Abstract
The cells in animals face unique demands beyond those encountered by their unicellular eukaryotic ancestors. For example, the forces engendered by the movement of animals places stresses on membranes of a different nature than those confronting free-living cells. The integration of cells into tissues, as well as the integration of tissue function into whole animal physiology, requires specialisation of membrane domains and the formation of signalling complexes. With the evolution of mammals, the specialisation of cell types has been taken to an extreme with the advent of the non-nucleated mammalian red blood cell. These and other adaptations to animal life seem to require four proteins—spectrin, ankyrin, 4.1 and adducin—which emerged during eumetazoan evolution. Spectrin, an actin cross-linking protein, was probably the earliest of these, with ankyrin, adducin and 4.1 only appearing as tissues evolved. The interaction of spectrin with ankyrin is probably a prerequisite for the formation of tissues; only with the advent of vertebrates did 4.1 acquires the ability to bind spectrin and actin. The latter activity seems to allow the spectrin complex to regulate the cell surface accumulation of a wide variety of proteins. Functionally, the spectrin–ankyrin–4.1–adducin complex is implicated in the formation of apical and basolateral domains, in aspects of membrane trafficking, in assembly of certain signalling and cell adhesion complexes and in providing stability to otherwise mechanically fragile cell membranes. Defects in this complex are manifest in a variety of hereditary diseases, including deafness, cardiac arrhythmia, spinocerebellar ataxia, as well as hereditary haemolytic anaemias. Some of these proteins also function as tumor suppressors. The spectrin–ankyrin–4.1–adducin complex represents a remarkable system that underpins animal life; it has been adapted to many different functions at different times during animal evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Discovery of the spectrin–ankyrin–4.1–adducin system
Mammalian red blood cells have no nuclei or other internal organelles; thus, they have no major biosynthetic repair mechanisms. Despite this, they survive in circulation for about 120 days. This implies that their membranes have some adaptation that allows them to survive for this length of time—an unsupported lipid bilayer would be unable to endure the rigours of circulation.
Spectrin
Membranes can be isolated from human erythrocytes by hypotonic lysis (Dodge et al. 1963): since they retain the “ghostly” outline of the original cell, these are usually referred to as ghosts. Ghosts, however, become unstable when they are exposed to very low ionic strength, slightly alkaline solutions: for example, incubation of ghosts at 37° for 30 min in a solution of 0.1 mM sodium phosphate pH 8 results in rapid fragmentation of the membranes and the formation of inside–out vesicles (Steck et al. 1970). Among the proteins that are released from red cell membranes under these conditions are a pair of high molecular weight polypeptides named spectrin (Marchesi and Steers 1968; Tillack et al. 1970; another word for a ghost is a spectre, hence spectrin). These high molecular weight peptides are referred to now as α- and β-spectrins (280 and 246 kDa based on their sequences), although other literature named them band 1 and band 2, respectively, because they were represented in the two highest molecular mass proteins on sodium dodecyl sulphate (SDS) polyacrylamide gels (Fairbanks et al. 1971).
Analysis of the 37°C extract of red cell membranes indicated that α- and β-spectrins formed a dimer (Gratzer and Beaven 1975; Ralston 1975; Shotton et al. 1978; Ungewickell and Gratzer 1978; Ralston and Dunbar 1979). However, if the extraction was done at 4°, spectrin was recovered predominantly as a tetramer comprising two dimers. Careful analysis of the chemical equilibrium between dimers and tetramers revealed that tetramers were the most abundant physiological form of spectrin; however, at 37°C, when spectrin was diluted by being removed from the membrane, the tetramers rapidly dissociated to dimers (e.g. Ungewickell and Gratzer 1978). Higher-order oligomers could also form; thus, in whole red cells, the highly concentrated nature of spectrin attached to the membrane means that there is an equilibrium between dimers, tetramers, hexamers and so on, with the tetramer apparently most abundant (Morrow and Marchesi 1981; Shahbakhti and Gratzer 1986).
Ankyrin—a linker between spectrin and the membrane
Spectrin seems to be important to the integrity of the red cell membrane, since its loss from the membrane results in fragmentation. How is it attached to the membrane? Bennett and Branton (1977) conducted a pioneering series of experiments in which they demonstrated that, in physiological salt solutions, spectrin can bind to the cytoplasmic face of inside–out vesicles. The “spectrin receptor” was a protein since it was susceptible to proteolysis and present at approximately one per tetramer (Bennett and Stenbuck 1979a). This receptor was identified as the protein ankyrin (Bennett and Stenbuck 1979a, 1980b; Yu and Goodman 1979). Ankyrin itself could be removed from membranes with concentrated salt solutions and thus was a peripheral protein. An “ankyrin receptor” was identified as Band 3, an anion (HCO -3 /Cl-) exchanger essential in carbon dioxide transport processes (Bennett and Stenbuck 1979b, 1980a). More recently, it has become clear that ankyrin binds a number of other transmembrane proteins including the Rhesus complex which probably functions as a transporter for small neutral substrates (Nicolas et al. 2003).
4.1, adducin and the actin junctional complex
Along with spectrin, some other proteins are released from the membrane in very low salt solutions. Among these is actin (Tilney and Detmers 1975). Mature human erythrocytes contain essentially only β-actin (Pinder et al. 1978b). Does spectrin bind actin? Early data indicated that spectrin could interact with actin although the mode was unclear (Pinder et al. 1975; Tilney and Detmers 1975; Kirkpatrick 1976; Sheetz et al. 1976) and the affinity was very weak (Ohanian et al. 1984). Furthermore, spectrin could act as a bridge between cytoplasmic actin and the membrane (Cohen et al. 1978; Cohen and Foley 1980; Fowler et al. 1981). The weak affinity prompted a number of workers to investigate whether there were factors that modulated the interaction with F-actin. Among the other proteins present in the red cell membrane low ionic strength extract is protein 4.1, which appears on SDS gels as a closely spaced doublet of bands at approximately 80 kDa (Yu et al. 1973). Protein 4.1 binds to spectrin (Tyler et al. 1980) and promotes the binding of spectrin to actin (Ungewickell et al. 1979; Fowler and Taylor 1980). Ohanian and coworkers (1984) quantified the interactions: in the absence of 4.1, the spectrin–F-actin interaction was characterised by a Ka approximately 1 × 103 M-1; in its presence, a ternary complex formed with an affinity around 1012 M-2.
Two further polypeptides present in the low salt extract had apparent molecular masses around 110 kDa. These were found to represent subunits of a calmodulin-binding phosphoprotein that contained a spectrin–actin-binding activity (Palfrey and Waseem 1985; Gardner and Bennett 1986; Ling et al. 1986; Mische et al. 1987). These were named α- and β-adducin because they can “adduce” (Latin adducere: ad-, to bring to + ducere, to lead) the formation of a spectrin–actin complex (Gardner and Bennett 1987). The protein is primarily a heterotetramer, and the interaction of the two subunits is required for high-affinity spectrin/actin binding (Matsuoka et al. 2000). The calmodulin-binding activity is confined to a basic 22 amino acid sequence near the C-terminal which has close sequence similarity to the calmodulin-binding region of myristoylated alanine-rich C-kinase substrate (MARCKS; Matsuoka et al. 1996). Its protein interactions are also regulated by protein kinase C, protein kinase A and by rho-kinase (Matsuoka et al. 1996, 1998; Waseem and Palfrey 1988, 1990; Kimura et al. 1998).
Further actin-binding proteins identified in the low salt extract protein include 4.9 (also known as dematin; Beaven et al. 1985a; Siegel and Branton 1985; Husain-Chishti et al. 1989), tropomyosin (Fowler and Bennett 1984) and tropomodulin (Fowler 1990).
The triton-resistant cytoskeleton and the state of erythrocyte actin
Further evidence for the presence of complexes between spectrin, ankyrin, 4.1, F-actin and other proteins came from extraction of red cell membranes with the detergent Triton X-100. The Triton X-100 insoluble residue represents a cytoskeletal structure, which retains the core cytoskeletal proteins plus a number of transmembrane proteins (Yu et al. 1973). Fractions of, for example, the anion exchanger band 3 (Yu et al. 1973; Sheetz 1979), glycophorin C (Reid et al. 1990) and Rhesus complex (Bruce et al. 2003) are retained in the detergent cytoskeleton: these can be removed by washing with concentrated salt solutions in the presence of detergent, a treatment which also removes ankyrin. The resulting high salt cytoskeleton, or shell, retains spectrin, 4.1, adducin and proteins of the actin complex (Sheetz 1979).
Electron microscopy of cytoskeletons or shells revealed a more or less hexagonal network of long spectrin filaments (mainly, tetramers, plus hexamers and higher-order oligomers) interconnected by junction points (Byers and Branton 1985; Beaven et al. 1985b; Liu et al. 1990a; Ursitti and Fowler 1994). Ankyrin-based complexes sat near to the sites where spectrin dimers formed tetramers (Liu et al. 1987), consistent with the electron microscopy of purified spectrin–ankyrin complexes (Tyler et al. 1979). Actin as well as its other binding partners was found at junction points where spectrin molecules come together (Ursitti and Fowler 1994).
An unusual aspect of the actin junction points is that no long actin filaments were visible in intact preparations (Tilney and Detmers 1975; Byers and Branton 1985). This is not because erythrocyte actin is incapable of forming long filaments: limited proteolysis releases constraints on actin polymerisation, and long filaments are then readily formed (Tilney and Detmers 1975). Pinder and Gratzer (1983) investigated the nature of actin in red cell membranes and concluded that it was restricted to short filaments (12 to 16 monomers) that would be of the right length to accommodate a single tropomyosin. The minus-ends of the filaments are blocked by tropomodulin (Fowler 1990). Whether the plus-ends were capped depended on whether the ghosts were prepared in the presence of Mg2+: the plus-end was uncapped in the absence of Mg2+; the presence of 2 mM Mg2+ in the lysis buffer was found to preserve the actin filaments in a capped state (Kuhlman 2000). The plus-end capping was found to be attributable to adducin, which was released from the membranes in the absence of Mg2+.
Several factors probably contribute to keeping the actin filaments short in the junctional complex. The plus-end capping activity of adducin together with tropomodulin may cap the filaments in red cells (Kuhlman et al. 1996). Spectrin and 4.1 together have an F-actin severing and capping activity (Pinder et al. 1984). In addition, the ubiquitous capping protein capZ is present in red cells (Kuhlman and Fowler 1997). However, capZ appears to be displaced from the end of actin filaments by adducin, and in mature cells, it is only cytoplasmic (Kuhlman and Fowler 1997).
In summary, we now have a picture of a red cell cytoskeleton that forms a roughly hexagonal array on the cytoplasmic face of the membrane. Spectrin filaments are cross-linked by actin-containing junction points. Ankyrin forms an adaptor between spectrin and the membrane; ankyrin cross-links links with transporters that include the Rhesus complex and the anion exchanger. In addition, as will be described below, the actin junctions are also linked to the plasma membrane via transmembrane complexes. Moreover, spectrin and protein 4.1 bind PIP2 (An et al. 2005a, 2006b). Spectrin also binds to some aminophospholipids including phosphatidylserine (Haest et al. 1978; Mombers et al. 1979; Cohen et al. 1986; Michalak et al. 1993; An et al. 2004). The interaction of spectrin with aminophospholipids modulates membrane stability (Manno et al. 2002). A minority of β-spectrin is palmitoylated in red cells, and this seems to strengthen its association with the membrane (Mariani et al. 1993).
Genes and proteins
Spectrins
The sequences of human α- and β-spectrin from red blood cells were first obtained by direct protein sequencing (Speicher et al. 1983; Speicher and Marchesi 1984) and, subsequently, by cDNA cloning (Linnenbach et al. 1986; Cioe et al. 1987; Winkelmann et al. 1988, 1990a; Sahr et al. 1989, 1990;). More recently, the sequences of spectrin genes from many different organisms have been revealed by genomics.
Mammals have seven spectrin genes: two α-genes (SPTA1 and SPTAN1 encoding αI- and αII-spectrins, respectively), four “conventional” β-genes (SPTB, SPTBN1, SPTBN2 and SPTBN4 encoding βI to βIV, respectively) and SPTBN5 encoding one β-heavy (βV)-spectrin. Invertebrates have a single α-, “conventional” β- and βV-spectrin genes. During the evolution of the invertebrates, there were two rounds of whole genome duplication (the 2R hypothesis), i.e. for each ancestral gene, vertebrates could have up to four paralogues (Kasahara 2007). The expectation of four paralogues seems to be met by the conventional β-genes, but not by the α- or βV-genes, which are clearly not advantageous when retained in full complement. Moreover, the duplication of α genes that we see represented in mammalian genomes is a late evolutionary event. The genomes of amphibia, birds and reptiles only contain single α-spectrin genes, and it seems that the duplication of α occurred with the advent of the mammals since it is found in all three branches of the mammalia (Salomao et al. 2006). In the lineage leading to the bony fish, a further round of duplication occurred (Van de Peer 2004), but it does not seem to have been advantageous to retain function of all the duplicates: for example, a duplicate of the α-gene is present in zebra fish as a non-expressed pseudogene (Salomao et al. 2006).
Spectrins can be defined by their domain structure. The bulk of the polypeptide comprises successive repeating units of approximately 106 amino acids (Speicher and Marchesi 1984; Fig. 1a,c). These repeating units are now referred to as spectrin repeats: these are folded as triple helical bundles (Yan et al. 1993; Pascual et al. 1996). Typically, α-spectrin contains about 20 complete repeats, conventional β16 and β-heavy about 29. In spectrin chains, successive triple helices connect through the junction of helix C of one repeat to helix A of the next in an uninterrupted helix (see Fig. 1; e.g. Kusunoki et al. 2004). In addition, as explained more fully below, partial triple helices define the sites where spectrin dimers self-associate.
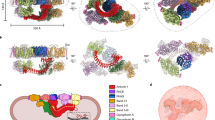
The structure of spectrin. a A spectrin dimer. Spectrin α- and β-chains associate side-by-side and antiparallel. Sites of interaction between the chains are indicated with a grey box. Some examples of protein and lipid interaction sites are also annotated. b The actin-binding domain of β-spectrin. A model is shown generated by Phyre of the CH1 and CH2 domains. Sequences equivalent to known actin-binding sites in utrophin are shown in yellow. Note that this structure also binds 4.1 and PIP2. c The structure of two spectrin triple helical repeats. The structure shows repeats 8–9 of βI-spectrin. C1167, in the linker region between the two tandem triple helices, is indicated: this residue becomes available to chemical modification when red cell membranes are subject to shear stress. From the PDB file 1S35. d The structure of the interactive domains in spectrin and ankyrin. The binding site in β-spectrin for ankyrin is located in repeats 14–15. The region in ankyrin that binds spectrin is the ZU5 domain. Two residues which when mutated give rise to hereditary pyropoikilocytosis are indicated: these destabilise spectrin repeats adjacent to the binding site. From the PDB file 3KBU. e The Pleckstrin homology domain. The domain is shown in cartoon representation with IP3 bound. From the PDB file 1BTN. f The structure of the site where spectrin tetramers form. To form a spectrin tetramer by the interaction of two dimers, a single helix from α interacts with two helices from β to recapitulate a full triple helix. Two residues which when mutated disrupt the formation of tetramers are indicated: mutations at these sites can result in elliptocytosis. From the PDB file 3LBX
At the N-terminus of β, there is a pair of calponin homology (CH) domains which together form an actin-binding domain (ABD; Karinch et al. 1990; Carugo et al. 1997; Banuelos et al. 1998). The ABD also binds 4.1, and the formation of a three-way complex between 4.1, spectrin and actin effectively strengthens the interaction of spectrin with actin (An et al. 2005a). The ABD also binds the lipid PIP2, and this promotes 4.1 binding (An et al. 2005a; Fig. 1b).
In α-spectrins, there is an SH3 domain inserted between repeats 9 and 10 (Musacchio et al. 1992). At the C-terminus is a calmodulin-like domain which contains four EF hands, of which two bind calcium (Trave et al. 1995). These have recently been reported to bind erythrocyte protein 4.2 (Korsgren et al. 2009).
To form a dimer, α- and β-spectrin chains interact via two repeats close to the C-terminus of α (repeats 20 and 21) and the N-terminus of β (repeats 1 and 2; see Fig. 1 and Ursitti et al. 1996; Viel et al. 1998). This forms an anti-parallel dimer, with the calmodulin-like domain opposed to the ABD.
To form a tetramer, at the other end of the dimer, the partial repeats in both α (repeat 1) and β (repeat 17) can interact with the corresponding partial repeats in another dimer to form a tetramer (Tse et al. 1990; Kotula et al. 1993; Cherry et al. 1999; Ipsaro et al. 2010). Thus, the tetramer is formed by “reconstituting” a full triple helix from the pair of partial triple helices (Fig. 1f).
Repeats 14 and 15 of the conventional β-spectrins bind ankyrin (Kennedy et al. 1991; Davis et al. 2008; Ipsaro et al. 2009; Stabach et al. 2009). Because there are two β-spectrins in each tetramer, each tetramer potentially binds two ankyrins: there is evidence of cooperativity between ankyrins in binding to spectrin (Cianci et al. 1988; Fig. 1d).
In α, the region between repeats 9 and 11 (which includes the SH3 domain) contains a plethora of binding sites. These include sites for binding the lipid phosphatidylserine (An et al. 2005b). The proteins that interact here include the non-receptor tyrosine kinase src (Nicolas et al. 2002; Nedrelow et al. 2003), a low molecular weight tyrosine phosphatase (Nicolas et al. 2002), tes and evl (Rotter et al. 2005; Bournier et al. 2006) and e3b1 (Ziemnicka-Kotula et al. 1998). These interactions of the SH3 domain may be linked to the control of cell motility (Merilainen et al. 1993). In addition, a differentially spliced sequence binds calmodulin in αII-spectrin (Simonovic et al. 2006).
The C-terminal region of β-spectrins can be subject to differential messenger ribonucleic acid (mRNA) splicing, which regulates both membrane binding and regulatory properties (Winkelmann et al. 1990b; Hayes et al. 2000). The ancestral β-spectrin common to all eumetazoans contained a C-terminal pleckstrin homology domain which binds to PIP2 via its head group IP3 (Macias et al. 1994; Zhang et al. 1995; Fig. 1e). This domain, additionally, seems to have a role in sorting spectrins in polarised cells (Das et al. 2008). In mammalian βI- and βII-spectrins, splicing can eliminate the PH domain and replace it with a short, relatively unstructured region containing phosphorylation sites (Winkelmann et al. 1990b; Hayes et al. 2000; Tang and Speicher 2004; Bignone et al. 2007). These short C-termini seem to have arisen independently after the duplication events encompassed in the 2R hypothesis, and certainly, the sequences, gene structures and phosphorylation sites in these regions are quite different from each other (Bignone et al. 2007). The function of phosphorylation of the short βI-spectrin C-terminal region is not known; it does not appear to regulate the formation of tetramers (Ungewickell and Gratzer 1978; Shahbakhti and Gratzer 1986), but it seems to be linked to the mechanical properties of red cell membranes (Manno et al. 1995). On the other hand, phosphorylation of the short βII-spectrin has a functional correlate, namely, regulation of the interaction of formation of spectrin tetramers (Bignone et al. 2007). Phosphorylation of the short C-terminal region by protein kinase A leads to a reduction in the affinity of αII and βII at the site where tetramers form. This phosphorylation appears to occur during neurite outgrowth.
Although there are multiple vertebrate spectrin polypeptides, detailed biochemical and biophysical comparisons between them remain incomplete. The best characterised polypeptides in those terms are the two mammalian α-spectrins (αI- and αII-spectrins) and βI- and βII-spectrins.
When spectrin was isolated from red cells at 37°C, it was recovered as a dimer (Ungewickell and Gratzer 1978). On the other hand, when spectrin was isolated from the brain, primarily, it was a tetramer (Bennett et al. 1982a; Glenney et al. 1982d; Davis and Bennett 1983). This is because the principal constituents of each (αI–βI from red cell; αII–βII from brain) vary in their relative affinity at the site were tetramers form. Experiments with fragments of spectrin containing the binding sites indicated that as monomers, αI and βI associate with K D approximately 800 nM (Kotula et al. 1993; Nicolas et al. 1998; Cherry et al. 1999; Bignone and Baines 2003). By comparison, equivalent fragments of αII- and βII-spectrins associated with K D approximately 10 nM (Bignone and Baines 2003). The affinity is primarily dictated by the partial repeats (one helix from α and two from β) that directly associate to recapitulate a full triple helix. In addition, experiments with chimeras revealed that the adjacent full triple helical repeat affected affinity as well (Bignone and Baines 2003).
The relatively low affinity of the red cell spectrin tetramer raises the question of whether or not spectrin tetramers—- even though they are highly concentrated on red cell membranes—- dissociate during circulation. Mohandas and co-workers (An et al. 2002) have investigated this by sealing into ghosts fragments of spectrin that contain the partial repeats. They found that at 37°, the partial repeats became incorporated into the membrane, i.e. they associated at the ends of spectrin dimers. As a result, the fragments acted as dominant negative inhibitors of the formation of tetramers. Furthermore, these fragments acted to destabilise the red cell membrane, and their affinity was reflected in their potency in this respect. Fragments of αI-spectrin were two orders of magnitude less potent in terms of destabilising red cell membranes against shear stress than fragments of αII-spectrin (Salomao et al. 2006).
In evolution, αI-spectrin arose by duplication of the pre-existing α-spectrin gene at about the same time as non-nucleated mammalian red cells appeared (Salomao et al. 2006). Evidently, it has been neo-functionalised to support the rapid making and breaking of spectrin tetramers in non-nucleated red cells undergoing shearing forces in circulation.
The biophysical properties of αI- and αII-spectrins vary. Experiments to measure the thermal denaturation of fragments of spectrin polypeptides revealed that, in general, the triple helical repeats in αII-spectrin denature at a higher temperatures than those of αI-spectrin (An et al. 2006a, c). In particular, in αI and βI, some repeats in the centre of the molecule are probably on the edge of being unfolded even at 37°. αI repeats 4, 6, 8, 11 and 12 all had mid-points of thermal denaturation below 37°. Experiments on a construct of βI-spectrin repeats 5–9 showed substantial loss of α-helicity even below 37°. Even more strikingly, in this construct, two repeats lost resistance to pulling in the atomic force microscope at or below 33°, indicating that, physiologically, they are probably in a relatively unfolded state.
To test the state of these repeats in whole membranes, Johnson et al. (2007b) investigated the accessibility of cysteine residues. In the linker region between βI-spectrin repeats 8 and 9, cysteine 1167 is occluded in the folded structure (PDB: 1 S35; Fig. 1c). When red cell membranes were subjected to a shear stress, this cysteine became available to a chemical reagent. In all, at least six cysteines became available for chemical modification upon shearing the cell membranes. The implication here is that spectrin responds to the shearing forces encountered in circulation by at least partially unfolding and acting to some extent as a spring. In this sense, spectrin has the potential to act as a sensor for mechanical forces acting on a cell membrane. Mutations that affect folding also have an effect on red cell stability. The mutation Q471P in αI-spectrin is in the linker between repeats 4 and 5. The effect of this mutation is to destabilise the folding of spectrin; this manifests itself in the hereditary haemolytic anaemia elleptocytosis (Johnson et al. 2007a).
Spectrin polypeptides can also form “mixed” tetramers. This was indicated first by the observation that chicken epithelial spectrin has a common α-subunit, but two β-subunits (i.e. basolateral βII and apical TW-260/βV; Glenney et al. 1982a). In vitro, the subunits of native spectrin tetramers can readily be separated by the use of moderate concentrations of urea, followed by hydroxyapatite chromatography (Yoshino and Marchesi 1984). After separation, they could reform tetramers once returned to physiological salt solutions. Davis and Bennett (1983) showed that the separated subunits of spectrin purified from brain (i.e. mostly αII- and βII-spectrin) could form hybrid tetramers with erythrocyte αI- and βI-spectrins. In many tissues, multiple-spectrin genes are expressed: can they form mixed tetramers? Clark et al. (1995) showed by immunoprecipitation that spectrin from cerebellum could contain hybrid tetramers in which two different spectrin β-subunits could co-exist.
Ankyrins
Vertebrates have three ankyrin genes (ANK1-3) encoding ankyrin-R, ankyrin-B and ankyrin-G, respectively. Ankyrin-R is the mammalian erythrocyte ankyrin (Lux et al. 1990; Otto et al. 1991; Peters et al. 1995; Kordeli et al. 1995). Invertebrates generally have a single ankyrin, but in the lineage from which arthropods descend, a gene duplication occurred, thus they have two ankyrins (Hortsch et al. 2002; Hopitzan et al. 2006).
Ankyrins are subject to regulation by extensive differential mRNA splicing, but their canonical forms (about 200–250 kDa) have a common domain structure (Fig. 2). An N-terminal region contains 24 ank repeats, short helix–loop–helix structures which form ligand-binding sites (Michaely et al. 2002; Fig. 2a and b). Ank repeats are widespread in evolution, and very commonly, groups of 2–4 sequential ank repeats can bind ligands with high affinity (Li et al. 2006; Stumpp and Amstutz 2007; Lowe et al. 2008). Because canonical ankyrins contain 24 repeats, they have the potential to cross-link more than one membrane protein: as an example of this, in heart, ankyrin-B forms a complex with the Na/Ca exchanger (NCX1), IP3 receptor and the Na/K-ATPase (Mohler et al. 2003).
Ankyrin structure. a The domain structure of ankyrin. The domains common to ankyrins include ank repeats, ZU5 and death domains. The ank repeats bind membrane proteins (some examples—the Rh proteins, clathrin, L1/neurofascin—are indicated). The 24 tandem repeats can be divided into four domains (D1–D4), which have distinctive activities. The ZU5 domain binds spectrin (see Fig. 1). The death domain binds fas. In some muscle forms of the protein, a domain that binds obscurin and titin (OTBD) can be present. Many further splice variants of ankyrin occur. For example, the ank repeats can be lost by mRNA splicing; a large insert between the ZU5 and death domains is present in giant forms. b The structure of ank repeats. Each repeat is a helix–loop–helix structure. From the PDB file 1N11. c The ZU5 domain. From the PDB file 3F59. d The death domain. Residue R1423 is required for the interactive properties of this domain. From the PDB file 2YVI
More centrally, a ZU5 domain binds spectrin (Ipsaro et al. 2009, 2010; Fig. 2c). This domain was originally defined as common to ZO-1 and Unc5-like netrin receptors (Interpro: IPR000906). It is about 150 amino acids in length and is folded with a core containing two β-sheets; two short helices abut one face of the sheets, the whole structure being connected by a series of solvent-exposed loops (Ipsaro et al. 2009). A death domain, a bundle of six α-helices, which forms another site for protein–protein interactions, is found more towards the C-terminus (Mohler et al. 2002; Del Rio et al. 2004; Fig. 2d).
Beyond these common elements of structure in the canonical forms, there is tremendous sequence variation via differential mRNA splicing. For example, some forms lack the ank repeats in part or in whole (e.g. Peters et al. 1995; Gagelin et al. 2002; Hopitzan et al. 2005). In the case of some small isoforms found in muscle saroplasmic reticulum (ank1.5, 17.5 kDa), a short N-terminal transmembrane segment anchors it to the membrane, but it lacks all the canonical domains (Bagnato et al. 2003; Kontrogianni-Konstantopoulos et al. 2003; Porter et al. 2005). However, some muscle ankyrins contain a 76 amino acid domain that binds obscurin and (possibly) titin (OTBD; Hopitzan et al. 2006; Borzok et al. 2007). In giant ankyrins, differentially spliced inserts raise the size to over 400 kDa: in the case of the giant ankyrin-G found at nodes of Ranvier, the insert is thought to be extended, potentially allowing this polypeptide to communicate between the plasma membrane and deep into the interior of the axon (Kordeli et al. 1995).
Protein 4.1
Protein 4.1, like spectrin and ankyrin, appeared early in animal evolution; single copies characterise all known invertebrate eumetazoan genomes (Hoover and Bryant 2000). As with spectrin and ankyrin, vertebrates have more copies of 4.1 genes. All four have been retained: the gene EPB41 encodes 4.1R; EPB41L1 encodes 4.1N; EPB41L2 encodes 4.1G; EPB41L3 encodes 4.1B (Conboy et al. 1986; Parra et al. 1998, 2000; Tran et al. 1999; Walensky et al. 1999). Protein 4.1R is so named because it is the abundant red cell 4.1, although it is expressed in many other cell types. The other three are essentially absent from red cells, but expressed widely in other cell types (Taylor-Harris et al. 2005a).
The four vertebrate proteins have a common domain structure (Fig. 3). Two of these domains—the 4.1 protein, ezrin, radixin and moesin (FERM) domain (Chishti et al. 1998) and the C-terminal domain (CTD; Scott et al. 2001)—can bind transmembrane proteins, and one—the spectrin–actin-binding (SAB) domain (Correas et al. 1986a, b; Discher et al. 1993, 1995)—provides the cytoskeletal linkage. Protein 4.1N is an exception to the last of these: its SAB is so divergent that it does not bind spectrin–actin (Gimm et al. 2002). Adjacent to the FERM domain is a FERM-adjacent (FA) domain (Baines 2006).
The general domain structure of protein 4.1. a The distinctive domains of protein 4.1 are a FERM domain that binds certain membrane proteins, calmodulin and PIP2; a FERM adjacent (FA) domain which, in 4.1R, is a substrate for phosphorylation by protein kinase A and protein kinase C; a spectrin actin-binding (SAB) domain; and a C-terminal domain (CTD) which binds certain membrane proteins. Between the major structural domains are regions relatively conserved between 4.1 proteins (U1–U3). U1, also known as the headpiece, also binds Ca2+/calmodulin. b The structure of the FERM domain. A surface rendering is shown. Sites associated with the interactions of AE1 (the anion exchanger band 3), glycophorin C (GpC), p55 and PIP2 are indicated. From the PDB file 1GG3
The FERM domain is so named because it is common to 4.1 (four-point-one), ezrin, moesin and radixin (Chishti et al. 1998). The fold consists of three globular units of approximately 100 amino acids each arranged as a cloverleaf-like structure (Han et al. 2000a). The N-terminal lobe (lobe A) has a ubiquitin-like fold, the central lobe (lobe B) has an α-helical fold like acyl-CoA-binding protein, and the C-terminal lobe (lobe C) has a fold like a pleckstrin homology domain (Han et al. 2000b). Since the determination of the structures of prototypical FERM domains, it has become apparent that FERM domains are widespread in evolution, and can be very divergent in sequence, while retaining the same fold (Tepass 2009).
Interspersed between the common domains are unconserved regions that show far less sequence identity between the individual proteins. These regions, U1, U2 and U3, are generally not well understood in terms of their functions. In 4.1R, the U1 region binds calmodulin (Leclerc and Vetter 1998) and the centrosomal P4.1-associated protein (CPAP; Hung et al. 2000). This region has also been reported to modulate nuclear import of 4.1R (Gascard et al. 1998, 1999; Luque and Correas 2000), suggesting regulation of protein 4.1 properties and function through intramolecular interactions between functional domains. Protein kinase C-dependent phosphorylation of a Ser residue within the FA region has been established to modulate interactions of adjacent FERM and SAB domains with selected binding partners (Manno et al. 2005). The U2 region has also been recently reported to confer upon 4.1B its anti-proliferative properties (Robb et al. 2005). As yet, no function has been assigned to the U3 region.
In the case of 4.1R, the major 80 kDa erythrocyte form appears as two closely spaced bands on SDS gels, known as a and b forms. They are related by deamidation of two asparagine residues, a process that occurs progressively as red cells age (Inaba et al. 1992). In this sense, the 4.1Ra: 4.1Rb ratio is a marker for red cell age (Inaba et al. 1992).
One of the common aspects of the 4.1 proteins is that they are subject to extensive tissue- and development-specific mRNA splicing. This is best established for 4.1R, where of the >20 exons of the gene, more than half are subject to splicing (Conboy 1999). This regulates cytoskeletal interaction and intracellular targeting (Conboy 1999; Gascard et al. 1999; Luque et al. 1999; Luque and Correas 2000). In the kidney (Ramez et al. 2003; Gascard et al. 2004) and heart (Taylor-Harris et al. 2005b), all the 4.1 proteins are subject to splice regulation, and this now appears to be common to most, if not all, tissues (Parra et al. 2004).
The FERM domain represents a site where numerous regulatory pathways converge. It has binding sites for calmodulin (Nunomura and Takakuwa 2006), phosphatidylserine (An et al. 2001) and PIP2 (An et al. 2006b); phosphorylation affects its activities (Manno et al. 2005), as noted above; and the U1 region, which is alternatively spliced, regulates its activities (Nunomura et al. 2009). As we shall see below, the FERM domain contains distinct binding sites for several membrane proteins, and these are differentially regulated by these factors. The FERM domain itself can also be spliced: the N-terminal lobe can be lost if initiation of translation is begun in exon 8 (Gascard et al. 1998; Luque et al. 1998). The 4.1 FERM domain therefore represents a most unusual nexus of regulation in response to signals regulating gene expression, signalling through protein kinases and Ca2+ and via the availability of phosphorylated lipids.
Adducin
Vertebrates have three adducin genes (ADD1–3, encoding α, β and γ polypeptides, respectively; in bony fish, some duplicates of these genes exist; Joshi et al. 1991; Lin et al. 1995; Tisminetzky et al. 1995; Katagiri et al. 1996; Citterio et al. 1999); invertebrates have just one adducin gene (e.g. the hu-li tai shao gene in Drosophila melanogaster; Yue and Spradling 1992).
Vertebrate adducin is a heterotetramer of α–β or α–γ polypeptides (Matsuoka et al. 2000). Each polypeptide comprises three regions: an N-terminal globular head region that is resistant to limited proteolysis and has an aldolase-like fold (aldolase-II superfamily), a short interconnecting (neck) region; and a protease-sensitive tail which contains a MARKS-like domain that binds calmodulin and can be phosphorylated by protein kinase C (Fig. 4).
Adducin structure. The domain structure of an adducin polypeptide is indicated. Adducins contain a domain homologous to the aldolase-II family. At the C-terminus is a short basic region that contains protein kinase substrate sites that is related to the membrane and calmodulin-binding site in MARCKS: this region is required for interaction with spectrin and actin; it also binds Ca2+/calmodulin. Regions showing high sequence similarity between adducin polypeptides are indicated in grey boxes. The central one of these forms oligomers (primarily tetramers). The central region of the protein also contains phosphorylation sites for the kinase ROCK which regulates the interaction with spectrin
The tail domain seems to provide multiple protein–protein interactive sites including those for spectrin and actin. However, it appears to require dimerisation/oligomerisation to be functional: this is driven by the neck domain (Matsuoka et al. 2000). Calmodulin and protein kinase C are negative regulators of adducin interactions with spectrin and actin (Matsuoka et al. 1996, 1998).
Adducin is an F-actin plus-end capping protein (Kuhlman et al. 1996), and it promotes the binding of spectrin to actin (Gardner and Bennett 1987; Li et al. 1998). The regulation of adducin’s plus-end capping activity may be of relevance in motile cells and tissue development, since Ca2+ signals would be predicted to expose the plus-ends of actin filaments. Adducin associates with the sides and plus-ends of actin filaments, both alone and with enhanced affinity in the presence of spectrin (Li et al. 1998). Thus, a major mode of association of adducin and spectrin is in a ternary complex at the plus-ends of actin filaments.
In D. melanogaster, hu-li tai shao (hts) mutations give rise to sterile females (Yue and Spradling 1992; Whittaker et al. 1999; Petrella et al. 2007). In oogenesis, mitotic germ cells contain a specialised organelle, the fusome. Post-mitotic cells lose the fusome as F-actin-rich ring canals form. Hts mutants affect both the fusome and ring canals. Surprisingly, the fly adducin encodes a polyprotein (Ovhts) which is cleaved to yield products that associate with the ring canal late in the oogenesis process (Ov-hts-RC) or the fusome (Ovhts-fus) earlier. Hts mutations result do not accumulate F-actin, and ring canal development is arrested.
The nature of red cell membrane–cytoskeleton complexes
Ankyrin-based complex
An interaction between ankyrin and the red cell membrane was first discovered by Bennett and Stenbuck (1979b): they found that ankyrin co-immunoprecipitated from red cell membrane extracts with the anion exchanger. Bruce and coworkers (2003) analysed the nature of the ankyrin-based complex using immunoprecipitation and also compared the protein complexes in human disease conditions characterised by mutations in one or other of the genes concerned. They concluded that ankyrin coordinates the formation of a complex that they termed a metabolon for carbon dioxide transport (see Fig. 5a, b). This metabolon is hypothesised to contain transporters both for carbon dioxide and the product of the carbonic anhydrase reaction, HCO -3 , as well as carbonic anhydrase itself. Ankyrin binds to the large N-terminal cytoplasmic domain of the anion exchanger (Bennett and Stenbuck 1980a, b). Nicolas et al. (2003) suggest it binds directly to the Rhesus complex as well, but Satchwell et al. (2009) consider that the interaction is indirect via protein 4.2 and CD47 (see below).
Major erythrocyte membrane–cytoskeletal complexes. a The spectrin–actin complex and linkages to membrane macrocomplexes. Spectrin tetramers are indicated, bound at their ends to short actin filaments. The actin filaments are long enough to bind a tropomyosin and are capped at the minus-end by tropomodulin. Proteins 4.1 and adducin regulate the interaction of spectrin with actin. For simplicity in this diagram, most of the 4.1 proteins are omitted from the right-hand junctional complex. Ankyrin links to β-spectrin and joins to membrane macrocomplexes. b The ankyrin-based complex: a hypothetical CO2 metabolon (Bruce et al. 2003). Ankyrin and protein 4.2 bridge between the anion exchanger band 3 (AE1) and the rhesus proteins. Although still controversial, as indicated in the text, one view is that the rhesus complex can act as a CO2 channel. Aquaporin is the water channel and potentially a CO2 channel as well. Together, these provide the substrates for carbonic anhydrase, an isoform of which is bound to the C-terminal region of band 3. The product of the carbonic anhydrase reaction is HCO -3 , which can be exchanged out of the cell for Cl-. c The junctional complex. The spectrin–actin junction is linked to membrane proteins via 4.1, adducin and dematin. Several 4.1 proteins are bound to each junction point; each is capable of interacting with various membrane proteins. Adducin can bind band 3 (AE1); it also forms a complex with dematin that binds to the glucose transporter glut1. Note also the overlap in the proteins of the junctional complex and of the ankyrin-based complex
Linkage to ankyrin is required for the efficient sorting of these membrane proteins during maturation of red cells: at enucleation, in the absence of ankyrin-attachment, the anion exchanger and Rh complex are missorted to the nuclear remnant (Salomao et al. 2010). It has been suggested that the Rhesus complex is a transporter for small neutral molecules. In the kidney, it appears that the Rhesus complex has a major role in ammonia transport, and in erythrocytes, it transports ammonia and methylamine (Kustu and Inwood 2006; Ripoche et al. 2006). Endeward and co-workers (2008) suggest that Rhesus-null individuals have a reduction of about 50% in carbon dioxide transport capacity, indicating that carbon dioxide is a substrate for transport by the Rhesus complex. On the other hand, Missner et al. (2008) and Ripoche et al. (2006) consider it highly unlikely that the Rh complex (or indeed any other channel) act as physiological CO2 transporters.
The anion exchanger binds carbonic anhydrase II in its C-terminal region (Vince and Reithmeier 1998; Adachi et al. 2009). Thus, carbon dioxide entering the cell, potentially via the Rhesus complex, would be close to carbonic anhydrase, the product of this reaction being immediately available for transport out of the cell in exchange for Cl-. (This description refers to red cells passing through actively metabolising tissues; the reverse process would apply in lung tissue).
In addition to the transporters, other transmembrane proteins are present. Glycophorin A is type 1 transmembrane protein, which has a chaperone-like activity for band 3 during trafficking through the secretory pathway and remains in complex with it in the mature red cell (e.g. Hassoun et al. 1998; Williamson and Toye 2008). CD47, a marker-of-self that is suggested to prevent clearance by macrophages in circulation, is also present in the complex (Bruce et al. 2002, 2003; Dahl et al. 2004; Satchwell et al. 2009). Protein 4.2, another peripheral membrane protein, can bind to the cytoplasmic domain of the anion exchanger as well as CD 47 (Satchwell et al. 2009).
The other substrate for carbonic anhydrase is water. Aquaporin is present in the red cell membrane (Preston and Agre 1991; Preston et al. 1992). It is suggested that in addition to water transport, it provides carbon dioxide transport capacity that is not accounted for by the Rhesus complex (Nakhoul et al. 1998; Prasad et al. 1998; Blank and Ehmke 2003; Endeward et al. 2006), although as with the Rh complex, this has been disputed (e.g. Ripoche et al. 2006; Missner et al. 2008). Whether or not CO2 is appreciably transported via AQP1 in erythrocytes remains a topic characterised by opposing views.
Complexes between the actin-based junction and the membrane
A large number of proteins are currently suggested to be associated with the junctional complex; some of these are indicated schematically in Fig. 5c. Protein 4.1-deficient human red cells were noted two decades ago to be lacking in glycophorin C, indicating a requirement for protein 4.1R in stable membrane accumulation of this type 1 protein (Reid et al. 1990; Gascard and Cohen 1994). Further analysis of corresponding knockout mice revealed reductions in additional proteins including the Rhesus polypeptide Rh, Plasmodium falciparum receptor Duffy and putative transporter XK (Salomao et al. 2008). Although band 3 was not lost from these membranes, an alteration to its conformation was suggested by the observation that cell surface exposure of band 3 antigens was altered (Salomao et al. 2008). Protein 4.1 binds to the cytoplasmic N-terminal domain of band 3 (Pasternack et al. 1985; Jons and Drenckhahn 1992). Since there are multiple (approximately 6) 4.1 proteins per junctional complex (Pinder and Gratzer 1983), the junctional complex has the capacity to cross-link a large number of membrane proteins.
The activities of 4.1R in binding membrane proteins are differentially regulated by the presence or absence of the differentially spliced U1 headpiece and by PIP2 binding. This has been analysed carefully for interaction with band 3 and glycophorin C: these bind to lobes A and B of the FERM domain, respectively (Han et al. 2000a). In general, each signal tends to have opposite effects on FERM domain interaction with the two membrane proteins. Thus, PIP2 promotes binding to glycophorin C, but inhibits binding to band 3 (An et al. 2006b). U1 promotes binding to band 3, but inhibits glycophorin C binding (Nunomura et al. 2009). The U1 headpiece contains a Ca2+/calmodulin-binding site (Leclerc and Vetter 1998; Nunomura et al. 2009); in the presence of Ca2+/calmodulin, neither band 3 nor glycophorin C bind to 4.1R containing the headpiece. In mature circulating red cells, PIP2 is present on the cytoplasmic leaflet of the membrane, and little of the isoforms of 4.1R that contain the headpiece are retained: the net effect is to promote glycophorin C interaction with 4.1R and to inhibit binding to band 3. But in erythropoiesis, the U1-containing isoform of 4.1R is more abundant; thus, it might be suggested that the band 3 interaction is more important during this stage (Chasis et al. 1996; Nunomura et al. 2009). This is supported by experiments in zebra fish which demonstrated a requirement for the 4.1R binding site in band 3 for mitosis during erythropoiesis (Paw et al. 2003).
The haematological phenotypes of mice deficient in either dematin or adducin are surprisingly mild (Gilligan et al. 1999; Muro et al. 2000; Robledo et al. 2008). Partly, this may be due to compensating up-regulation of γ-adducin in the case of β-adducin knockout, although α-adducin knockouts lack all adducin in erythrocytes. But crossing the two strains gave a strong spherocytosis phenotype. Chen and co-workers (2007) and Khan et al. (2008) have identified an interaction of dematin and adducin together with the glucose transporter Glut1 in humans. Mouse membranes have a much lower level of Glut1 than human, and it is suggested that in mouse erythrocytes, adducin and dematin may bind to other solute transporters.
A further linkage between the junctional complex and the membrane has been revealed by Low and co-workers (Anong et al. 2009). They have identified binding between the anion exchanger band 3 and adducin. Moreover, protein 4.2, which binds to band 3, has recently been found to bind the C-terminal region of α-spectrin in the EF-hand region (Korsgren et al. 2009). This part of spectrin is close to the junctional complex and forms yet another potential linkage to the membrane. Significantly, mice with a spectrin mutation that disrupts this interaction (strain sph(1J)/sph(1J) have very fragile red cells (Korsgren et al. 2009).
In all, it appears that the actin-based junctional complex coordinates membrane linkages to a large range of membrane proteins. Some of these appear to have roles in red cell stability -—for example, rupture of the adducin-band 3 linkage is reported to weaken red cell membranes (Anong et al. 2009) and the combined loss of adducin and dematin alters cell shape and membrane stability (Khan et al. 2008). On the other hand, only the domain of protein 4.1 that binds to spectrin and actin is required for red cell membrane stability (Discher et al. 1993); thus, it seems likely that the function of 4.1-transmembrane protein complexes is to ensure that transmembrane proteins are trapped at the cell surface after their synthesis, thus ensuring the cell surface display of antigens.
Physiological consequences of mutations in red cell membrane proteins
Two broad classes of phenotypes arise from mutations in membrane–cytoskeletal proteins (Tse and Lux 1999; An and Mohandas 2008). One of these classes causes the cells to lose surface area and so become progressively smaller and relatively spherical. These give rise to the condition hereditary spherocytosis (Eber and Lux 2004). The second class of mutations gives rise to shape changes, and in particular, they reduce the ability of the cell to reform a round biconcave disc after passage through narrow capillaries. These mutations are characteristic of the condition hereditary elliptocytosis (Gallagher 2004).
Mutations of the former class are typically associated with loss of interaction with the ankyrin-based complex (Eber and Lux 2004). They include mutations close to the ankyrin binding site in spectrin that destabilise the folding structure in that region, presumably weakening the interaction when the cells are subjected to shearing forces in circulation (although it should be pointed out that in vitro purified fragments of spectrin that contain these mutations interact with ankyrin with nearly normal affinity). Mutations in ankyrin itself are common in human spherocytosis, as are mutations in transmembrane proteins of the ankyrin-based complex, e.g. band 3, or loss of Rhesus proteins (the Rhesus-null phenotype), or protein 4.2. These are sometimes described as the “vertical” interactions in red cell membranes.
Elliptocytogenic mutations are associated with “horizontal” interactions, i.e. in the formation of the network that lines the membrane (Gallagher 2004). Typically, these are associated with the formation of spectrin tetramers, or in the spectrin–actin-binding activity or abundance of protein 4.1. In the most severe cases of elliptocytosis, membrane fragmentation can occur; these aggravated cases are sometimes described as hereditary pyropoikilocytosis because the cells become unstable in the warm.
Alterations in the red cell membrane cytoskeleton in malaria
Malaria remains one of the most deadly human diseases. The malaria parasite P. falciparum is widely regarded as one of the strongest forces for evolution of the human genome since the emergence of our species. Since the parasite spends part of its life cycle within the red blood cell, is the erythrocyte cytoskeleton involved in this process?
A key element of the intra-erythrocytic stage of the parasite’s life cycle is that the infected cells are sequestered within the vascular system, thus avoiding the spleen where they would be more likely to be removed from circulation. Recent data indicates that several proteins exported from P. falciparum can bind to the membrane cytoskeleton and by altering its properties contribute to pathogenesis. The parasite has evolved mechanisms that make the red blood cells sticky (i.e. they have increased adhesiveness to vascular walls) and they become more rigid and less deformable. Conceptually, the latter could be associated with alterations to the spectrin system.
A protein that has been associated with alteration to the erythrocyte’s cytoskeleton apparatus is the ring-infected erythrocyte surface antigen (RESA). RESA is exported from the parasite and binds to repeat 16 of β-spectrin (Pei et al. 2007a). This is directly adjacent to the site where β-spectrin interacts with α to form tetramers. An active fragment of RESA stabilises spectrin tetramers in vitro and also in situ in whole red cell membranes. This is accompanied by increased rigidity of the cells and also resistance to thermal degradation. Resealed red cell ghosts containing the RESA fragment within them also display resistance to further invasion by P. falciparum merozoites; thus, RESA confers two advantages on the parasite: first in stiffening the cells so that they are retained within narrow blood vessels, and second in preventing further invasion of the infected cell by other parasites, as well as possibly protecting the cells against the high temperatures encountered during febrile crises.
Later in the parasite’s development, the red blood cell membrane becomes weakened to enable the escape of the parasites. A protein potentially implicated in this is P. falciparum erythrocyte membrane protein 3 (PfEMP3). PfEMP3 binds to the C-terminal region of α-spectrin, a site close to the point where spectrin attaches to actin and protein 4.1R (Pei et al. 2007b). An active peptide of PfEMP3 reduces the formation of the spectrin–actin–4.1R ternary complex in vitro. Erythrocyte ghosts resealed with an active fragment of PfEMP3 within reveal an extensive reduction in shear resistance. Since PfEMP3 is expressed in the later stages of the parasite development, it might be hypothesised that it contributes to the release of the parasite.
Many other parasite proteins probably interact with the membrane skeleton. Among them, Pf332 binds actin (Waller et al. 2010) and mature parasite-infected erythrocyte surface antigen (MESA) binds protein 4.1R (Waller et al. 2003). The challenge for the future is to understand the full repertoire of parasite proteins that interact with the red cell cytoskeleton and how their expression relates to alterations in the key parameters that allow effective invasion of cells (presumably the cytoskeleton has to be penetrated), parasitised cells to escape immune surveillance and the release of parasites when they are fully mature. In principle, these interactions should be targets for drug discovery.
Some mutations in red cell cytoskeleton proteins have been selected in populations within malaria-endemic regions. In South East Asia, ovalocytosis arises from band 3 mutations that give stiffer membrane (Liu et al. 1990b; Mohandas et al. 1992). These have a protective effect on cerebral malaria. Analysis of populations in Benin, West Africa, has revealed high levels of hereditary elliptocytosis, associated with mutations in αI- and βI-spectrins (Glele-Kakai et al. 1996).
Discovery of spectrin outside red cells (“non-erythroid” spectrins)
It was clear in the1970s that some nucleated cells could contain spectrin-like proteins. Pinder et al. (1978a) showed that the nucleated erythrocytes of some invertebrates contained immunologically cross-reactive proteins. However, initial surveys of cultured mammalian cells failed to reveal spectrins (Hiller and Weber 1977).
This changed with the advent of sensitive immunoblotting methods and immunofluorescence in the late 1970s and early 1980s. Spectrin was discovered independently by several investigators and variously termed brain (or non-erythroid) spectrin (Goodman et al. 1981; Bennett et al. 1982b; Burridge 1982; Lazarides and Nelson 1982), fodrin (Levine and Willard 1981; Glenney et al. 1982a) or calspectin (Kakiuchi et al. 1982).
The term fodrin still occasionally use today, and it is generally taken to refer to polypeptides termed here αII- and βII-spectrin. Spectrin identified in embryonic liver was termed “embryonic liver fodrin” or ELF (Mishra et al. 1999).
The identification of spectrin-related proteins in many tissues strongly implies that spectrin is not simply an erythrocyte protein but has descended from a lineage of animal proteins that arose before the advent of erythrocytes.
Gene cloning technology has revealed some of the evolutionary history of spectrin, ankyrin, 4.1 and adducin, and since it sheds light on their functions, it is relevant here to consider a little of this.
The spectrin-associated cytoskeleton in evolution
The evolution of animals required the emergence of adaptations for, e.g. cell differentiation and polarisation, the assembly of signalling and cell adhesion systems, as well as systems that provide the cell with resilience to the forces engendered by animal motility. Some of these, surprisingly, arose even before the emergence of metazoa. For example, in choanoflagellates, colonial protozoa that are thought to represent a lineage from which metazoa diverged (King 2005), there are a surprising number of signalling and cell adhesion systems (King et al. 2003). This suggests that some of the systems that were prerequisite for the ultimate appearance of animals actually had functions at a simpler stage of evolution.
Annotation of the genome of the choanoflagellate Monosiga brevicollis revealed a protein very similar to spectrin (King et al. 2008). This genome predicted α-, β- and βV-spectrin chains, although the β-spectrin does not obviously have an ankyrin-binding site (Baines 2009). It seems likely that in choanoflagellates, the functions of spectrin that are required for the formation of tissues had not yet evolved. However, the presence of an ABD in the choanoflagellate spectrin, as well as the potential for the protein to form tetramers, suggests that the fundamental actin-cross-linking activity of spectrin may have evolved very early (Baines 2009).
The genomes of other simple animals are now available: for example, the placozoan Trichoplax adherens (Srivastava et al. 2008) and sea anemone Nematostella vectensis (cnidarian; Putnam et al. 2007). Both of these have spectrin genes (α, β and β-heavy) although only Nematostella has an ankyrin with the potential to bind spectrin. It appears that spectrin–ankyrin interactions evolved at a similar time to the appearance of complex tissues. A protein 4.1 gene is also present in Nematostella, although it lacks the spectrin–actin-binding domain. This indicates that protein 4.1 probably evolved independently of a requirement to bind spectrin; indeed, the spectrin–actin-binding domain is a late evolutionary development—Pfam (Bateman et al. 2002) indicates that this domain probably evolved with the vertebrates.
The spectrin–ankyrin–4.1 system shows a remarkable gain of function in evolution: the formation of tissues may well have required an interaction between spectrin and ankyrin, and the gain of size of the vertebrates seems to have coincided with the strengthening of the spectrin–actin junction by the gain of spectrin–actin-binding activity of 4.1.
The protein accumulator model
Both ankyrin and protein 4.1 appear to be necessary for the stable accumulation of certain membrane proteins at the cell surface. It was noted above that they are required for the accumulation of the anion exchanger, Rh complex, Duffy and others in the mature erythrocyte membrane (e.g. Salomao et al. 2008, 2010): in their absence, they are lost being missorted during erythroid maturation. There are also many examples outside mammalian erythrocytes of a requirement for these proteins in the correct acculation of proteins at requisite points on cell surfaces. As will be detailed further below, ankyrin and 4.1 are required in many different cell types for the stable accumulation of a wide variety of transmembrane proteins.
Observations from worms and flies indicate that one of the functions of spectrin and ankyrin, at least, is to stabilise cell–cell junctions (e.g. Dubreuil et al. 1996; Hammarlund et al. 2000; Moorthy et al. 2000; Norman and Moerman 2002), particularly, those based around cell adhesion molecules of the L1 family. Ankyrin binds to such cell adhesion molecules and seems to be required for strengthening cell adhesions against the forces generated by the movement of animals (Davis and Bennett 1994; Dubreuil et al. 1996; Chen et al. 2001). One possibility is that ankyrin recruits spectrin to sites of activated L1 thereby cross-linking them: since spectrin tetramers each contain two ankyrin-binding sites, spectrin has the potential to cross-link L1 molecules. Individual spectrin tetramers might be joined together via F-actin, creating a cross-linked “Velcro-like” adhesion. Weakness of cell junctions in the absence of spectrin was indicated observations that Caenorhabditis elegans deficient in β-spectrin was paralysed in part because muscles pull away from the body wall (Hammarlund et al. 2000; Moorthy et al. 2000).
In skeletal muscle, there is a role for both ankyrin-B and ankyrin-G in the organisation and stability of the costameres, sites inking the internal cytoskeleton to the stress-resilient extracellular matrix. Here, β-dystroglycan and the dystrophin–glycoprotein complex require ankyrins for stable targeting to the plasma membrane (Ayalon et al. 2008). β-dystroglycan is a type I transmembrane protein and binds ankyrin-G. Dystrophin, a giant actin-binding protein that is part of the spectrin superfamily, binds ankyrin-B. In the absence of ankyrin-B, both β-dystroglycan and dystrophin remain intracellular and seem to require ankyrin-B for delivery to the cell surface. Both proteins require ankyrin-G for retention at the costamere. β-dystroglycan and dystrophin are key elements in the pathogenesis of muscular dystrophy. A Becker muscular dystrophy mutation E3335N reduced binding of both ankyrin-G and ankyrin-B to dystrophin.
These observations on cell adhesion also suggest mechanisms for targeting and accumulating membrane proteins at points on the cell surface specified by cell adhesions. Again, since spectrin tetramers bivalent for ankyrin, it should be possible for spectrin to cross-link ankyrin-bound cell adhesion molecules to different types of membrane protein also bound to ankyrin. One such membrane protein might be the sodium pump. In fruit flies, β-spectrin is required for accurate targeting of the sodium pump to the plasma membrane of copper cells in the gut. One hypothesis is that spectrin can cross-link the ankyrin-bound pump to cell adhesion molecules that specify its requisite position (Pinder and Baines 2000). In the absence of cross-linking to spectrin, the pump does not receive “permission” to remain at the cell surface.
Protein 4.1 also binds to cell adhesion molecules. Among these, proteins of the neurexin family in both mammals and invertebrates bind protein 4.1 (e.g. Baumgartner et al. 1996; Menegoz et al. 1997; Poliak et al. 1999; Biederer and Sudhof 2001; Denisenko-Nehrbass et al. 2003; Horresh et al. 2008; Laprise et al. 2009). The CTD binds a number of receptors including ionotropic glutamate receptors (Shen et al. 2000; Coleman et al. 2003) and certain G-protein coupled receptors (e.g. Binda et al. 2002; Lu et al. 2004a). There is clearly the potential for protein 4.1 to cross-link receptors to cell adhesion molecules. It is interesting to note that the spectrin–actin-binding domain of protein 4.1 is a late evolutionary adaptation of protein 4.1. Indeed, in fruitflies, coracle, the fly 4.1, is clearly not associated with spectrin (Fehon et al. 1994). The FERM and CTDs are common to all 4.1 proteins; thus, it seems likely that cross-linking membrane proteins, rather than cytoskeleton–membrane interactions is the fundamental role of protein 4.1.
A cartoon summarising the “protein accumulator” model is shown in Fig. 6.
The protein accumulator model for spectrin and ankyrin function. Adapted from Pinder and Baines (2000). In this model, interaction of cells via cell adhesion molecules (CAMs) leads to activation of the CAMs, which then recruit ankyrin from the cytoplasm. These, in turn, recruit spectrin tetramers which cross-link the ankyrin-bound CAMs. The cross-links are further strengthened by spectrin molecules binding to actin filaments. Because spectrin has two ankyrin-binding sites per tetramer, each spectrin has the potential to bind additional ankyrins that might be bound to other transmembrane proteins, for instance, the Na/K-ATPase. This would trap such transmembrane proteins and promote their stable incorporation into the plasma membrane. It could also be imagined that proteins 4.1 and adducin would modulate the spectrin–actin linkages and trap further transmembrane proteins in this complex
A table of some of the transmembrane proteins known to interact with ankyrin or 4.1 is given in Table 1.
Physiology of the spectrin–ankyrin–4.1–adducin system
In the following section, some elements of the functions of the spectrin–ankyrin–4.1–adducin complex are discussed in relation to the physiology of some key cell and tissue types. The focus is on epithelia, nerve and cardiac muscle. These have each been the subject of intense investigation for 2–3 decades and reveal many principles underlying the function of the spectrin complex. In the space available in this review, it is not possible to cover function in all tissues; thus, these have simply been chosen as exemplars. Nevertheless, the reader should be aware that the proteins of the spectrin complex are present in all animal cells.
A role for spectrin, ankyrin, adducin and protein 4.1 in the polarisation of function in epithelial cells
-
(a)
Basolateral domain
Since proteins of the spectrin complex arose coincidentally with the formation of tissues, they might be predicted to have roles in the formation of apical or basolateral domains. However, proposals that spectrin is required for the formation of all polarised cells were not supported by knockdown experiments in C. elegans. Elimination of worm β-spectrin resulted in a variety of phenotypic alterations—most strikingly in muscle and nerve—nevertheless, epithelia appeared to polarise normally and the process of secretion appeared unaffected since the cuticle was deposited normally (Hammarlund et al. 2000; Moorthy et al. 2000). Similarly, mutations in fruit fly alpha-spectrin indicated that, although the epithelia formed, spectrin-stabilised cell–cell junctions are critical to cell shape and tissue organisation (Lee et al. 1993). However, in mammalian systems, clear evidence has been found for a requirement for βII-spectrin, ankyrin and protein 4.1 in the formation of lateral membranes.
Nelson and co-workers identified detergent-resistant complexes between spectrin, ankyrin, E-cadherin and the Na/K-ATPase that formed during kidney cell polarisation (Nelson and Veshnock 1987; Morrow et al. 1989; Nelson and Hammerton 1989; Nelson et al. 1990).
Over-expression of fragments of spectrin gave disruption of Caco-2 cell epithelial morphology, indicating a fundamental role for spectrin in epithelial biogenesis (Hu et al. 1995). Knockdown of βII-spectrin, ankyrin or 4.1R in monoloayers of human bronchial epithelial cells results in loss of lateral membrane and an apparent collapse of these cells (Kizhatil and Bennett 2004; Kizhatil et al. 2007b; Yang et al. 2009). The role of 4.1, in particular, is conserved in evolution since the fruit fly 4.1, coracle, promotes basolateral membrane stability (Laprise et al. 2009), although this implies spectrin independence of its function. Interaction of ankyrin with spectrin is clearly required since a mutant ankyrin-G that does not bind spectrin cannot participate in lateral membrane biogenesis (Kizhatil et al. 2007b).
Spectrin, protein 4.1 and ankyrin participate in the formation of cadherin-based junctions. Ankyrin-G links βII-spectrin to E-cadherin, and this interaction was found to be required required for exit of E-cadherin from the Golgi apparatus and for accumulation of E-cadherin at sites of cell–cell contact in embryonic cells and cultured epithelia (Kizhatil et al. 2007a, b; Kizhatil and Bennett 2004). In 4.1R knockout mice, histological examination revealed impairment of cell–cell junctions in the stomach epithelia, and the gastric glands were disorganised (Yang et al. 2009). Protein 4.1R binds to the adherens junction protein β-catenin (Yang et al. 2009). α-Catenin binds (αII–βII)-spectrin (Pradhan et al. 2001) at a site close to the N-terminal of αII-spectrin, i.e. closely adjacent to the site in βII-spectrin in a spectrin tetramer that binds actin. All these data indicate an important role for (αII–βII)-spectrin, ankyrin-G and 4.1R in connecting the cadherin/β-catenin complex to the internal cytoskeleton and in ensuring the formation and integrity of adherens junctions.
Adducin seems to have a role in stabilising epithelial junctions once they have formed. Early observations on adducin in epithelial cells revealed that it was recruited to sites of E-cadherin cell adhesions (Kaiser et al. 1989). Knockdown and rescue experiments suggest it stabilises junctions once they have formed. Interaction of adducin with sites of cell adhesion was dependent on spectrin, but the presence of adducin increased detergent resistance of spectrin (Abdi and Bennett 2008). In the absence of adducin, the basal surface area was found to expand, and E-cadherin diffusion was increased (Abdi and Bennett 2008).
The delivery of membrane proteins to apical or basolateral domains in epithelial cells is of central importance to their function. As well as E-cadherin, other membrane proteins in epithelia that require ankyrin linkage for passage through the secretory pathway and delivery to the basolateral surface include the Na/K-ATPase (Nelson and Veshnock 1987; Morrow et al. 1989; Hu et al. 1995; Kizhatil and Bennett 2004). A 25 amino acid cytoplasmic sequence within the pump has been found to bind to ankyrin. When this sequence was used to replace the cytoplasmic domain of the VSV G protein, it conferred ankyrin-R dependence on passage of the chimera through the Golgi. The Na/K-ATPase also requires a Golgi form of 4.1B for passage through the secretory pathway (Kang et al. 2009). These data indicate functions for 4.1 and ankyrin proteins in a fundamental process, trafficking of transmembrane proteins, required in all eukaryotic cells, not just epithelia. Whether this represents an adaptation of the trafficking apparatus to fit the needs of vectorial delivery of membrane cargoes in polarised metazoan cells or whether the function of ankyrin/4.1 has been adapted to play a more fundamental role in membrane trafficking remains to be seen.
If the spectrin/ankyrin/4.1 complex is damaged in some way, polarisation might be lost. Several workers have suggested that this is the case in kidney epithelia after ischemia (Doctor et al. 1993; Alejandro et al. 1995; Woroniecki et al. 2003). In ischemic tissues, cytoplasmic Ca2+ concentrations rise, activating the protease calpain. Spectrin, ankyrin and 4.1 are all calpain substrates, but a site in αII-spectrin, in particular, close to the centre of the polypeptide is exquisitely sensitive to calpain. In ischemic cells, the spectrin–ankyrin linkage was found to be lost, even though ankyrin remained bound to the pump. Breaking of the linkage coincided with loss of the polarised distribution of the pump (Woroniecki et al. 2003).
It has been speculated that calpain cleavage of spectrin is important in physiological modelling of tissues during development. However, mice with a spectrin knock-in that render them resistant to calpain cleavage develop normally and have no major phenotypes (Meary et al. 2007), arguing against such roles.
-
(b)
Apical domain
A role for spectrin in the apical domain of epithelial cells was suggested by the work of Glenney and co-workers (1982b) who isolated a spectrin-related protein from chicken intestinal terminal web: they named this protein TW-260/240. In the terminal web, it appeared to span the actin filaments that emerged from the microvilli. The contour length of the protein was about 263 nm, much greater than that of spectrin tetramers (approximately 190 nm; Glenney et al. 1982c). The protein appeared to be a tetramer of elongated subunits and generally resembled spectrin. The α-subunit (TW-240) by immunological analysis and peptide mapping seemed to be identical to the α-subunit of brain spectrin (Glenney et al. 1982c; Glenney and Glenney 1984), i.e. it was αII-spectrin. Comparison of the contour length of the intact TW-260/240 molecule with spectrin indicated that the size of the β-subunit was probably of the order of 1.7 times greater than that of red cell β-spectrin (Glenney et al. 1982c). The sequence of βV-spectrin (or βH-spectrin) contains approximately 1.7 times more amino acids than that of erythrocyte β-spectrin (Stabach and Morrow 2000); thus, it is likely that TW-260 actually represents a β-heavy (βH)-subunit (although the author can find no evidence in the public databases that TW-260 has been sequenced to date). The protein did not seem to be linked to membranes in the same way as red blood cell spectrin, and correspondingly, the β-subunit (TW-260) did not bind ankyrin (Howe et al. 1985). Nor, indeed, was its interaction promoted by protein 4.1 (Coleman et al. 1987), although βV-spectrin in cochlear outer hair cells apparently forms a complex with protein 4.1G (Legendre et al. 2008).
A spectrin-related gene was identified in the karst locus in fruit flies (Thomas et al. 1998). This gene encoded a high molecular mass β-heavy subunit: correspondingly, it is referred to as βH. The equivalent gene in C. elegans is SMA-1 (McKeown et al. 1998).
Karst mutations are largely larval lethal, although there are adult escapers with rough eyes (many of which lacked photoreceptor R7), bent wings, tracheal defects and infertility (Thomas et al. 1998). In the mid-gut, immunological analysis revealed that βH-spectrin was a terminal web protein, indicating its relationship to chicken TW-260 (Thomas et al. 1998). Absence of βH-spectrin was accompanied by the breakup of the zonula adherens during mid-oogenesis in follicle cells (Thomas et al. 1998; Zarnescu and Thomas 1999). It also prevented stable recruitment of α-spectrin to the apical domain, although it did not result in loss of apical–basal polarity (Thomas et al. 1998; Zarnescu and Thomas 1999). The fly β-spectrin interacted with the crumbs protein, an apical transmembrane protein required in the assembly of the zonula adherens, as well as for apical–basal polarity (Medina et al. 2002). A complex of crumbs, discs lost, moesin and βH-spectrin was immunoprecipitated from fly embryos, indicating the existence of a multi-protein complex (Medina et al. 2002). As with the role of β-spectrin in the basal domain, the role of apical βH seems to be related to stabilising a region of cell adhesion. In the retina, the cell adhesion molecule roughest is required in refinement of the retinal lattice (Lee et al. 2010). Over-expression of a fragment of βH-spectrin disrupted the zonula adherens and displaced roughest (Lee et al. 2010). Combining karst and roughest mutations gave catastrophic effects on retinal development, indicating that apical spectrin links to both zonula adherens assembly and roughest morphogenesis (Lee et al. 2010).
In C. elegans, the βH-spectrin SMA-1 was also required for morphogenetic events. SMA-1 mutants elongated more slowly than wild-type embryos, and SMA-1 was first expressed in epithelia (McKeown et al. 1998). It appeared to have a role in the apical domain of epidermal cells, where it was required to maintain the association between actin and the apical membrane (McKeown et al. 1998; Praitis et al. 2005). Similarly, in flies, βH-spectrin was required in wound healing in the software in which an injured epithelial sheet closes its whole via a “purse-string” mechanism (Campos et al. 2010). SMA-1 was also required for morphogenesis of the lumen of epithelial tubes (Gobel et al. 2004).
In the brush border, βH-spectrin was also found to be required for maintenance of Rab5 endosomes, and an apical proton vacuolar-ATPase was lost from both the brush border and Rab5 endosomes (Phillips and Thomas 2006). βH-spectrin probably has a role in protein sorting in the endocytic pathway. Interestingly, a possibly parallel relationship to protein sorting occurs in mammals where transient receptor potential channel protein TRPC4 interacts with βV- and αII-spectrin, and this interaction is required for intracellular sorting (Odell et al. 2008).
Mechanistically, it is suggested that β-spectrin and βH-spectrin act in an antagonistic way to balance apical and basolateral domains (Chen et al. 2009). Epithelial polarity is generally considered to be governed by three protein complexes: the apical polarity complexes (par3/par6/aPKC and crumbs/pals1/patj) and the lateral complex (scribble/dlg/lgl; Assemat et al. 2008). As noted above, βH-spectrin in flies interacts with crumbs. Interestingly, human 4.1R binds tothe human homologue of the Drosophila discs large tumor suppressor (hdlg; Lue et al. 1994), providing a link to the lateral polarity complex. A topic for the future will be to understand better the mechanistic links between the spectrin cytoskeleton and the polarity complexes, not only in epithelia but also in additional polarised cells, including nerve cells.
Spectrin, ankyrin and protein 4.1 in nerve cells
Nervous tissue was one of the earliest sources of material for investigation of spectrin, ankyrin, adducin and protein 4.1 outside red blood cells.
Purification of spectrin from brain revealed that it had an actin-binding activity (Bennett et al. 1982a; Burridge 1982; Glenney et al. 1982d; Burns et al. 1983), although unlike erythrocyte spectrin, it could bind actin with high affinity even in the absence of protein 4.1; but protein 4.1 still promoted the binding of brain spectrin to F-actin (Burns et al. 1983).
An ankyrin was also purified from brain and this was termed ankyrin-B (B for brain; Davis and Bennett 1984a, b). This could bind to brain spectrin at a site equivalent to the ankyrin-binding site in erythrocyte β-spectrin. Brain ankyrin itself was found to bind to protein sites on brain membranes stripped of peripheral membrane proteins by treatment with concentrated potassium iodide solutions (Davis and Bennett 1986; Davis et al. 1989). The most abundant binding sites for ankyrin on brain membranes were identified as polypeptides of the neurofascin/L1 family (Davis and Bennett 1993, 1994), but it was clear that brain ankyrin bound to other proteins as well including voltage-gated sodium channels (Srinivasan et al. 1988; Lambert et al. 1997; Zhou et al. 1998; Garrido et al. 2003).
The significance of βII-spectrin to mammalian tissue development has been addressed in mice. βII-spectrin knockout mice die in utero, with major defects in the formation of the brain, heart and other organs (Tang et al. 2003). By comparison, mice of the βI-spectrin mutant strain ja/ja, while extremely anaemic from loss of erythrocyte spectrin, survive till a few days after birth (Bodine et al. 1984); this indicates that βI-spectrin is probably not required for the formation of major brain structures.
Axon outgrowth
Analysis of C. elegans in which spectrin has been knocked down revealed that, among other phenotypic anomalies leading to the animal’s paralysis, axons did not find their targets; they were described as “wandering” (Hammarlund et al. 2000; Moorthy et al. 2000). Subsequent analysis using time-lapse microscopy revealed that the axons were able to form normally and exhibited growth cones, but the axons broke frequently. After breakage of the first axon outgrowth, more axons formed, seemingly at random over the nerve cell body, and, where they extended, they were not guided precisely (Hammarlund et al. 2007). It seems likely that spectrin is required for maintenance of axon integrity in the face of the forces generated by the movement of the animal. To that extent, these data reveal a common theme in the function of spectrin in both nerve cells and erythrocytes.
In mammals, there is evidence for a requirement for spectrin function, as well as that of ankyrin, in initiation of axon outgrowth. Knockdown of ankyrin in rat primary nerve cells blocked axon outgrowth; this was rescued by transfection with human ankyrin-GFP, but not by a mutant that cannot bind spectrin (Nishimura et al. 2003). It is suggested that ankyrin recruits spectrin to sites where cell adhesion molecules are engaged with cell adhesion molecules that permit axon outgrowth; spectrin would therefore be hypothesised to be recruited to such sites and, in turn, linked to actin. This would stabilise treadmilling filaments so that growth at their plusends push out the leading edge of the cell. It is notable in this context that αII-spectrin interacts with a number of proteins of the motility apparatus of crawling cells (Ziemnicka-Kotula et al. 1998; Rotter et al. 2005; Bournier et al. 2006): this is a topic that remains to be explored further.
Further evidence for involvement of spectrin in axon (neurite) outgrowth comes from experiments with the model cell line PC12. Upon serum starvation and exposure to nerve growth factor, these rat pheochromocytoma cells cease proliferating and differentiate into a sympathetic nerve cell type which extend axon-like neurites (Greene and Tischler 1976). Transfection of these cells with fragments of βII-spectrin (which act as dominant negative inhibitors of tetramer formation) blocked NGF-induced neurite outgrowth (Bignone et al. 2007). Interestingly, a mutant resembling a phosphorylated spectrin fragment that was able to bind α-spectrin only weakly did not block axon outgrowth; a non-phosphoryatable mutant that bound α-spectrin strongly (and hence was a strong inhibitor of spectrin function) was a strong inhibitor of axon outgrowth. The mutation was at a site recognised by both protein kinase A and protein kinase CKII: protein kinase A has attracted much attention in regulation of nerve axon outgrowth, and it is tempting to speculate that spectrin is one of its key substrates in this respect.
Assembly of the signalling systems that transmit action potentials
Nodes of ranvier
The observation in the late 1980s that ankyrin could bind to voltage-gated Na+ channels (Srinivasan et al. 1988) indicated a role for the spectrin–ankyrin system in nerve cell signalling. Subsequently, it became clear that a specialised giant ankyrin, ankyrin-G 480, which has a large insertion in the C-terminal region, was enriched at nodes of Ranvier (Kordeli et al. 1995). Ankyrins also bind the nodal Kv3.1 and KCNQ K+ channels (Devaux et al. 2003, 2004; Pan et al. 2006; Xu et al. 2007). Both the K+ and voltage-gated Na+ channels require ankyrin-G to assemble at nodes (Pan et al. 2006). Both groups of vertebrate channels contain similar ankyrin-binding motifs that appear to have evolved subsequent to the appearance of vertebrates, at a similar evolutionary point to the appearance of myelin (Hill et al. 2008). Furthermore, several other key elements of the node—the Na/K-ATPase, Na/Ca exchanger and L1/neurofascin cell adhesion molecules—also bind ankyrin (Lambert et al. 1997).
The most abundant spectrin at nodes comprises αII–βIV polypeptides (Berghs et al. 2000; Jenkins and Bennett 2002; Komada and Soriano 2002; Dzhashiashvili et al. 2007; Uemoto et al. 2007). Mice lacking βIV-spectrin had a quivering phenotype (qv/qv strain) with auditory and motor neuropathies, presumably caused by mislocalised channels that depend on the ankyrin–spectrin complex (Parkinson et al. 2001; Komada and Soriano 2002).
In the paranodal and juxtaparanodal regions, the abundant spectrins are αII–βII; thus, there is microscale compartmentation of spectrins with the nodal region (Ogawa et al. 2006; Voas et al. 2007). The targeting mechanisms and functional selectivities of the different spectrin populations have yet to be clearly established.
Proteins 4.1 are also present in nodes of Ranvier at paranodes and juxtaparanodes. Protein 4.1B bound CASPR1 and CASPR2, two cell adhesion molecules of the NCP (neurexin/paranodin/CASPR) family at juxtaparanodes (Gollan et al. 2002; Denisenko-Nehrbass et al. 2003). Protein 4.1B also colocalised with Kv1 (Shaker type) K+ channels at juxtaparanodes (Poliak et al. 2001; Horresh et al. 2010). It is thought that 4.1B forms part of a scaffold that controls the targeting and retention of these membrane proteins at that point. Interestingly, CASPR2 seemed to be actively retrieved from plasma membrane in somatodendritic compartments, and this was required for its axon targeting: interaction with 4.1B seemed essential for this (Bel et al. 2009).
An evolutionary correlate of this has been drawn with the fruit fly 4.1 coracle. Coracle is enriched in pleated septate junctions in flies which are the functional equivalent of the vertebrate tight junctions. Coracle and a group of transmembrane proteins—the cell adhesion molecules neurexin and neuroglian (an L1/neurofascin family member) and the Na/K-ATPase—formed an interdependent complex (Genova and Fehon 2003).
A further component of the paranode/juxtaparanode is β-adducin (Ogawa and Rasband 2009). This polypeptide colocalised with CASPR2, Kv1.2 and βII-spectrin. However, it is hard to be sure of the functional significance of β-adducin at these points since mice deficient in β-adducin showed no obvious developmental defects: perhaps, as in erythrocytes (Gilligan et al. 1999), γ-adducin compensates.
In summary, the major transmembrane protein complexes at nodes of Ranvier, as well as paranodes and juxtaparanodes that dictate both the ion fluxes underpinning action potential transmission, as well as the cell adhesions that provide tight association between the axonal plasma membrane and myelinating cells, are closely linked to the spectrin–ankyrin–4.1–adducin complex. It is likely that these interactions converged in evolution as a prerequisite to the appearance of salutatory conduction of action potentials.
Axon initial segment
Nerve cells are highly polarised cells, with a single axon and multiple dendrites. A key region in determining this polarity is the axon initial segment (AIS), which acts as a diffusion barrier between the somatodendritic compartment and the axon (Ogawa and Rasband 2008). Here, transmembrane proteins that are responsible for initiating action potentials are clustered together with molecules that specify adhesion to other cells and are linked to the underlying cytoskeleton, in an array conceptually similar to that described above for the node of Ranvier. Indeed, the AIS is also characterised by βIV-spectrin and ankyrin-G (Jenkins and Bennett 2001).
The key role of ankyrin-G in assembling the AIS is mediated via its mutually interdependent interactions with cell adhesion molecules that include the neurofascin/L1 family member Nf186 and the neuron glia-related CAM (NrCAM; Ango et al. 2004; Dzhashiashvili et al. 2007; Hedstrom et al. 2007). These interactions are required for the establishment of the AIS diffusion barrier that separates axonal and dendritic compartments and clusters the channels required for action potentials (Lemaillet et al. 2003; Boiko et al. 2007; Nishimura et al. 2007; Song et al. 2009).
Ankyrin-G binds AIS Na+ and K+ channels and seems to be intimately involved in generating the clusters required for action potential firing (Bouzidi et al. 2002; Garrido et al. 2003; Xu and Shrager 2005; Chung et al. 2006; Rasmussen et al. 2007; Van Wart et al. 2007; Hedstrom et al. 2008). The importance of these interactions has recently been emphasised by the observation that both βIV-spectrin and ankyrin-G are targets for the Ca2+-activated protease calpain (Schafer et al. 2009). Calpain-catalysed proteolysis of these proteins is observed in AIS of injured nerves and is associated with loss of neuronal polarity and clusters of Na+ channels. These observations are consistent with findings on ankyrin-G-deficient cerebellar nerve cells: these lose polarity and develop sproutings on their axons resembling dendritic spines in their morphology, in the presence of dendritic protein markers and in forming contacts with presynaptic glutamatergic boutons (Sobotzik et al. 2009).
Similar data have been obtained for small interfering RNA silencing of ankyrin-G in hippocampal cultures in vitro: these cells also develop spine-like structures and synapses on their axons. Somatodenditic markers, such as the K+/Cl- cotransporter KCC2 and the microtubule-associated protein MAP2, enter the axon (Hedstrom et al. 2008).
Synaptic structure and stability
A role for spectrin in the function of synapses was suggested by early observations that brain spectrin was a major calmodulin-binding component of the post-synaptic density (Carlin et al. 1983). Post-synaptic spectrin interacts with an extensive PSD cytoskeletal scaffold and directly with certain receptors including ionotropic glutamate receptors (Wechsler and Teichberg 1998). In fruit flies, α-spectrin and β-spectrin are required for normal synaptic development and organisation (Pielage et al. 2006).
Recent genetic screens in fruit flies have identified α-spectrin, β-spectrin and ankyrin-2 as essential components for normal synaptic stability, with a presynaptic function. A giant ankyrin-2 (ank2-L: 4,083 amino acids) was distinguished in neuromuscular junctions, where it was organised in a presynaptic lattice on the membrane (Pielage et al. 2008). This protein contains a large insert in the C-terminal region. Ank2-L was required for synaptic stability: in its absence, the homophilic cell adhesion molecule fasiclin II was disorganised, and the presynaptic nerve ending appeared to disassemble. Correspondingly, electrophysiological analysis indicated major defects in synaptic transmission. In the absence of ank2-L, the presynaptic microtubule lattice became disorganised. Interestingly, one of the early observations on the activities of non-erythroid ankyrins was that they bound microtubules (Bennett and Davis 1981): this seems to be the functional correlate of this.
It should be noted that there are several other isoforms of Drosophila ank2 that are spatially segregated. Ank2-short (137 kDa) was only found in neuronal cell bodies, while Ank2-long isoforms (also described at ank2-M: about 400 kDa) were present in axons (Hortsch et al. 2002).
Spectrin/ankyrin/4.1 and the regulation of heart beating
One of the first tissues in which spectrin was discovered was the heart muscle cells (cardiomyocytes; Goodman et al. 1981). Spectrin appeared in striated muscles to be concentrated in regular plasma membrane accumulations named costameres (Craig and Pardo 1983; Pardo et al. 1983a, b; Craig and Pardo 1983), aligned with the Z-line and linked to the internal contractile apparatus via intermediate filament complexes. Actin is present in costameres, but unlike the contractile apparatus which contains α-actin, costameres contain γ-actin (Craig and Pardo 1983). Externally, they are linked to the stress-tolerant extracellular matrix via cell adhesion molecules that include integrins (Hilenski et al. 1992). Since they link the extracellular matrix to internal actin-rich structures, they are often likened conceptually to focal adhesions (Samarel 2005). Costameres represent sites of biomechanical signal transmission, and altered signalling via costameres is associated with hypertrophy (Sussman et al. 2002).
αI-, αII-, βI- and βII-spectrins are present on cardiomyocyte plasma membranes (Isayama et al. 1993; Flick and Konieczny 2000; Hayes et al. 2000; Bennett et al. 2004; Baines and Pinder 2005). These proteins are strikingly compartmentalised: both α-spectrins were present at lateral plasma membranes and close to T-tubules, but only αII-spectrin was present at intercalated discs (ID; Baines and Pinder 2005; Bennett et al. 2006). These are the sites where contractile forces are transmitted between cells. The relatively stiff αII-spectrin polypeptide might be hypothesised to be fitted for a role in maintaining plasma membrane structure at IDs; the relatively springy αI-spectrin might supplement αII-spectrin on lateral membranes to accommodate the membrane distortions that take place on contraction. In addition, αII-spectrin was found to be aligned with the terminal sarcomere in cardiomyocyte myofibrils, suggesting a mechanism for direct communication between intercalated disc and contractile apparatus (Bennett et al. 2006).
βI-spectrin is also present on the plasma membrane; thus, a proportion of the cardiomyocyte spectrin therefore presumably is similar to the erythrocyte protein. The βI-spectrin isoform that is dominant is the long, muscle-specific form that contains the PH domain. βI-spectrin has a potential link to cardiomyopathy and heart failure in that it binds to muscle LIM protein (MLP; Arber et al. 1997; Flick and Konieczny 2000). MLP binds triple helical repeat 7 of βI-spectrin (Flick and Konieczny 2000).
βII-spectrin is present in two isoforms: the long C-terminal (βIIΣ1) and the short C-terminal (βIIΣ2) forms. The plasma membrane form is primarily long; the short form is mainly internal and associated with Z-disks and M-lines. αII-spectrin is also a component of the Z-disk structure as judged by immunoelectron microscopy; thus, Z-disks probably contain (αII-βIIΣ2)2 tetramers. On the other hand, at the M-line, there is apparently no α-spectrin; there have been a number of reports of α-less β-spectrins in muscles, but the functional significance of these is unknown (and indeed whether or not there are other proteins that block the α-binding sites in these β-spectrins).
Ankyrins in heart: ankyrin-linked cardiomyopathies associated with loss of channel targeting
Ankyrin-B (Mohler et al. 2003) and ankyrin-G (Mohler et al. 2004b) were found to be expressed in heart, where immunofluorescence placed them close to T-tubules and intercalated discs, although relatively little of both was observed at lateral plasma membranes. Ankyrin-B seemed to join NCX1, the Na/K-ATPase and IP3 receptor in a complex that linked between the plasma membrane/T-tubule and an internal membrane compartment possibly linked to the sarcoplasmic reticulum (Mohler et al. 2005). Ankyrin-B mice were haploid-insufficient. Even in the heterozygous state, ankyrin-B +/- mice had an arrhythmia and an extended period in the electrocardiogram (ECG) equivalent to the human QT interval. The arrhythmia phenotype was exacerbated by exercise. Adrenergic stimulation with exercise led to occasions of sudden deaths. In this sense, this appeared to be a long QT syndrome and was originally classified as long QT syndrome type 4 (Mohler et al. 2003). However, more recently, it has been referred to as “ankyrin-B syndrome” (Mohler and Bennett 2005). Several channels, including the NCX1, the Na/K-ATPase and the IP3 receptor, were not correctly located in these mice (Tuvia et al. 1999; Chauhan et al. 2000; Mohler et al. 2003).
In a human pedigree with an inactivating mutation (E1425G) in ankyrin-B, a remarkably similar arrhythmia has been found, characterised by bradycardia, atrial fibrillation, cardiac conduction disease and increased risk for catecholaminergic sudden death. Ankyrin-B also had a role in sinus node disease (SND). In the sinoatrial node, NCX1, Na/K-ATPase and inositol trisphosphate (IP3) receptor all required ankyrin-B for correct localisation, as did the voltage-gated calcium channel α-subunit Cav1.3 (Hund and Mohler 2008; Le Scouarnec et al. 2008). Ankyrin-B therefore has a critical role in cardiac pacing via its transporter-targeting activity. One possibility is that ankyrin-B-mediated connection between these proteins is essential for the efficient extrusion of calcium from the cell and that the defects in ankyrin-B syndrome arise from dysregulation of intracellular calcium homeostasis (Mohler and Bennett 2005).
A second ankyrin-related condition is a variant of Brugada syndrome, in which the inactivating mutation E1053K in the voltage-gated Na+ channel Nav1.5 eliminates interaction with ankyrin-G (Mohler et al. 2004b). This channel is the principal voltage-gated sodium channel in the heart, and ankyrin binds to it via an intracellular loop between domains two and three of the channel (Garrido et al. 2003; Lemaillet et al. 2003). Ankyrin-G is required for targeting and expression of the channel.
The function of ankyrin in heart clearly demonstrates its requirement in targeting and localisation pathways for multiple membrane proteins. These heart complexes are analogous to those described above in brain; indeed, complexes of ankyrin-B, NCX1, IP3 receptor and αII–βII-spectrin can be imunoprecipitated from brain (Lencesova et al. 2004).
Protein 4.1R and control of heartbeat
All four 4.1 genes are expressed in heart. Of these, 4.1R has been localised to plasma membrane (including intercalated discs) and striated structures that are probably T-tubules (Taylor-Harris et al. 2005b). Homozygous mice deficient in protein 4.1R had a bradycardia and an extended interval in the ECG equivalent to the QT period (Stagg et al. 2008). In this sense, they resembled ankyrin-deficient mice. Analysis of their physiology revealed defects in multiple ion transport processes including, among others, a reduction in the current density for sodium/calcium exchange, elongation of the period of the action potential and increase in the persistent sodium current density. The latter was probably associated with a failure to inactivate Nav1.5 fully. Analysis of the protein content of the mouse revealed a reduction in the total content of Nav1.5 as well as a (presumably compensating) up-regulation of protein 4.1G. It seems very likely that protein 4.1 and ankyrin have an overlapping spectrum of roles in the heart in control of the cellular accumulation and localisation of diverse membrane proteins. As yet, no human correlate of the mouse data exists. It will be interesting in the future to establish if mutations in 4.1R can be associated with human cardiac conditions.
Summary and perspectives
In this review, numerous examples have been cited, which indicate a very fundamental role for the proteins spectrin, ankyrin, 4.1 and adducin in the distinctive functions of cells in animals. We have also seen that there is good evidence that these proteins arose with the animals and that their interactions evolved as animals gained tissues, as well as subsequently during the evolution of the vertebrates. The implication is that these proteins are very likely to be essential to all animal life. As a consequence, defects in these proteins caused by mutation or by modifications, such as proteolysis, are associated with numerous disease states ranging from hereditary anaemia to heart disease.
Since their disease associations are so diverse, we can expect to find new mutations associated with diseases related to membrane function that have not been expected until now. The 4.1 proteins are enigmatic in their relationship to human diseases: while a link to heart disease is suggested by the mouse 4.1R model (Stagg et al. 2008), to the author’s knowledge, thus far, no single nucleotide polymorphism (SNP) analysis links any of the 4.1 genes to inherited heart disease. Indeed, the only clearly 4.1-linked condition is hereditary anaemia (Gallagher 2004).
An emerging issue is the relationship between the spectrin complex and progression through the cell cycle and, therefore, a potential involvement in cancer. Since the cell cycle is governed by mechanisms common to all eukaryotic cells, it might not be expected that animal-specific proteins would have a major role in such a fundamental process. But knockdown experiments have recently revealed a requirement for αII-spectrin in progression through G1 in WM-266 human melanoma cells (Metral et al. 2009). The precise implications of this are not clear at present, since, for instance, fruit flies with α-spectrin mutations survive until first instar larvae and then die from defects in tissue organisation (Lee et al. 1993). Both 4.1R (Robb et al. 2003) and 4.1B (Tran et al. 1999; Gutmann et al. 2000) have both been implicated as human tumor (carcinoma) suppressors. Given their functions in the biogenesis and maintenance of cell adhesions, this is perhaps not surprising. But in addition, 4.1R is associated with the mitotic spindles and centrosomes as well as interphase microtubules of mammalian cells (Correas and Avila 1988; Mattagajasingh et al. 1999; Perez-Ferreiro et al. 2001, 2004; Delhommeau et al. 2002; Krauss et al. 2004), and the D. melanogaster orthologue coracle is required for imaginal cell proliferation (Ward et al. 2001).
One of the problems with investigating the structural/functional relationships in these proteins is that some of the most powerful genetic tools are unavailable because of the animal basis of their function; thus, high throughput tools using bacteria or yeast are, at present, not usable. However, the availability of genomewide screens in, for instance, fruit flies will make identification of further candidate roles for these proteins faster.
Are these proteins candidates for drug discovery directly? In principle, one might imagine that these proteins might be interesting candidates for drug discovery because of their important roles in establishing signalling systems. Historically, however, the pharmaceutical industry had little success with developing medicines based on protein–protein interaction. It seems rather unlikely that direct drug discovery will follow from this work (although the author would be delighted to be proved wrong!).
On the other hand, there are potential applications for these proteins that so far have not been explored. For example, both ankyrin and protein 4.1 are required for the cellular accumulation and intracellular targeting (as well as full activity) of many membrane proteins, including G protein-coupled receptors and voltage-gated sodium channels. These are important targets for pharmaceutical discovery. In programmes for discovering new medicines, the first step is often to express the target protein of interest in a recombinant animal cell. A consideration that must emerge from the work reviewed here is that the ankyrin/4.1 content of such cells must be considered in relation to establishing high-level expression of fully active and regulated target protein. Likewise, the production of pharmaceutical proteins from animal cells in culture typically requires large-scale culture under conditions of quite high shear stress. It may be possible to engineer animal cells, for production purposes, whose membranes are fitted to withstand shear forces, taking advantage of knowledge of the spectrin system.
References
Abdi KM, Bennett V (2008) Adducin promotes micrometer-scale organization of beta2-spectrin in lateral membranes of bronchial epithelial cells. Mol Biol Cell 19(2):536–545
Adachi H, Ito D, Kurooka T, Otsuka Y, Arashiki N, Sato K, Inaba M (2009) Structural implications of the EL(KIQ)(L/C)LD(A/G)DD sequence in the C-terminal cytoplasmic tail for proper targeting of anion exchanger 1 to the plasma membrane. Jpn J Vet Res 57(3):135–146
Alejandro VSJ, Nelson WJ, Huie P, Sibley RK, Dafoe D, Kuo P, Scandling JD, Myers BD (1995) Postischemic injury, delayed function and Na+/K+-ATPase distribution in the transplanted kidney. Kidney Int 48(4):1308–1315
An X, Mohandas N (2008) Disorders of red cell membrane. Br J Haematol 141(3):367–375
An XL, Takakuwa Y, Manno S, Han BG, Gascard P, Mohandas N (2001) Structural and functional characterization of protein 4.1R–phosphatidylserine interaction: potential role in 4.1R sorting within cells. J Biol Chem 276(38):35778–35785
An X, Lecomte MC, Chasis JA, Mohandas N, Gratzer W (2002) Shear-response of the spectrin dimer-tetramer equilibrium in the red blood cell membrane. J Biol Chem 277(35):31796–31800
An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N (2004) Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 43(2):310–315
An X, Debnath G, Guo X, Liu S, Lux SE, Baines A, Gratzer W, Mohandas N (2005a) Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4, 5-bisphosphate. Biochemistry 44(31):10681–10688
An X, Guo X, Gratzer W, Mohandas N (2005b) Phospholipid binding by proteins of the spectrin family: a comparative study. Biochem Biophys Res Commun 327(3):794–800
An X, Guo X, Zhang X, Baines AJ, Debnath G, Moyo D, Salomao M, Bhasin N, Johnson C, Discher D, Gratzer WB, Mohandas N (2006a) Conformational stabilities of the structural repeats of erythroid spectrin and their functional implications. J Biol Chem 281(15):10527–10532
An X, Zhang X, Debnath G, Baines AJ, Mohandas N (2006b) Phosphatidylinositol-4, 5-biphosphate (PIP2) differentially regulates the interaction of human erythrocyte protein 4.1 (4.1R) with membrane proteins. Biochemistry 45(18):5725–5732
An X, Zhang X, Salomao M, Guo X, Yang Y, Wu Y, Gratzer W, Baines AJ, Mohandas N (2006c) Thermal stabilities of brain spectrin and the constituent repeats of subunits. Biochemistry 45(45):13670–13676
Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ (2004) Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119(2):257–272
Anong WA, Franco T, Chu H, Weis TL, Devlin EE, Bodine DM, An X, Mohandas N, Low PS (2009) Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 114(9):1904–1912
Arber S, Hunter JJ, Ross J Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P (1997) MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88(3):393–403
Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D (2008) Polarity complex proteins. Biochim Biophys Acta 1778(3):614–630. doi:10.1016/j.bbamem.2007.08.029
Ayalon G, Davis JQ, Scotland PB, Bennett V (2008) An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135(7):1189–1200
Bagnato P, Barone V, Giacomello E, Rossi D, Sorrentino V (2003) Binding of an ankyrin-1 isoform to obscurin suggests a molecular link between the sarcoplasmic reticulum and myofibrils in striated muscles. J Cell Biol 160(2):245–253
Baines AJ (2006) A FERM-adjacent (FA) region defines a subset of the 4.1 superfamily and is a potential regulator of FERM domain function. BMC Genomics 7:85
Baines AJ (2009) Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans 37(Pt 4):796–803
Baines AJ, Pinder JC (2005) The spectrin-associated cytoskeleton in mammalian heart. Front Biosci 10:3020–3033
Banuelos S, Saraste M, Carugo KD (1998) Structural comparisons of calponin homology domains: implications for actin binding. Structure 6(11):1419–1431
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL (2002) The Pfam protein families database. Nucleic Acids Res 30(1):276–280
Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquetehrismann R, Prokop A, Bellen HJ (1996) A Drosophila neurexin is required for septate junction and blood–nerve barrier formation and function. Cell 87(6):1059–1068
Beaven GH, Jean-Baptiste L, Ungewickell E, Baines AJ, Shahbakhti F, Pinder JC, Lux SE, Gratzer WB (1985a) An examination of the soluble oligomeric complexes extracted from the red cell membrane and their relation to the membrane cytoskeleton. Eur J Cell Biol 36(2):299–306
Beaven GH, Jean-Baptiste L, Ungewickell E, Baines AJ, Shahbakhti F, Pinder JC, Lux SE, Gratzer WB (1985b) An examination of the soluble oligomeric complexes extracted from the red cell membrane and their relation to the membrane cytoskeleton. Eur J Cell Biol 36(2):299–306
Bel C, Oguievetskaia K, Pitaval C, Goutebroze L, Faivre-Sarrailh C (2009) Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J Cell Sci 122(Pt 18):3403–3413
Bennett V, Branton D (1977) Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem 252(8):2753–2763
Bennett V, Davis J (1981) Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc Natl Acad Sci USA 78(12):7550–7554
Bennett V, Stenbuck PJ (1979a) Identification and partial purification of ankyrin, the high affinity membrane attachment site for human erythrocyte spectrin. J Biol Chem 254(7):2533–2541
Bennett V, Stenbuck PJ (1979b) The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature 280(5722):468–473
Bennett V, Stenbuck PJ (1980a) Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem 255(13):6424–6432
Bennett V, Stenbuck PJ (1980b) Human erythrocyte ankyrin. Purification and properties. J Biol Chem 255(6):2540–2548
Bennett V, Davis J, Fowler WE (1982a) Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature 299(5879):126–131
Bennett V, Davis J, Fowler WE (1982b) Brain spectrin. A membrane associated protein related in structure and function to erythrocyte spectrin. Nature 299:126–131
Bennett PM, Baines AJ, Lecomte MC, Maggs AM, Pinder JC (2004) Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils. J Muscle Res Cell Motil 25(2):119–126
Bennett PM, Maggs AM, Baines AJ, Pinder JC (2006) The transitional junction: a new functional subcellular domain at the intercalated disc. Mol Biol Cell 17(4):2091–2100
Berghs S, Aggujaro D, Dirkx R Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M (2000) betaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol 151(5):985–1002
Biederer T, Sudhof TC (2001) Cask and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem 276(51):47869–47876
Bignone PA, Baines AJ (2003) Spectrin alphaII and betaII isoforms interact with high affinity at the tetramerization site. Biochem J 374(Pt 3):613–624
Bignone PA, King MD, Pinder JC, Baines AJ (2007) Phosphorylation of a threonine unique to the short C-terminal isoform of betaII-spectrin links regulation of alpha–beta spectrin interaction to neuritogenesis. J Biol Chem 282(2):888–896
Binda AV, Kabbani N, Lin R, Levenson R (2002) D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol 62(3):507–513
Blank ME, Ehmke H (2003) Aquaporin-1 and HCO3(-)-Cl- transporter-mediated transport of CO2 across the human erythrocyte membrane. J Physiol 550(Pt 2):419–429
Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B (2007) Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci 27(3):590–603
Borzok MA, Catino DH, Nicholson JD, Kontrogianni-Konstantopoulos A, Bloch RJ (2007) Mapping the binding site on small ankyrin 1 for obscurin. J Biol Chem 282(44):32384–32396. doi:10.1074/jbc.M704089200
Bourguignon LY, Lokeshwar VB, He J, Chen X, Bourguignon GJ (1992) A CD44-like endothelial cell transmembrane glycoprotein (GP116) interacts with extracellular matrix and ankyrin. Mol Cell Biol 12(10):4464–4471
Bourguignon LY, Jin H, Iida N, Brandt NR, Zhang SH (1993) The involvement of ankyrin in the regulation of inositol 1, 4, 5- trisphosphate receptor-mediated internal Ca2+ release from Ca2+ storage vesicles in mouse T-lymphoma cells. J Biol Chem 268(10):7290–7297
Bournier O, Kroviarski Y, Rotter B, Nicolas G, Lecomte MC, Dhermy D (2006) Spectrin interacts with EVL (enabled/vasodilator-stimulated phosphoprotein-like protein), a protein involved in actin polymerization. Biol Cell 98(5):279–293
Bouzidi M, Tricaud N, Giraud P, Kordeli E, Caillol G, Deleuze C, Couraud F, Alcaraz G (2002) Interaction of the Nav1.2a subunit of the voltage-dependent sodium channel with nodal ankyrinG. In vitro mapping of the interacting domains and association in synaptosomes. J Biol Chem 277(32):28996–29004
Bruce LJ, Ghosh S, King MJ, Layton DM, Mawby WJ, Stewart GW, Oldenborg PA, Delaunay J, Tanner MJ (2002) Absence of CD47 in protein 4.2-deficient hereditary spherocytosis in man: an interaction between the Rh complex and the band 3 complex. Blood 100(5):1878–1885
Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ (2003) A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101(10):4180–4188
Burns NR, Ohanian V, Gratzer WB (1983) Properties of brain spectrin (fodrin). FEBS Lett 153(1):165–168
Burridge K (1982) Nonerythroid spectrins: actin–membrane attachment proteins in many cell types. J Cell Biol 95:478–486
Byers TJ, Branton D (1985) Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci USA 82(18):6153–6157
Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A (2010) Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics 184(1):129–140
Carlin RK, Bartelt DC, Siekevitz P (1983) Identification of fodrin as a major calmodulin-binding protein in postsynaptic density preparations. J Cell Biol 96:443–448
Carugo KD, Banuelos S, Saraste M (1997) Crystal structure of a calponin homology domain. Nat Struct Biol 4(3):175–179
Chasis JA, Coulombel L, McGee S, Lee G, Tchernia G, Conboy J, Mohandas N (1996) Differential use of protein-4.1 Translation initiation sites during erythropoiesis—implications for a mutation-induced stage-specific deficiency of protein-4.1 during erythroid development. Blood 87(12):5324—5331
Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO (2000) Abnormal cardiac Na(+) channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circ Res 86(4):441–447
Chen L, Ong B, Bennett V (2001) LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol 154(4):841–855
Chen H, Khan AA, Liu F, Gilligan DM, Peters LL, Messick J, Haschek-Hock WM, Li X, Ostafin AE, Chishti AH (2007) Combined deletion of mouse dematin-headpiece and beta-adducin exerts a novel effect on the spectrin–actin junctions leading to erythrocyte fragility and hemolytic anemia. J Biol Chem 282(6):4124–4135
Chen TW, Chen G, Funkhouser LJ, Nam SC (2009) Membrane domain modulation by Spectrins in Drosophila photoreceptor morphogenesis. Genesis 47(11):744–750
Cherry L, Menhart N, Fung LW (1999) Interactions of the alpha-spectrin N-terminal region with beta-spectrin. Implications for the spectrin tetramerization reaction. J Biol Chem 274(4):2077–2084
Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Bretscher A, Fehon RG, Gusella AF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Arpin M, Louvard D, Tonks NK, Anderson JM, Fanning AS, Bryant PJ, Woods DF, Hoover KB (1998) The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 23(8):281–282
Chung HJ, Jan YN, Jan LY (2006) Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA 103(23):8870–8875
Cianci CD, Giorgi M, Morrow JS (1988) Phosphorylation of ankyrin down-regulates its cooperative interaction with spectrin and protein 3. J Cell Biochem 37(3):301–315
Cioe L, Laurila P, Meo P, Krebs K, Goodman S, Curtis PJ (1987) Cloning and nucleotide sequence of a mouse erythrocyte beta-spectrin cDNA. Blood 70(4):915–920
Cioffi DL, Wu S, Alexeyev M, Goodman SR, Zhu MX, Stevens T (2005) Activation of the endothelial store-operated ISOC Ca2+ channel requires interaction of protein 4.1 with TRPC4. Circ Res 97(11):1164–1172
Citterio L, Azzani T, Duga S, Bianchi G (1999) Genomic organization of the human gamma adducin gene. Biochem Biophys Res Commun 266(1):110–114
Clark MB, Ma Y, Bloom ML, Barker JE, Zagon IS, Zimmer WE, Goodman SR (1995) Brain alpha-erthroid spectrin—identification, compartmentalization, and beta-spectrin associations (vol 663, p 223, 1994). Brain Res 700(1–2):308–308
Cohen CM, Foley SF (1980) Spectrin-dependent and -independent association of F-actin with the erythrocyte membrane. J Cell Biol 86(2):694–698
Cohen CM, Jackson PL, Branton D (1978) Actin–membrane interactions: association of G-actin with the red cell membrane. J Supramol Struct 9(1):113–124
Cohen AM, Liu SC, Derick LH, Palek J (1986) Ultrastructural studies of the interaction of spectrin with phosphatidylserine liposomes. Blood 68(4):920–926
Coleman TR, Harris AS, Mische SM, Mooseker MS, Morrow JS (1987) Beta spectrin bestows protein 4.1 sensitivity on spectrin–actin interactions. J Cell Biol 104(3):519–526
Coleman SK, Cai C, Mottershead DG, Haapalahti JP, Keinanen K (2003) Surface expression of GluR-D AMPA receptor is dependent on an interaction between its C-terminal domain and a 4.1 protein. J Neurosci 23(3):798–806
Conboy J (1999) The role of alternative pre-mRNA splicing in regulating the structure and function of skeletal protein 4.1. Proc Soc Exp Biol Med 220(2):73–78
Conboy J, Kan YW, Shohet SB, Mohandas N (1986) Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci USA 83(24):9512–9516
Correas I, Avila J (1988) Erythrocyte protein-4.1 associates with tubulin. Biochem J 255(1):217–221
Correas I, Leto TL, Speicher DW, Marchesi VT (1986a) Identification of the functional site of erythrocyte protein 4.1 involved in spectrin–actin associations. J Biol Chem 261(7):3310–3315
Correas I, Speicher DW, Marchesi VT (1986b) Structure of the spectrin–actin binding-site of erythrocyte protein 4.1. J Biol Chem 261(28):3362–3366
Craig SW, Pardo JV (1983) Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil 3(5–6):449–462
Dahl KN, Parthasarathy R, Westhoff CM, Layton DM, Discher DE (2004) Protein 4.2 is critical to CD47–membrane skeleton attachment in human red cells. Blood 103(3):1131–1136
Das A, Base C, Manna D, Cho W, Dubreuil RR (2008) Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. J Biol Chem 283(18):12643–12653
Davis J, Bennett V (1983) Brain spectrin. Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem 258(12):7757–7766
Davis JQ, Bennett V (1984a) Brain ankyrin. A membrane-associated protein with binding sites for spectrin, tubulin, and the cytoplasmic domain of the erythrocyte anion channel. J Biol Chem 259(21):13550–13559
Davis JQ, Bennett V (1984b) Brain ankyrin. Purification of a 72, 000 Mr spectrin-binding domain. J Biol Chem 259(3):1874–1881
Davis JQ, Bennett V (1986) Association of brain ankyrin with brain membranes and isolation of active proteolytic fragments of membrane-associated ankyrin-binding protein(s). J Biol Chem 261(34):16198–16206
Davis JQ, Bennett V (1993) Ankyrin-binding activity of nervous system cell adhesion molecules expressed in adult brain. J Cell Sci Suppl 17:109–117
Davis JQ, Bennett V (1994) Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem 269(44):27163–27166
Davis J, Davis L, Bennett V (1989) Diversity in membrane binding sites of ankyrins. Brain ankyrin, erythrocyte ankyrin, and processed erythrocyte ankyrin associate with distinct sites in kidney microsomes. J Biol Chem 264(11):6417–6426
Davis L, Abdi K, Machius M, Brautigam C, Tomchick DR, Bennett V, Michaely P (2008) Localization and structure of the ankyrin-binding site on beta 2-spectrin. J Biol Chem
Del Rio M, Imam A, DeLeon M, Gomez G, Mishra J, Ma Q, Parikh S, Devarajan P (2004) The death domain of kidney ankyrin interacts with Fas and promotes Fas-mediated cell death in renal epithelia. J Am Soc Nephrol 15(1):41–51
Delhommeau F, Vasseur-Godbillon C, Leclerc P, Schischmanoff PO, Croisille L, Rince P, Moriniere M, Benz EJ Jr, Tchernia G, Tamagnini G, Ribeiro L, Delaunay J, Baklouti F (2002) A splicing alteration of 4.1R pre-mRNA generates 2 protein isoforms with distinct assembly to spindle poles in mitotic cells. Blood 100(7):2629–2636
Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA (2003) Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci 17(2):411–416
Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS (2003) Kv3.1b is a novel component of CNS nodes. J Neurosci 23(11):4509–4518
Devaux JJ, Kleopa KA, Cooper EC, Scherer SS (2004) KCNQ2 is a nodal K+ channel. J Neurosci 24(5):1236–1244
Discher D, Parra M, Conboy JG, Mohandas N (1993) Mechanochemistry of the alternatively spliced spectrin–actin binding domain in membrane skeletal protein 4.1. J Biol Chem 268(10):7186–7195
Discher DE, Winardi R, Schischmanoff PO, Parra M, Conboy JG, Mohandas N (1995) Mechanochemistry of protein 4.1 s spectrin–actin-binding domain—ternary complex interactions, membrane-binding, network integration, structural strengthening. J Cell Biol 130(4):897–907
Bodine DMt, Birkenmeier CS, Barker JE (1984) Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell 37(3):721–729
Doctor RB, Bennett V, Mandel LJ (1993) Degradation of spectrin and ankyrin in the ischemic rat kidney. Am J Physiol 264(4 Pt 1):C1003–C1013
Dodge JT, Mitchell C, Hanahan DJ (1963) The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100:119–130
Dubreuil RR, MacVicar G, Dissanayake S, Liu C, Homer D, Hortsch M (1996) Neuroglian-mediated cell adhesion induces assembly of the membrane skeleton at cell contact sites. J Cell Biol 133(3):647–655
Dzhashiashvili Y, Zhang Y, Galinska J, Lam I, Grumet M, Salzer JL (2007) Nodes of Ranvier and axon initial segments are ankyrin G-dependent domains that assemble by distinct mechanisms. J Cell Biol 177(5):857–870
Eber S, Lux SE (2004) Hereditary spherocytosis-defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin Hematol 41(2):118–141
Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G (2006) Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20(12):1974–1981
Endeward V, Cartron JP, Ripoche P, Gros G (2008) RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22(1):64–73
Fairbanks G, Steck TL, Wallach DF (1971) Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry 10(13):2606–2617
Fehon RG, Dawson IA, Artavanistsakonas S (1994) A Drosophila homolog of membrane–skeleton protein–4.1 is associated with septate junctions and is encoded by the coracle gene. Development 120(3):545–557
Flick MJ, Konieczny SF (2000) The muscle regulatory and structural protein MLP is a cytoskeletal binding partner of betaI-spectrin. J Cell Sci 113(Pt 9):1553–1564
Fowler VM (1990) Tropomodulin: a cytoskeletal protein that binds to the end of erythrocyte tropomyosin and inhibits tropomyosin binding to actin. J Cell Biol 111(2):471–481
Fowler VM, Bennett V (1984) Erythrocyte membrane tropomyosin. Purification and properties. J Biol Chem 259(9):5978–5989
Fowler V, Taylor DL (1980) Spectrin plus band 4.1 cross-link actin. Regulation by micromolar calcium. J Cell Biol 85(2):361–376
Fowler VM, Luna EJ, Hargreaves WR, Taylor DL, Branton D (1981) Spectrin promotes the association of F-actin with the cytoplasmic surface of the human erythrocyte membrane. J Cell Biol 88(2):388–395
Gagelin C, Constantin B, Deprette C, Ludosky MA, Recouvreur M, Cartaud J, Cognard C, Raymond G, Kordeli E (2002) Identification of Ank(G107), a muscle-specific ankyrin-G isoform. J Biol Chem 277(15):12978–12987
Gallagher PG (2004) Hereditary elliptocytosis: spectrin and protein 4.1R. Semin Hematol 41(2):142–164
Gardner K, Bennett V (1986) A new erythrocyte membrane-associated protein with calmodulin-binding activity: identification and purification. J Biol Chem 261:1339–1348
Gardner K, Bennett V (1987) Modulation of spectrin–actin assembly by erythrocyte adducin. Nature 328(6128):359–362
Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B (2003) A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300(5628):2091–2094
Gascard P, Cohen CM (1994) Absence of high-affinity band 4.1 binding sites from membranes of glycophorin C- and D-deficient (leach phenotype) erythrocytes. Blood 83(4):1102–1108
Gascard P, Lee G, Coulombel L, Auffray I, Lum M, Parra M, Conboy JG, Mohandas N, Chasis JA (1998) Characterization of multiple isoforms of protein 4.1R expressed during erythroid terminal differentiation. Blood 92(11):4404–4414
Gascard P, Nunomura W, Lee G, Walensky LD, Krauss SW, Takakuwa Y, Chasis JA, Mohandas N, Conboy JG (1999) Deciphering the nuclear import pathway for the cytoskeletal red cell protein 4.1R. Mol Biol Cell 10(6):1783–1798
Gascard P, Parra MK, Zhao Z, Calinisan VR, Nunomura W, Rivkees SA, Mohandas N, Conboy JG (2004) Putative tumor suppressor protein 4.1B is differentially expressed in kidney and brain via alternative promoters and 5' alternative splicing. Biochim Biophys Acta 1680(2):71–82
Genova JL, Fehon RG (2003) Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol 161(5):979–989. Epub 2003 Jun 2002
Gilligan DM, Lozovatsky L, Gwynn B, Brugnara C, Mohandas N, Peters LL (1999) Targeted disruption of the beta adducin gene (Add2) causes red blood cell spherocytosis in mice. Proc Natl Acad Sci USA 96(19):10717–10722
Gimm JA, An X, Nunomura W, Mohandas N (2002) Functional characterization of spectrin–actin-binding domains in 4.1 family of proteins. Biochemistry 41(23):7275–7282
Girault JA, Oguievetskaia K, Carnaud M, Denisenko-Nehrbass N, Goutebroze L (2003) Transmembrane scaffolding proteins in the formation and stability of nodes of Ranvier. Biol Cell 95(7):447–452
Glele-Kakai C, Garbarz M, Lecomte MC, Leborgne S, Galand C, Bournier O, Devaux I, Gautero H, Zohoun I, Gallagher PG, Forget BG, Dhermy D (1996) Epidemiological studies of spectrin mutations related to hereditary elliptocytosis and spectrin polymorphisms in Benin. Br J Haematol 95(1):57–66
Glenney JR Jr, Glenney P (1984) Comparison of spectrin isolated from erythroid and non-erythroid sources. Eur J Biochem 144(3):529–539
Glenney JR, Glenney P, Weber K (1982a) Erythroid spectrin, brain fodrin, and intestinal brush-border proteins (tw-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci USA Biol Sci 79(13):4002–4005
Glenney JR Jr, Glenney P, Osborn M, Weber K (1982b) An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell 28(4):843–854
Glenney JR Jr, Glenney P, Weber K (1982c) Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci USA 79(13):4002–4005
Glenney JR Jr, Glenney P, Weber K (1982d) F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem 257(16):9781–9787
Gobel V, Barrett PL, Hall DH, Fleming JT (2004) Lumen morphogenesis in C. elegans requires the membrane–-cytoskeleton linker erm-1. Dev Cell 6(6):865–873. doi:10.1016/j.devcel.2004.05.018
Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E (2002) Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol 157(7):1247–1256. Epub 2002 Jun 1224
Goodman SR, Zagon IS, Kulikowski RR (1981) Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci USA 78(12):7570–7574
Gratzer WB, Beaven GH (1975) Properties of the high-molecular-weight protein (spectrin) from human-erythrocyte membranes. Eur J Biochem 58(2):403–409
Greene LA, Tischler AS (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73(7):2424–2428
Gutmann DH, Donahoe J, Perry A, Lemke N, Gorse K, Kittiniyom K, Rempel SA, Gutierrez JA, Newsham IF (2000) Loss of DAL-1, a protein 4.1-related tumor suppressor, is an important early event in the pathogenesis of meningiomas. Hum Mol Genet 9(10):1495–1500
Haest CW, Plasa G, Kamp D, Deuticke B (1978) Spectrin as a stabilizer of the phospholipid asymmetry in the human erythrocyte membrane. Biochim Biophys Acta 509(1):21–32
Hammarlund M, Davis WS, Jorgensen EM (2000) Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J Cell Biol 149(4):931–942
Hammarlund M, Jorgensen EM, Bastiani MJ (2007) Axons break in animals lacking beta-spectrin. J Cell Biol 176(3):269–275
Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK (2000a) Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol 7(10):871–875
Han BG, Nunomura W, Takakuwa Y, Mohandas N, Jap BK (2000b) Protein 4.1R core domain structure and insights into regulation of cytoskeletal organization. Nat Struct Biol 7(10):871–875
Hassoun H, Hanada T, Lutchman M, Sahr KE, Palek J, Hanspal M, Chishti AH (1998) Complete deficiency of glycophorin A in red blood cells from mice with targeted inactivation of the band 3 (AE1) gene. Blood 91(6):2146–2151
Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, Kordeli E, Baines AJ (2000) Identification of a novel C-terminal variant of beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci 113(Pt 11):2023–2034
Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN (2007) Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J Cell Biol 178(5):875–886
Hedstrom KL, Ogawa Y, Rasband MN (2008) AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol 183(4):635–640
Hilenski LL, Ma XH, Vinson N, Terracio L, Borg TK (1992) The role of beta 1 integrin in spreading and myofibrillogenesis in neonatal rat cardiomyocytes in vitro. Cell Motil Cytoskeleton 21(2):87–100
Hill AS, Nishino A, Nakajo K, Zhang G, Fineman JR, Selzer ME, Okamura Y, Cooper EC (2008) Ion channel clustering at the axon initial segment and node of Ranvier evolved sequentially in early chordates. PLoS Genet 4(12):e1000317
Hiller G, Weber K (1977) Spectrin is absent in various tissue culture cells. Nature 266(5598):181–183
Hoover KB, Bryant PJ (2000) The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr Opin Cell Biol 12(2):229–234
Hopitzan AA, Baines AJ, Ludosky MA, Recouvreur M, Kordeli E (2005) Ankyrin-G in skeletal muscle: tissue-specific alternative splicing contributes to the complexity of the sarcolemmal cytoskeleton. Exp Cell Res 309(1):86–98
Hopitzan AA, Baines AJ, Kordeli E (2006) Molecular evolution of ankyrin: gain of function in vertebrates by acquisition of an obscurin/titin-binding-related domain. Mol Biol Evol 23(1):46–55
Horresh I, Poliak S, Grant S, Bredt D, Rasband MN, Peles E (2008) Multiple molecular interactions determine the clustering of Caspr2 and Kv1 channels in myelinated axons. J Neurosci 28(52):14213–14222
Horresh I, Bar V, Kissil JL, Peles E (2010) Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci 30(7):2480–2489
Hortsch M, Paisley KL, Tian MZ, Qian M, Bouley M, Chandler R (2002) The axonal localization of large Drosophila ankyrin2 protein isoforms is essential for neuronal functionality. Mol Cell Neurosci 20(1):43–55
Howe CL, Sacramone LM, Mooseker MS, Morrow JS (1985) Mechanisms of cytoskeletal regulation: modulation of membrane affinity in avian brush border and erythrocyte spectrins. J Cell Biol 101(4):1379–1385
Hu RJ, Moorthy S, Bennett V (1995) Expression of functional domains of beta G-spectrin disrupts epithelial morphology in cultured cells. J Cell Biol 128(6):1069–1080
Hund TJ, Mohler PJ (2008) Ankyrin-based targeting pathway regulates human sinoatrial node automaticity. Channels (Austin) 2(6):404–406
Hung LY, Tang CJ, Tang TK (2000) Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol 20(20):7813–7825
Husain-Chishti A, Faquin W, Wu CC, Branton D (1989) Purification of erythrocyte dematin (protein 4.9) reveals an endogenous protein kinase that modulates actin-bundling activity. J Biol Chem 264(15):8985–8991
Inaba M, Gupta KC, Kuwabara M, Takahashi T, Benz EJ Jr, Maede Y (1992) Deamidation of human erythrocyte protein 4.1: possible role in aging. Blood 79(12):3355–3361
Ipsaro JJ, Huang L, Mondragon A (2009) Structures of the spectrin–ankyrin interaction binding domains. Blood 113(22):5385–5389
Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragon A, Speicher DW (2010) Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood Mar 2. [Epub ahead of print] doi:10.1182/blood-2010-1101-261396
Isayama T, Goodman SR, Zagon IS (1993) Localization of spectrin isoforms in the adult mouse heart. Cell Tissue Res 274(1):127–133
Jenkins SM, Bennett V (2001) Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol 155(5):739–746
Jenkins SM, Bennett V (2002) Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc Natl Acad Sci USA 99(4):2303–2308
Johnson CP, Gaetani M, Ortiz V, Bhasin N, Harper S, Gallagher PG, Speicher DW, Discher DE (2007a) Pathogenic proline mutation in the linker between spectrin repeats: disease caused by spectrin unfolding. Blood 109(8):3538–3543
Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE (2007b) Forced unfolding of proteins within cells. Science 317(5838):663–666
Jons T, Drenckhahn D (1992) Identification of the binding interface involved in linkage of cytoskeletal protein 4.1 to the erythrocyte anion exchanger. EMBO J 11(8):2863–2867
Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V (1991) Primary structure and domain organization of human alpha and beta adducin. J Cell Biol 115(3):665–675
Kaiser HW, O’Keefe E, Bennett V (1989) Adducin: Ca++-dependent association with sites of cell–cell contact. J Cell Biol 109(2):557–569
Kakiuchi S, Sobue K, Morimoto K, Kanda K (1982) A spectrin-like calmodulin-binding protein (calspectin) of brain. Biochem Int 5(6):755–762
Kang Q, Wang T, Zhang H, Mohandas N, An X (2009) A Golgi-associated protein 4.1B variant is required for assimilation of proteins in the membrane. J Cell Sci 122(Pt 8):1091–1099
Karinch AM, Zimmer WE, Goodman SR (1990) The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem 265(20):11833–11840
Kasahara M (2007) The 2R hypothesis: an update. Curr Opin Immunol 19(5):547–552
Katagiri T, Ozaki K, Fujiwara T, Shimizu F, Kawai A, Okuno S, Suzuki M, Nakamura Y, Takahashi E, Hirai Y (1996) Cloning, expression and chromosome mapping of adducin-like 70 (ADDL), a human cDNA highly homologous to human erythrocyte adducin. Cytogenet Cell Genet 74(1–2):90–95
Kennedy SP, Warren SL, Forget BG, Morrow JS (1991) Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin. J Cell Biol 115(1):267–277
Khan AA, Hanada T, Mohseni M, Jeong JJ, Zeng L, Gaetani M, Li D, Reed BC, Speicher DW, Chishti AH (2008) Dematin and adducin provide a novel link between the spectrin cytoskeleton and human erythrocyte membrane by directly interacting with glucose transporter-1. J Biol Chem 283(21):14600–14609
Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, Iwamatsu A, Kaibuchi K (1998) Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem 273(10):5542–5548
King N (2005) Choanoflagellates. Curr Biol 15(4):R113–R114
King N, Hittinger CT, Carroll SB (2003) Evolution of key cell signaling and adhesion protein families predates animal origins. Science 301(5631):361–363
King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, Fairclough S, Hellsten U, Isogai Y, Letunic I, Marr M, Pincus D, Putnam N, Rokas A, Wright KJ, Zuzow R, Dirks W, Good M, Goodstein D, Lemons D, Li W, Lyons JB, Morris A, Nichols S, Richter DJ, Salamov A, Sequencing JG, Bork P, Lim WA, Manning G, Miller WT, McGinnis W, Shapiro H, Tjian R, Grigoriev IV, Rokhsar D (2008) The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451(7180):783–788
Kirkpatrick FH (1976) Interaction of spectrin with muscle actin detected by spin labelling. Biochem Biophys Res Commun 69(1):225–229
Kizhatil K, Bennett V (2004) Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. J Biol Chem 279(16):16706–16714
Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V (2007a) Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 282(36):26552–26561
Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V (2007b) Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem 282(3):2029–2037
Komada M, Soriano P (2002) [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol 156(2):337–348
Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ (2003) Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell 14(3):1138–1148
Kordeli E, Lambert S, Bennett V, Ankyrin G (1995) A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem 270(5):2352–2359
Korsgren C, Peters LL, Lux SE (2009) Protein 4.2 binds to the carboxyterminal EF-hands of erythroid alpha spectrin in a calcium and calmodulin dependent manner. J Biol Chem
Kotula L, DeSilva TM, Speicher DW, Curtis PJ (1993) Functional characterization of recombinant human red cell alpha-spectrin polypeptides containing the tetramer binding site. J Biol Chem 268(20):14788–14793
Krauss SW, Lee G, Chasis JA, Mohandas N, Heald R (2004) Two protein 4.1 domains essential for mitotic spindle and aster microtubule dynamics and organization in vitro. J Biol Chem 279(26):27591–27598
Kuhlman PA (2000) Characterization of the actin filament capping state in human erythrocyte ghost and cytoskeletal preparations. Biochem J 349(Pt 1):105–111
Kuhlman PA, Fowler VM (1997) Purification and characterization of an alpha 1 beta 2 isoform of CapZ from human erythrocytes: cytosolic location and inability to bind to Mg2+ ghosts suggest that erythrocyte actin filaments are capped by adducin. Biochemistry 36(44):13461–13472. doi:10.1021/bi970601b
Kuhlman PA, Hughes CA, Bennett V, Fowler VM (1996) A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem 271(14):7986–7991
Kustu S, Inwood W (2006) Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol 13(1–2):103–110
Kusunoki H, MacDonald RI, Mondragon A (2004) Structural insights into the stability and flexibility of unusual erythroid spectrin repeats. Structure (Camb) 12(4):645–656
Lambert S, Davis JQ, Bennett V (1997) Morphogenesis of the node of Ranvier: co-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci 17(18):7025–7036
Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U (2009) Yurt, coracle, neurexin IV and the Na(+), K(+)-ATPase form a novel group of epithelial polarity proteins. Nature 459(7250):1141–1145
Lazarides E, Nelson WJ (1982) Expression of spectrin in nonerythroid cells. Cell 31:505–508
Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ (2008) Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 105(40):15617–15622
Leclerc E, Vetter S (1998) Characterization of a calcium-dependent calmodulin-binding domain in the 135-kD human protein 4.1 isoform. Eur J Biochem 258(2):567–571
Lee JK, Coyne RS, Dubreuil RR, Goldstein LS, Branton D (1993) Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol 123(6 Pt 2):1797–1809
Lee HG, Zarnescu DC, MacIver B, Thomas GH (2010) The cell adhesion molecule Roughest depends on beta(heavy)-spectrin during eye morphogenesis in Drosophila. J Cell Sci 123(Pt 2):277–285
Legendre K, Safieddine S, Kussel-Andermann P, Petit C, El-Amraoui A (2008) alphaII-betaV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. J Cell Sci 121(Pt 20):3347–3356
Lemaillet G, Walker B, Lambert S (2003) Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem 278(30):27333–27339
Lencesova L, O’Neill A, Resneck WG, Bloch RJ, Blaustein MP (2004) Plasma membrane–cytoskeleton–endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem 279(4):2885–2893
Levine J, Willard M (1981) Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol 90:631–643
Li X, Matsuoka Y, Bennett V (1998) Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS-related domain and a newly defined oligomerization domain. J Biol Chem 273(30):19329–19338
Li J, Mahajan A, Tsai MD (2006) Ankyrin repeat: a unique motif mediating protein–protein interactions. Biochemistry 45(51):15168–15178
Lin B, Nasir J, McDonald H, Graham R, Rommens JM, Goldberg YP, Hayden MR (1995) Genomic organization of the human alpha-adducin gene and its alternately spliced isoforms. Genomics 25(1):93–99
Ling E, Gardner K, Bennett V (1986) Protein kinase C phosphorylates a recently identified membrane skeleton- associated calmodulin-binding protein in human erythrocytes. J Biol Chem 261(30):13875–13878
Linnenbach AJ, Speicher DW, Marchesi VT, Forget BG (1986) Cloning of a portion of the chromosomal gene for human erythrocyte alpha-spectrin by using a synthetic gene fragment. Proc Natl Acad Sci USA 83(8):2397–2401
Liu SC, Derick LH, Palek J (1987) Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J Cell Biol 104(3):527–536
Liu SC, Derick LH, Agre P, Palek J (1990a) Alteration of the erythrocyte membraneskeletal ultrastructure in hereditary spherocytosis, hereditary elliptocytosis, and pyropoikilocytosis. Blood 76(1):198–205
Liu SC, Zhai S, Palek J, Golan DE, Amato D, Hassan K, Nurse GT, Babona D, Coetzer T, Jarolim P et al (1990b) Molecular defect of the band 3 protein in southeast Asian ovalocytosis. N Engl J Med 323(22):1530–1538
Lopez C, Metral S, Eladari D, Drevensek S, Gane P, Chambrey R, Bennett V, Cartron JP, Le Van KC, Colin Y (2005) The ammonium transporter RhBG: requirement of a tyrosine-based signal and ankyrin-G for basolateral targeting and membrane anchorage in polarized kidney epithelial cells. J Biol Chem 280(9):8221–8228
Lowe JS, Palygin O, Bhasin N, Hund TJ, Boyden PA, Shibata E, Anderson ME, Mohler PJ (2008) Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol 180(1):173–186
Lu D, Yan H, Othman T, Rivkees SA (2004a) Cytoskeletal protein 4.1G is a binding partner of the metabotropic glutamate receptor subtype 1alpha. J Neurosci Res 78(1):49–55
Lu D, Yan H, Othman T, Turner CP, Woolf T, Rivkees SA (2004b) Cytoskeletal protein 4.1G binds to the third intracellular loop of the A1 adenosine receptor and inhibits receptor action. Biochem J 377(Pt 1):51–59
Lue RA, Marfatia SM, Branton D, Chishti AH (1994) Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA 91(21):9818–9822
Luque CM, Correas I (2000) A constitutive region is responsible for nuclear targeting of 4.1R: modulation by alternative sequences results in differential intracellular localization. J Cell Sci 113(Pt 13):2485–2495
Luque CM, Lallena MJ, Alonso MA, Correas I (1998) An alternative domain determines nuclear localization in multifunctional protein 4.1. J Biol Chem 273(19):11643–11649
Luque CM, Lallena MJ, Perez-Ferreiro CM, de Isidro Y, De Carcer G, Alonso MA, Correas I (1999) The N-terminal 209-aa domain of high molecular-weight 4.1R isoforms abrogates 4.1R targeting to the nucleus. Proc Natl Acad Sci USA 96(26):14925–14930
Lux SE, John KM, Bennett V (1990) Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature 344(6261):36–42
Macias MJ, Musacchio A, Ponstingl H, Nilges M, Saraste M, Oschkinat H (1994) Structure of the pleckstrin homology domain from beta-spectrin. Nature 369(6482):675–677
Manno S, Takakuwa Y, Nagao K, Mohandas N (1995) Modulation of erythrocyte membrane mechanical function by beta-spectrin phosphorylation and dephosphorylation. J Biol Chem 270(10):5659–5665
Manno S, Takakuwa Y, Mohandas N (2002) Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine–skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA 99(4):1943–1948
Manno S, Takakuwa Y, Mohandas N (2005) Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem 280(9):7581–7587
Marchesi VT, Steers E Jr (1968) Selective solubilization of a protein component of the red cell membrane. Science 159(811):203–204
Mariani M, Maretzki D, Lutz HU (1993) A tightly membrane-associated subpopulation of spectrin is 3H-palmitoylated. J Biol Chem 268(17):12996–13001
Matsuoka Y, Hughes CA, Bennett V (1996) Adducin regulation. Definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem 271(41):25157–25166
Matsuoka Y, Li X, Bennett V (1998) Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin–actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol 142(2):485–497
Matsuoka Y, Li X, Bennett V (2000) Adducin: structure, function and regulation. Cell Mol Life Sci 57(6):884–895
Mattagajasingh SN, Huang SC, Hartenstein JS, Snyder M, Marchesi VT, Benz EJ (1999) A nonerythroid isoform of protein 4.1R interacts with the nuclear mitotic apparatus (NuMA) protein. J Cell Biol 145(1):29–43
Maximov A, Tang TS, Bezprozvanny I (2003) Association of the type 1 inositol (1, 4, 5)-trisphosphate receptor with 4.1N protein in neurons. Mol Cell Neurosci 22(2):271–283
McKeown C, Praitis V, Austin J (1998) sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development 125(11):2087–2098
Meary F, Metral S, Ferreira C, Eladari D, Colin Y, Lecomte MC, Nicolas G (2007) A mutant alphaII-spectrin designed to resist calpain and caspase cleavage questions the functional importance of this process in vivo. J Biol Chem 282(19):14226–14237
Medina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A (2002) Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol 158(5):941–951
Menegoz M, Gaspar P, LeBert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Amos F, Girault JA (1997) Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron 19(2):319–331
Merilainen J, Palovuori R, Sormunen R, Wasenius VM, Lehto VP (1993) Binding of the alpha-fodrin SH3 domain to the leading lamellae of locomoting chicken fibroblasts. J Cell Sci 105(Pt 3):647–654
Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte M-C (2009) {alpha}II-Spectrin is critical for cell adhesion and cell cycle. J Biol Chem 284(4):2409–2418. doi:10.1074/jbc.M801324200
Michaely P, Tomchick DR, Machius M, Anderson RG (2002) Crystal structure of a 12 ANK repeat stack from human ankyrinR. EMBO J 21(23):6387–6396
Michalak K, Bobrowska M, Sikorski AF (1993) Interaction of bovine erythrocyte spectrin with aminophospholipid liposomes. Gen Physiol Biophys 12(2):163–170
Mische SM, Mooseker MS, Morrow JS (1987) Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin–actin binding. J Cell Biol 105(6 Pt 1):2837–2845
Mishra L, Cai T, Yu P, Monga SP, Mishra B (1999) Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene 18(2):353–364
Missner A, Kugler P, Saparov SM, Sommer K, Mathai JC, Zeidel ML, Pohl P (2008) Carbon dioxide transport through membranes. J Biol Chem 283(37):25340–25347
Mohandas N, Winardi R, Knowles D, Leung A, Parra M, George E, Conboy J, Chasis J (1992) Molecular basis for membrane rigidity of hereditary ovalocytosis. A novel mechanism involving the cytoplasmic domain of band 3. J Clin Invest 89(2):686–692
Mohler PJ, Bennett V (2005) Ankyrin-based cardiac arrhythmias: a new class of channelopathies due to loss of cellular targeting. Curr Opin Cardiol 20(3):189–193
Mohler PJ, Gramolini AO, Bennett V (2002) The ankyrin-B C-terminal domain determines activity of ankyrin-B/G chimeras in rescue of abnormal inositol 1, 4, 5-trisphosphate and ryanodine receptor distribution in ankyrin-B (-/-) neonatal cardiomyocytes. J Biol Chem 277(12):10599–10607
Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V (2003) Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421(6923):634–639
Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V (2004a) Inositol 1, 4, 5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem 279(13):12980–12987
Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V (2004b) Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA 101(50):17533–17538
Mohler PJ, Davis JQ, Bennett V (2005) Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3(12):e423
Mombers C, Verkleij AJ, de Gier J, van Deenen LL (1979) The interaction of spectrin–actin and synthetic phospholipids. II. The interaction with phosphatidylserine. Biochim Biophys Acta 551(2):271–281
Moorthy S, Chen L, Bennett V (2000) Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J Cell Biol 149(4):915–930
Morrow JS, Marchesi VT (1981) Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol 88(2):463–468
Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M (1989) Ankyrin links fodrin to the alpha subunit of Na, K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol 108(2):455–465
Muro AF, Marro ML, Gajovic S, Porro F, Luzzatto L, Baralle FE (2000) Mild spherocytic hereditary elliptocytosis and altered levels of alpha- and gamma-adducins in beta-adducin-deficient mice. Blood 95(12):3978–3985
Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M (1992) Crystal structure of a Src-homology 3 (SH3) domain. Nature 359(6398):851–855
Nakhoul NL, Davis BA, Romero MF, Boron WF (1998) Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol 274(2 Pt 1):C543–C548
Nedrelow JH, Cianci CD, Morrow JS (2003) c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem 278(9):7735–7741
Nelson WJ, Hammerton RW (1989) A membrane–cytoskeletal complex containing Na+, K + -ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol 108(3):893–902
Nelson WJ, Veshnock PJ (1987) Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 328(6130):533–536
Nelson WJ, Shore EM, Wang AZ, Hammerton RW (1990) Identification of a membrane–cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin- Darby canine kidney epithelial cells. J Cell Biol 110(2):349–357
Nicolas G, Pedroni S, Fournier C, Gautero H, Craescu C, Dhermy D, Lecomte MC (1998) Spectrin self-association site: characterization and study of beta-spectrin mutations associated with hereditary elliptocytosis. Biochem J 332(Pt 1):81–89
Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B, Lecomte MC (2002) Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol 22(10):3527–3536
Nicolas V, Le Van KC, Gane P, Birkenmeier C, Cartron JP, Colin Y, Mouro-Chanteloup I (2003) Rh-RhAG/ankyrin-R, a new interaction site between the membrane bilayer and the red cell skeleton, is impaired by Rh(null)-associated mutation. J Biol Chem 278(28):25526–25533
Nishimura K, Yoshihara F, Tojima T, Ooashi N, Yoon W, Mikoshiba K, Bennett V, Kamiguchi H (2003) L1-dependent neuritogenesis involves ankyrinB that mediates L1-CAM coupling with retrograde actin flow. J Cell Biol 163(5):1077–1088
Nishimura K, Akiyama H, Komada M, Kamiguchi H (2007) betaIV-spectrin forms a diffusion barrier against L1CAM at the axon initial segment. Mol Cell Neurosci 34(3):422–430
Norman KR, Moerman DG (2002) Alpha spectrin is essential for morphogenesis and body wall muscle formation in Caenorhabditis elegans. J Cell Biol 157(4):665–677
Nunomura W, Takakuwa Y (2006) Regulation of protein 4.1R interactions with membrane proteins by Ca2+ and calmodulin. Front Biosci 11:1522–1539
Nunomura W, Takakuwa Y, Tokimitsu R, Krauss SW, Kawashima M, Mohandas N (1997) Regulation of CD44–protein 4.1 interaction by Ca2+ and calmodulin. Implications for modulation of CD44–ankyrin interaction. J Biol Chem 272(48):30322–30328
Nunomura W, Parra M, Hebiguchi M, Sawada KI, Mohandas N, Takakuwa Y (2009) Marked difference in membrane protein binding properties of the two isoforms of protein 4.1R expressed at early and late stages of erythroid differentiation. Biochem J 417(1):141–148
Odell AF, Van Helden DF, Scott JL (2008) The spectrin cytoskeleton influences the surface expression and activation of human transient receptor potential channel 4 channels. J Biol Chem 283(7):4395–4407
Ogawa Y, Rasband MN (2008) The functional organization and assembly of the axon initial segment. Curr Opin Neurobiol 18(3):307–313
Ogawa Y, Rasband MN (2009) Proteomic analysis of optic nerve lipid rafts reveals new paranodal proteins. J Neurosci Res 87(15):3502–3510
Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN (2006) Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci 26(19):5230–5239
Ohanian V, Wolfe LC, John KM, Pinder JC, Lux SE, Gratzer WB (1984) Analysis of the ternary interaction of the red cell membrane skeletal proteins spectrin, actin, and 4.1. Biochemistry 23(19):4416–4420
Otto E, Kunimoto M, McLaughlin T, Bennett V (1991) Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol 114(2):241–253
Palfrey HC, Waseem A (1985) Protein kinase C in the human erythrocyte. Translocation to the plasma membrane and phosphorylation of bands 4.1 and 4.9 and other membrane proteins. J Biol Chem 260(29):16021–16029
Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC (2006) A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26(10):2599–2613
Pardo JV, Siliciano JD, Craig SW (1983a) A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80(4):1008–1012
Pardo JV, Siliciano JD, Craig SW (1983b) Vinculin is a component of an extensive network of myofibril-sarcolemma attachment regions in cardiac muscle fibers. J Cell Biol 97(4):1081–1088
Parkinson NJ, Olsson CL, Hallows JL, McKee-Johnson J, Keogh BP, Noben-Trauth K, Kujawa SG, Tempel BL (2001) Mutant beta-spectrin 4 causes auditory and motor neuropathies in quivering mice. Nat Genet 29(1):61–65
Parra M, Gascard P, Walensky LD, Snyder SH, Mohandas N, Conboy JG (1998) Cloning and characterization of 4.1G (EPB41L2), a new member of the skeletal protein 4.1 (EPB41) gene family. Genomics 39:298–306
Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA, Snyder SH, Mohandas N, Conboy JG (2000) Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J Biol Chem 275(5):3247–3255
Parra M, Gee S, Chan N, Ryaboy D, Dubchak I, Mohandas N, Gascard PD, Conboy JG (2004) Differential domain evolution and complex RNA processing in a family of paralogous EPB41 (protein 4.1) genes facilitate expression of diverse tissue-specific isoforms. Genomics 84(4):637–646
Pascual J, Pfuhl M, Rivas G, Pastore A, Saraste M (1996) The spectrin repeat folds into a three-helix bundle in solution. FEBS Lett 383(3):201–207
Pasternack GR, Anderson RA, Leto TL, Marchesi VT (1985) Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem 260(6):3676–3683
Paw BH, Davidson AJ, Zhou Y, Li R, Pratt SJ, Lee C, Trede NS, Brownlie A, Donovan A, Liao EC, Ziai JM, Drejer AH, Guo W, Kim CH, Gwynn B, Peters LL, Chernova MN, Alper SL, Zapata A, Wickramasinghe SN, Lee MJ, Lux SE, Fritz A, Postlethwait JH, Zon LI (2003) Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat Genet 34(1):59–64
Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, Mohandas N, An X (2007a) The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood 110(3):1036–1042
Pei X, Guo X, Coppel R, Mohandas N, An X (2007b) Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) destabilizes erythrocyte membrane skeleton. J Biol Chem 282(37):26754–26758
Perez-Ferreiro CM, Luque CM, Correas I (2001) 4.1R proteins associate with interphase microtubules in human T cells: a 4.1R constitutive region is involved in tubulin binding. J Biol Chem 276(48):44785–44791
Perez-Ferreiro CM, Vernos I, Correas I (2004) Protein 4.1R regulates interphase microtubule organization at the centrosome. J Cell Sci 117(Pt 25):6197–6206
Peters LL, John KM, Lu FM, Eicher EM, Higgins A, Yialamas M, Turtzo LC, Otsuka AJ, Lux SE (1995) Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J Cell Biol 130(2):313–330
Petrella LN, Smith-Leiker T, Cooley L (2007) The Ovhts polyprotein is cleaved to produce fusome and ring canal proteins required for Drosophila oogenesis. Development 134(4):703–712
Phillips MD, Thomas GH (2006) Brush border spectrin is required for early endosome recycling in Drosophila. J Cell Sci 119(Pt 7):1361–1370
Pielage J, Fetter RD, Davis GW (2006) A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol 175(3):491–503
Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW (2008) A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron 58(2):195–209
Pinder JC, Baines AJ (2000) A protein accumulator. Nature 406(6793):253–254
Pinder JC, Gratzer WB (1983) Structural and dynamic states of actin in the erythrocyte. J Cell Biol 96(3):768–775
Pinder JC, Bray D, Gratzer WB (1975) Actin polymerisation induced by spectrin. Nature 258(5537):765–766
Pinder JC, Phethean J, Gratzer WB (1978a) Spectrin in primitive erythrocytes. FEBS Lett 92(2):278–282
Pinder JC, Ungewickell E, Bray D, Gratzer WB (1978b) The spectrin–actin complex and erythrocyte shape. J Supramol Struct 8(4):439–445
Pinder JC, Ohanian V, Gratzer WB (1984) Spectrin and protein 4.1 as an actin filament capping complex. FEBS Lett 169(2):161–164
Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E (1999) Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron 24(4):1037–1047
Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E (2001) Localization of Caspr2 in myelinated nerves depends on axon–glia interactions and the generation of barriers along the axon. J Neurosci 21(19):7568–7575
Porter NC, Resneck WG, O’Neill A, Van Rossum DB, Stone MR, Bloch RJ (2005) Association of small ankyrin 1 with the sarcoplasmic reticulum. Mol Membr Biol 22(5):421–432. doi:10.1080/09687860500244262
Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS (2001) alpha -Catenin binds directly to spectrin and facilitates spectrin–membrane assembly in vivo. J Biol Chem 276(6):4175–4181
Praitis V, Ciccone E, Austin J (2005) SMA-1 spectrin has essential roles in epithelial cell sheet morphogenesis in C-elegans. Dev Biol 283(1):157–170
Prasad GV, Coury LA, Finn F, Zeidel ML (1998) Reconstituted aquaporin 1 water channels transport CO2 across membranes. J Biol Chem 273(50):33123–33126
Preston GM, Agre P (1991) Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci USA 88(24):11110–11114
Preston GM, Carroll TP, Guggino WB, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256(5055):385–387
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS (2007) Sea Anemone Genome Reveals Ancestral Eumetazoan Gene Repertoire and Genomic Organization. Science 317(5834):86–94. doi:10.1126/science.1139158
Ralston GB (1975) The isolation of aggregates of spectrin from bovine erythrocyte membranes. Aust J Biol Sci 28(3):259–266
Ralston GB, Dunbar JC (1979) Salt and temperature-dependent conformation changes in spectrin from human erythrocyte membranes. Biochim Biophys Acta 579(1):20–30
Ramez M, Blot-Chabaud M, Cluzeaud F, Chanan S, Patterson M, Walensky LD, Marfatia S, Baines AJ, Chasis JA, Conboy JG, Mohandas N, Gascard P (2003) Distinct distribution of specific members of protein 4.1 gene family in the mouse nephron. Kidney Int 63(4):1321–1337
Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N (2007) Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci 120(Pt 6):953–963
Reid ME, Takakuwa Y, Conboy J, Tchernia G, Mohandas N (1990) Glycophorin C content of human erythrocyte membrane is regulated by protein 4.1. Blood 75(11):2229–2234
Ripoche P, Goossens D, Devuyst O, Gane P, Colin Y, Verkman AS, Cartron JP (2006) Role of RhAG and AQP1 in NH3 and CO2 gas transport in red cell ghosts: a stopped-flow analysis. Transfus Clin Biol 13(1–2):117–122
Robb VA, Li W, Gascard P, Perry A, Mohandas N, Gutmann DH (2003) Identification of a third Protein 4.1 tumor suppressor, Protein 4.1R, in meningioma pathogenesis. Neurobiol Dis 13(3):191–202
Robb VA, Gerber MA, Hart-Mahon EK, Gutmann DH (2005) Membrane localization of the U2 domain of Protein 4.1B is necessary and sufficient for meningioma growth suppression. Oncogene 24(11):1946–1957
Robledo RF, Ciciotte SL, Gwynn B, Sahr KE, Gilligan DM, Mohandas N, Peters LL (2008) Targeted deletion of {alpha}-adducin results in absent {beta}- and {gamma}-adducin, compensated hemolytic anemia, and lethal hydrocephalus in mice. Blood
Rotter B, Bournier O, Nicolas G, Dhermy D, Lecomte MC (2005) AlphaII-spectrin interacts with Tes and EVL, two actin-binding proteins located at cell contacts. Biochem J 388(Pt 2):631–638
Sahr KE, Tobe T, Scarpa A, Laughinghouse K, Marchesi SL, Agre P, Linnenbach AJ, Marchesi VT, Forget BG (1989) Sequence and exon–intron organization of the DNA encoding the alpha I domain of human spectrin. Application to the study of mutations causing hereditary elliptocytosis J Clin Invest 84(4):1243–1252
Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, Leto TL, Linnenbach AJ, Winkelmann JC, Speicher DW, Marchesi VT, Curtis PJ, Forget BG (1990) The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem 265(8):4434–4443
Salomao M, An X, Guo X, Gratzer WB, Mohandas N, Baines AJ (2006) Mammalian alphaI-spectrin is a neofunctionalized polypeptide adapted to small highly deformable erythrocytes. Proc Natl Acad Sci USA 103(3):643–648
Salomao M, Zhang X, Yang Y, Lee S, Hartwig JH, Chasis JA, Mohandas N, An X (2008) Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci USA 105(23):8026–8031
Salomao M, Chen K, Villalobos J, Mohandas N, An X, Chasis JA (2010) Hereditary spherocytosis and hereditary elliptocytosis: aberrant protein sorting during erythroblast enucleation. Blood Mar 25. [Epub ahead of print]
Samarel AM (2005) Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol 289(6):H2291–H2301
Satchwell TJ, Shoemark DK, Sessions RB, Toye AM (2009) Protein 4.2: a complex linker. Blood Cells Mol Dis 42(3):201–210
Schafer DP, Jha S, Liu F, Akella T, McCullough LD, Rasband MN (2009) Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J Neurosci 29(42):13242–13254
Scott C, Phillips GW, Baines AJ (2001) Properties of the C-terminal domain of 4.1 proteins. Eur J Biochem 268(13):3709–3717
Shahbakhti F, Gratzer WB (1986) Analysis of the self-association of human red cell spectrin. Biochemistry 25(20):5969–5975
Sheetz MP (1979) Integral membrane protein interaction with Triton cytoskeletons of erythrocytes. Biochim Biophys Acta 557(1):122–134
Sheetz MP, Painter RG, Singer SJ (1976) Relationships of the spectrin complex of human erythrocyte membranes to the actomyosins of muscle cells. Biochemistry 15(20):4486–4492
Shen L, Liang F, Walensky LD, Huganir RL (2000) Regulation of AMPA receptor GluR1 subunit surface expression by a 4. 1 N-linked actin cytoskeletal association. J Neurosci 20(21):7932–7940
Shotton D, Burke B, Branton D (1978) The shape of spectrin molecules from human erythrocyte membranes. Biochim Biophys Acta 536(1):313–317
Siegel DL, Branton D (1985) Partial purification and characterization of an actin-bundling protein, band 4.9, from human erythrocytes. J Cell Biol 100(3):775–785
Simonovic M, Zhang Z, Cianci CD, Steitz TA, Morrow JS (2006) Structure of the calmodulin alphaII-spectrin complex provides insight into the regulation of cell plasticity. J Biol Chem 281(45):34333–34340
Sobotzik JM, Sie JM, Politi C, Del Turco D, Bennett V, Deller T, Schultz C (2009) AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc Natl Acad Sci USA 106(41):17564–17569
Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM (2009) A selective filter for cytoplasmic transport at the axon initial segment. Cell 136(6):1148–1160
Speicher DW, Marchesi VT (1984) Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature 311(5982):177–180
Speicher DW, Davis G, Marchesi VT (1983) Structure of human erythrocyte spectrin. II. The sequence of the alpha-I domain. J Biol Chem 258(24):14938–14947
Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K (1988) Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature 333(6169):177–180
Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS (2008) The Trichoplax genome and the nature of placozoans. Nature 454(7207):955–960
Stabach PR, Morrow JS (2000) Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem 275(28):21385–21395
Stabach PR, Simonovic I, Ranieri MA, Aboodi MS, Steitz TA, Simonovic M, Morrow JS (2009) The structure of the ankyrin-binding site of beta-spectrin reveals how tandem spectrin-repeats generate unique ligand-binding properties. Blood 113(22):5377–5384
Stagg MA, Carter E, Sohrabi N, Siedlecka U, Soppa GK, Mead F, Mohandas N, Taylor-Harris P, Baines A, Bennett P, Yacoub MH, Pinder JC, Terracciano CMN (2008) Cytoskeletal protein 4.1R affects repolarization and regulates calcium handling in the heart. Circ Res 103(8):855–863. doi:10.1161/circresaha.108.176461
Steck TL, Weinstein RS, Straus JH, Wallach DF (1970) Inside-out red cell membrane vesicles: preparation and purification. Science 168(928):255–257
Stumpp MT, Amstutz P (2007) DARPins: a true alternative to antibodies. Curr Opin Drug Disc Dev 10(2):153–159
Sussman MA, McCulloch A, Borg TK (2002) Dance band on the titanic: biomechanical signaling in cardiac hypertrophy. Circ Res 91(10):888–898. doi:10.1161/01.res.0000041680.43270.f8
Tang HY, Speicher DW (2004) In vivo phosphorylation of human erythrocyte spectrin occurs in a sequential manner. Biochemistry 43(14):4251–4262
Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L (2003) Disruption of transforming growth factor-beta signaling in ELF beta- spectrin-deficient mice. Science 299(5606):574–577
Taylor-Harris PM, Felkin LE, Birks EJ, Franklin RC, Yacoub MH, Baines AJ, Barton PJ, Pinder JC (2005a) Expression of human membrane skeleton protein genes for protein 4.1 and betaIISigma2-spectrin assayed by real-time RT-PCR. Cell Mol Biol Lett 10(1):135–149
Taylor-Harris PM, Keating LA, Maggs AM, Phillips GW, Birks EJ, Franklin RC, Yacoub MH, Baines AJ, Pinder JC (2005b) Cardiac muscle cell cytoskeletal protein 4.1: analysis of transcripts and subcellular location–relevance to membrane integrity, microstructure, and possible role in heart failure. Mamm Genome 16(3):137–151
Tepass U (2009) FERM proteins in animal morphogenesis. Curr Opin Genet Dev 19(4):357–367
Thomas GH, Zarnescu DC, Juedes AE, Bales MA, Londergan A, Korte CC, Kiehart DP (1998) Drosophila betaHeavy-spectrin is essential for development and contributes to specific cell fates in the eye. Development 125(11):2125–2134
Tillack TW, Marchesi SL, Marchesi VT, Steers E Jr (1970) A comparative study of spectrin: a protein isolated from red blood cell membranes. Biochim Biophys Acta 200(1):125–131
Tilney LG, Detmers P (1975) Actin in erythrocyte ghosts and its association with spectrin. Evidence for a nonfilamentous form of these two molecules in situ. J Cell Biol 66(3):508–520
Tisminetzky S, Devescovi G, Tripodi G, Muro A, Bianchi G, Colombi M, Moro L, Barlati S, Tuteja R, Baralle FE (1995) Genomic organisation and chromosomal localisation of the gene encoding human beta adducin. Gene 167(1–2):313–316
Tran YK, Bogler O, Gorse KM, Wieland I, Green MR, Newsham IF (1999) A novel member of the NF2/ERM/4.1 superfamily with growth suppressing properties in lung cancer. Cancer Res 59(1):35–43
Trave G, Pastore A, Hyvonen M, Saraste M (1995) The C-terminal domain of alpha-spectrin is structurally related to calmodulin. Eur J Biochem 227(1–2):35–42
Tse WT, Lux SE (1999) Red blood cell membrane disorders. Br J Haematol 104(1):2–13
Tse WT, Lecomte MC, Costa FF, Garbarz M, Feo C, Boivin P, Dhermy D, Forget BG (1990) Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J Clin Invest 86(3):909–916
Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V (1999) Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol 147(5):995–1008
Tyler JM, Hargreaves WR, Branton D (1979) Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci USA 76(10):5192–5196
Tyler JM, Reinhardt BN, Branton D (1980) Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J Biol Chem 255(14):7034–7039
Uemoto Y, Suzuki S, Terada N, Ohno N, Ohno S, Yamanaka S, Komada M (2007) Specific role of the truncated betaIV-spectrin Sigma6 in sodium channel clustering at axon initial segments and nodes of Ranvier. J Biol Chem 282(9):6548–6555
Ungewickell E, Gratzer W (1978) Self-association of human spectrin. A thermodynamic and kinetic study. Eur J Biochem 88(2):379–385
Ungewickell E, Bennett PM, Calvert R, Ohanian V, Gratzer WB (1979) In vitro formation of a complex between cytoskeletal proteins of the human erythrocyte. Nature 280(5725):811–814
Ursitti JA, Fowler VM (1994) Immunolocalization of tropomodulin, tropomyosin and actin in spread human erythrocyte skeletons. J Cell Sci 107(Pt 6):1633–1639
Ursitti JA, Kotula L, DeSilva TM, Curtis PJ, Speicher DW (1996) Mapping the human erythrocyte beta-spectrin dimer initiation site using recombinant peptides and correlation of its phasing with the alpha-actinin dimer site. J Biol Chem 271(12):6636–6644
Van de Peer Y (2004) Tetraodon genome confirms Takifugu findings: most fish are ancient polyploids. Genome Biol 5(12):250
Van Wart A, Trimmer JS, Matthews G (2007) Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol 500(2):339–352
Viel A, Gee MS, Tomooka L, Branton D (1998) Motifs involved in interchain binding at the tail-end of spectrin. Biochim Biophys Acta 1384(2):396–404
Vince JW, Reithmeier RA (1998) Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. J Biol Chem 273(43):28430–28437
Voas MG, Lyons DA, Naylor SG, Arana N, Rasband MN, Talbot WS (2007) alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol 17(6):562–568
Walensky LD, Blackshaw S, Liao D, Watkins CC, Weier HU, Parra M, Huganir RL, Conboy JG, Mohandas N, Snyder SH (1999) A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci 19(15):6457–6467
Waller KL, Nunomura W, An X, Cooke BM, Mohandas N, Coppel RL (2003) Mature parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum binds to the 30-kDa domain of protein 4.1 in malaria-infected red blood cells. Blood 102(5):1911–1914
Waller KL, Stubberfield LM, Dubljevic V, Buckingham DW, Mohandas N, Coppel RL, Cooke BM (2010) Interaction of the exported malaria protein Pf332 with the red blood cell membrane skeleton. Biochim Biophys Acta 1798(5):861–871
Ward REt, Schweizer L, Lamb RS, Fehon RG (2001) The protein 4.1, ezrin, radixin, moesin (FERM) domain of Drosophila Coracle, a cytoplasmic component of the septate junction, provides functions essential for embryonic development and imaginal cell proliferation. Genetics 159(1):219–228
Waseem A, Palfrey HC (1988) Erythrocyte adducin. Comparison of the alpha- and beta-subunits and multiple-site phosphorylation by protein kinase C and cAMP-dependent protein kinase. Eur J Biochem 178(2):563–573
Waseem A, Palfrey HC (1990) Identification and protein kinase C-dependent phosphorylation of alpha-adducin in human fibroblasts. J Cell Sci 96(Pt 1):93–98
Wechsler A, Teichberg VI (1998) Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J 17(14):3931–3939
Whittaker KL, Ding D, Fisher WW, Lipshitz HD (1999) Different 3’ untranslated regions target alternatively processed hu-li tai shao (hts) transcripts to distinct cytoplasmic locations during Drosophila oogenesis. J Cell Sci 112(Pt 19):3385–3398
Williamson RC, Toye AM (2008) Glycophorin A: band 3 aid. Blood Cells Mol Dis 41(1):35–43
Winkelmann JC, Leto TL, Watkins PC, Eddy R, Shows TB, Linnenbach AJ, Sahr KE, Kathuria N, Marchesi VT, Forget BG (1988) Molecular cloning of the cDNA for human erythrocyte beta-spectrin. Blood 72(1):328–334
Winkelmann JC, Chang JG, Tse WT, Scarpa AL, Marchesi VT, Forget BG (1990a) Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem 265(20):11827–11832
Winkelmann JC, Costa FF, Linzie BL, Forget BG (1990b) Beta spectrin in human skeletal muscle. Tissue-specific differential processing of 3' beta spectrin pre-mRNA generates a beta spectrin isoform with a unique carboxyl terminus. J Biol Chem 265(33):20449–20454
Woroniecki R, Ferdinand JR, Morrow JS, Devarajan P (2003) Dissociation of spectrin–ankyrin complex as a basis for loss of Na-K-ATPase polarity after ischemia. Am J Physiol Renal Physiol 284(2):F358–F364
Xu X, Shrager P (2005) Dependence of axon initial segment formation on Na+ channel expression. J Neurosci Res 79(4):428–441
Xu M, Cao R, Xiao R, Zhu MX, Gu C (2007) The axon-dendrite targeting of Kv3 (Shaw) channels is determined by a targeting motif that associates with the T1 domain and ankyrin G. J Neurosci 27(51):14158–14170
Yan Y, Winograd E, Viel A, Cronin T, Harrison SC, Branton D (1993) Crystal structure of the repetitive segments of spectrin. Science 262(5142):2027–2030
Yang S, Guo X, Debnath G, Mohandas N, An X (2009) Protein 4.1R links E-cadherin/beta-catenin complex to the cytoskeleton through its direct interaction with beta-catenin and modulates adherens junction integrity. Biochim Biophys Acta 1788(7):1458–1465
Yoshino H, Marchesi VT (1984) Isolation of spectrin subunits and reassociation in vitro. Analysis by fluorescence polarization. J Biol Chem 259(7):4496–4500
Yu J, Goodman SR (1979) Syndeins: the spectrin-binding protein(s) of the human erythrocyte membrane. Proc Natl Acad Sci USA 76(5):2340–2344
Yu J, Fischman DA, Steck TL (1973) Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents. J Supramol Struct 1(3):233–248
Yue L, Spradling AC (1992) hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev 6(12B):2443–2454
Zarnescu DC, Thomas GH (1999) Apical spectrin is essential for epithelial morphogenesis but not apicobasal polarity in Drosophila. J Cell Biol 146(5):1075–1086
Zhang P, Talluri S, Deng H, Branton D, Wagner G (1995) Solution structure of the pleckstrin homology domain of Drosophila beta-spectrin. Structure 3(11):1185–1195
Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V (1998) AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143(5):1295–1304
Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L (1998) Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem 273(22):13681–13692
Acknowledgements
The authors are grateful to Dr. Marie-Christine Lecomte and Dr. Ekaterini Kordeli (INSERM, Paris) for their comments on the manuscript.
Conflict of Interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: David Robinson
Rights and permissions
About this article
Cite this article
Baines, A.J. The spectrin–ankyrin–4.1–adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma 244, 99–131 (2010). https://doi.org/10.1007/s00709-010-0181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0181-1