedir E, Khan I, Moraes RM (2001) Bioprospecting for podophyllotoxin. In: Janik J, Whipkey A (eds) Trends in new crops and new uses. ASHS, Alexandria, pp 545–549
Berlin GP, Miksche JP (1976) Botanical microtechnique and cytochemistry, 3rd edn. Iowa State University Press, Ames, Iowa, USA
Bhatt A, Rawal RS, Dhar U (2005) Germination improvement in Swertia angustifolia: a high value medicinal plant of Himalaya. Curr Sci 89(6):1008–1011
Brown DC, Thorpe TA (1995) Crop improvement through tissue culture. World J Microbiol Biotechnol 11:409–415
Bush EJ, Jones DW (1995) Asymmetric total synthesis of (−)-podophyllotoxin. J Chem Soc Perkin Trans 1:151–155
Chandra B, Palni LMS, Nandi SK (2006) Propagation and conservation of Picrorhiza kurrooa Royle ex Benth.: an endangered Himalayan medicinal herb of high commercial value. Biodivers Conserv 15:2325–2338
Choi YE, Kim JW, Yoon ES (1999) High frequency of plant production via somatic embryogenesis from callus or cell suspension cultures in Eleutherococcus senticosus. Ann Bot 83:309–314
Chugh A, Khurana PJ (2002) Gene expression during somatic embryogensis—recent advances. Curr Science 83:715–730
De Kroon H, Whigham DF, Watson MA (1991) Developmental ecology of mayapple: effects of rhizome severing, fertilization and timing of shoot senescence. Funct Ecol 5:360–368
Eudes F, Acharya S, Laroche A, Selinger LB, Cheng KJ (2003) A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tiss Org Cult 73:147–157
Feher A, Pasternak T, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Fehr A (2006) Why somatic plant cells start to form embryos? In Mujid A, Samaj J, eds., Somatic Embryogenesis., Plant Cell Monographs,Vol.2, Robinson DG, series ed., Springer-Verlag, Berlin Heidelberg, Germany, pp. 85–101
Fischer-Iglesias C, Sundberg B, Neuhaus G, Jones AM (2001) Auxin distribution and transport during embryonic pattern formation in wheat. Plant J 26:115–129
Fitch MM (1993) High frequency somatic embryogenesis and plant regeneration from papaya hypocotyls callus. Plant Cell Tiss Org Cult 32(2):205–212
Gaj MD (2001) Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana (L.) Heynh. Plant Cell Tiss Org Cult 64:39–46
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Kanchanapoom K, Domyoas P (1999) The origin and development of embryoids in oil palm (Elaeis guineensis Jacq.) embryo culture. Sci Asia 25:193–200
Kim SW, Oh SC, Liu JR (2003) Control of direct and indirect somatic embryogenesis by exogenous growth regulators in immature zygotic embryo cultures of rose. Plant Cell Tissue Org Cult 74(1):61–66
Kim YS, Lim S, Choi YE, Anbazhagan VR (2007) High frequency plant regeneration via somatic embryogenesis in Podophyllum peltatum L., an important medicinal plant for source of anticancer drug. Curr Sci 92:662–666
Krishnaraj S, Vasil IK (1995) Somatic embryogenesis in herbaceous monocots. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 417–471
Majumder A, Jha S (2009) Biotechnological approaches for the production of potential anticancer leads podophyllotoxin and paclitaxel: an overview. J Bio Sci 1:46–69
Merkle SA, Sotak RJ, Wiecko AT, Sommer HE (1990) Maturation and conversion of Liriodendron tulipifera somatic embryos. In Vitro Cell Dev Biol 26:1086–1093
Mikuła A, Tykarska T, Zielińska M, Kurać M, Rybczyński J (2004) Ultrastructural changes in zygotic embryos of Gentiana punctata L. during callus formation and somatic embryogenesis. Acta Biol Cracov Ser Bot 46:109–120
Moraes-Cerdeira RM, Dayan FE, Bedir E, Barrett H, Burandt C Jr, Canel C (2002) The lignans of Podophyllum. In: Rahman AU (ed) Studies in natural product chemistry, vol 26. Elsevier, New York, pp 149–182
Nadeem M, Palni LMS, Purohit AN, Pandey H, Nandi SK (2000) Propagation and conservation of Podophyllum hexandrum Royle: an important medicinal herb. Biol Conserv 92:121–129
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Orozco-Segovia A, Batis AI, Rojas-Aréchiga M, Mendoza A (2003) Seed biology of palms: a review. Palms 47:79–94
Paek KY, Chakrabarty D, Hahn EJ (2005) Application of bioreactor systems for large scale production of horticultural and medicinal plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, The Netherlands, pp 95–116
Pandey H, Nandi SK, Kumar A, Palni UT, Palni LMS (2007) Podophyllotoxin content in Podophyllum hexandrum Royle plants of known age of seed origin and grown at a lower altitude. Acta Physiol Plant 29:121–126
Pasternak T, Prinsen E, Ayaydin F, Miskolczi P, Potters G, Asard H, Van Onckelen H, Dudits D, Fehér A (2002) The role of auxin, pH and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa (Medicago sativa L.). Plant Physiol 129:1807–1819
Sadowska A, Wiweger M, Lata B, Obidoska G (1997) In vitro propagation of Podophyllum peltatum L. by the cultures of embrya and divided embrya. Biol Plant 39:331–336
Sajc L, Grubisic D, Vunjak-Novakovic G (2000) Bioreactors for plant engineering: an outlook for further research. Biochem Eng J 4:89–99
Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11(9):440–448
Samant SS, Dhar U, Palni LMS (1998) Medicinal plants of Himalaya: diversity distribution and potential values. Himvikas, Gyanodaya Prakashan, Nainital
Scherwinski-Pereira JE, Guedes RS, Silva RA, Fermino PCP, Luis ZG, Freitas EO (2012) Somatic embryogenesis and plant regeneration in açaí palm (Euterpe oleracea). Plant Cell Tiss Org Cult 109:501–508
Sivanandhan G, Mariashibu TS, Arun M, Rajesh M, Kasthurirengan S, Selvaraj N, Ganapathi A (2011) The effect of polyamines on the efficiency of multiplication and rooting of Withania somnifera (L.) Dunal and content of some withanolides in obtained plants. Acta Physiol Plant 33:2279–2288
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyaraj M, Kapil Dev G, Manickavasagam M, Selvaraj N, Ganapathi A (2012a) Optimization of elicitation conditions with methyl jasmonate and salicylic acid to improve the productivity of withanolides in the adventitious root culture of Withania somnifera (L.) Dunal. Appl Biochem Biotechnol 168:681–696
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Mariashibu TS, Manickavasagam M, Selvaraj N, Ganapathi A (2012b) Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind Crop Prod 37:124–129
Sultan P, Shawl AS, Ramteke PW, Jan A, Chisti N, Jabeen N, Shabir S (2006) In vitro propagation for mass multiplication of Podophyllum hexandrum: a high value medicinal herb. Asian J Plant Sci 5:179–184
van Uden W, Pras N, Visser JF, Malingre TM (1989) Detection and identification of podophyllotoxin produced by cell cultures derived from Podophyllum hexandrum Royle. Plant Cell Rep 8:165–168
Vasil IK (2008) A short history of plant biotechnology. Phytochem Rev 7:387–394
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Acknowledgments
The authors are thankful to the Life Science Research Board, Defence Research and Development Organisation (DRDO), Govt. of India for financial support (DLS/81/48222/LSRB–171 BTB/2008) used to carry out the present work. The corresponding author is thankful to the University Grants Commission (UGC), Govt. of India, for providing an emeritus fellowship under the BSR scheme. The first author is thankful to Prof. S.K. Nandi, G.B. Pant Institute of Himalayan Environment and Development, Almora, Uttarakhand, India, Prof. M.C. Nautiyal, H.N.B. Garhwal University, Garhwal Srinagar, Uttarakhand, India, and Dr. Lokho Puni IFS, Forest Research Institute, Dehradun, Uttaranchal for help with the collection of seeds at different locations. The first author is thankful to Dr. V. Nandhagopal, National College, Trichy, for his critical guidance in histology work. The authors are thankful to Dr. R. Boopathy, Head of the Department and Dr. S. Girija, Associate Professor, Department of Biotechnology, Bharathiar University, Coimbatore for providing HPLC facility.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Rajesh, M., Sivanandhan, G., Jeyaraj, M. et al. An efficient in vitro system for somatic embryogenesis and podophyllotoxin production in Podophyllum hexandrum Royle. Protoplasma 251, 1231–1243 (2014). https://doi.org/10.1007/s00709-014-0632-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0632-1