Abstract
Dystrophin and Spectrin are two proteins essential for the organization of the cytoskeleton and for the stabilization of membrane cells. The comparison of these two sister proteins, and with the dystrophin homologue utrophin, enables us to emphasise that, despite a similar topology with common subdomains and a common structural basis of a three-helix coiled-coil, they show a large range of dissimilarities in terms of genetics, cell expression and higher level structural organisation. Interactions with cellular partners, including proteins and membrane phospholipids, also show both strikingly similar and very different behaviours. The differences between dystrophin and spectrin are also illustrated by the large variety of pathological anomalies emerging from the dysfunction or the absence of these proteins, showing that they are keystones in their function of providing a scaffold that sustains cell structure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Dystrophin is a unique protein coded by a single gene, which is expressed in differentiated smooth and striated muscular and nervous cells. Spectrins, on the other hand, are a large family of several proteins, coded by several genes, leading to a high number of isoforms that are ubiquitously expressed in numerous cell types. Dystrophin and its homologue utrophin are each coded by a single gene, DMD and UTRN (Table 12.1) respectively. These two genes are associated with the expression of unique proteins (Koenig et al. 1987; Tinsley et al. 1992). However, in the case of dystrophin, several promoters allow the tissue-specific expression of shorter isoforms of dystrophin compared to the full length protein. Utrophin is only known as the full length transcript (Helliwell et al. 1992; Tinsley et al. 1992). Unlike the two single genes for dystrophin and utrophin, spectrins are coded by numerous genes. In mammals, two genes, SPTA1 and SPTAN1, code the two major α isoforms of spectrin, αI and αII, respectively. αI is characteristic of erythroid cells (Sahr et al. 1990). In contrast to SPTA1, alternative processing of the transcript of the SPTAN1 gene gives rise to at least four isoforms of αII spectrin (Cianci et al. 1999). Moreover, four genes (SPTB, SPTBN1, SPTBN2, SPTBN4) code for conventional β spectrins (β-erythrocytic and βI, II and IV, respectively; Winkelmann et al. 1990; Stankewich et al. 1998; Berghs et al. 2000) and one gene ( SPTBN5 ) codes for the large βV spectrin (Stabach and Morrow 2000). Variations in mRNA splicing in the case of isoforms βI, βII and βIV generate longer or shorter C-terminal regions (Berghs et al. 2000; Hayes et al. 2000;Table 12.1). The expression of the isoforms is tightly regulated in a tissue- and time-dependent manner, which is a consequence of the structural complexity of mammalian cells. Various intracellular locations (e.g. plasma membrane, Golgi apparatus, endoplasmic reticulum, nucleus) of spectrins reflect the variety of their functions. Only three spectrin isoforms are present in D. melanogaster and C. elegans, where the α subunit is encoded by a gene resembling mammalian SPTAN1 and the β subunits correspond to mammalian βII and βV (Dubreuil and Grushko 1998).
Dystrophin, utrophin and the spectrins share the unique fold of the so-called “spectrin repeat”, while they also have specific additional domains. Due to their similar spectrin repeats, they have been attributed to the same vast “spectrin” family of proteins (Parry et al. 1992). The spectrin family also includes α-actinin (see Ribiero Ede et al. 2014) which we will not discuss further in this Chapter. However, the general topology of dystrophin, utrophin and the spectrins is different, with dystrophin and utrophin existing as monomers and spectrins as homodimers or higher homo-oligomers of αβ-heterodimers. Their genes are all subject to mutations, but mutation types are very specific for each gene, with the vast majority being deletions of exons for dystrophin and point mutations for spectrins. The effect of out-of-frame mutations in the DMD gene is, in most cases, to cause the total absence of dystrophin in the musculature, which is highly deleterious and leads to Duchenne muscular dystrophy (DMD), with patients having an expected life span of less than 30 years. The effects of in-frame mutations are highly specific for the DMD gene and can lead to a shortened dystrophin, which partially maintains the dystrophin function and leads in most cases to Becker muscular dystrophy (BMD), with an expected life span that is very variable. In-frame point mutations of spectrins primarily affect the tetramerization processes.
Until now, the functions of dystrophin, utrophin and the spectrin proteins have not been well defined at the molecular level. They are largely thought to be scaffolding proteins interacting with numerous partners, some of them well documented, but there are probably many unknown partners. Their scaffolding function means that they are an important part of the structure of the cell cytoskeleton. However, understanding their function at a molecular level is only in its infancy, partly due to the large size of these proteins: from 71 to 427 kDa for the longest isoform of dystrophin and ranging between 246 and 430 kDa for spectrins.
12.2 Dystrophin, Utrophin and Spectrin Structural Domains
Dystrophin is a monomeric protein comprising 3685 residues in the longest isoform, which is present in muscle and heart cells (Fig. 12.1: Koenig et al. 1987, 1988). Several promotors allow for the tissue-specific expression of isoforms of various lengths. There are three full length isoforms. Dp427m is expressed in striated muscles, where it appears at the internal face of the sarcolemma. Dp427c is found in heart and glial cells (Koenig et al. 1989) and in brain and retina (Nudel et al. 1989; Chelly et al. 1990). Dp427p occurs in Purkinje cells and in skeletal muscle (Chelly et al. 1990). The shorter isoform, Dp160, is expressed in retina (D’Souza et al. 1995), Dp140 in brain, retina and kidney (Lidov et al. 1995), Dp116 in Schwann cells (Byers et al. 1993) and Dp71 in all tissues except skeletal muscles (Hugnot et al. 1992).
Dystrophin and spectrin structural domains. CH1 and CH2, calponin homology domains, constituting the actin-binding domain ABD1; H1–H4, unstructured domains called hinges 1–4; R1–24, spectrin repeats, constituting the central domain; WW, Tryptophan-rich domain, EFH1 and EFH2, EF hand domains 1 and 2 and ZZ, ZZ domain, constituting the Cys-rich domain; C-ter, C-terminal domain, SH3, SH3 domain, PH, PH domain
Dystrophin comprises four main domains (Le Rumeur et al. 2010). At the N-terminal end there is a first actin binding domain (ABD1) consisting of two calponin homology (CH) domains. It is followed by a very long central domain made of 24 spectrin repeats of about 100–110 residues each. They are interrupted by hinges, one between repeat 3 and 4 and one between repeats 19 and 20. A Cys-rich domain comprises WW, EFH1 and EFH and ZZ domains. The fourth domain is the C-terminal domain (Fig. 12.1). Only a few of these domains have been crystallized and have had their structures solved (Table 12.2). These are the first actin-binding domain ABD1, repeat 1 (R1) and the WW and EFH subdomains in complex with a peptide from β-dystroglycan, an essential partner of dystrophin (see below). Depending on the location of the promotor, the shorter isoforms do not maintain the ABD1 subdomain and do not present all the repeats. As an example, the shortest isoform, Dp71, consists only of the C-terminal end starting at the EFH1 domain.
Utrophin has a similar domain structure to Dystrophin, the only difference being that there are two repeats less than in dystrophin (Winder et al. 1995; Le Rumeur et al. 2010). Apart from the ABD1 domain, which is also found in many other proteins, the other domains show high specificity in terms of their structure and interactions (see below).
Spectrin is a large, mostly heterotetrameric protein with a characteristic modular structure of helical spectrin repeats (Fig. 12.1). While there are 20 full-length repeating units and one incomplete repeat in α spectrins, only 16 (or 29 in case of heavy isoforms) repeats, along with one incomplete repeat, occur in β spectrins. The incomplete segments together provide one full spectrin repeating unit after tetramer formation (Kotula et al. 1993; Ipsaro et al. 2010). However, spectrin is much more than just an assembly of spectrin repeats (Machnicka et al. 2014). The 280-kDa α-spectrin also contains EF hands (calmodulin-like domains) at its N-terminus, an SH3 domain within repeat 9, and a short insert (named CCC) within repeat 10 of αII, which includes sites for calmodulin binding and caspase/calpain cleavage (Czogalla and Sikorski 2005). The variety of β spectrins is reflected not only in a broad molecular weight range (246–430 kDa), but also by the presence or absence of C-terminal plecstrin homology (PH) domains. Moreover, an actin binding domain, which consists of tandem calponin homology (CH) domains, is located at the N-terminus of each of the β spectrins.
12.2.1 Common Features of Dystrophin and Spectrin
12.2.1.1 Classical ABD Domains at the N-terminal End of Dystrophin, Utrophin and β-spectrin
Dystrophin, utrophin and β-spectrins are F-actin interacting proteins through the presence of actin-binding-domains either constituted by two CH domains (respectively CH1 and CH2) situated at the N-terminal end of the mentioned proteins (ABD1 in the case of dystrophin) or constituted of spectrin repeats (ABD2) in dystrophin only.
The entire ABD1 domains of dystrophin and utrophin have been crystallized as open conformations stabilized as head-to-tail homodimers (Keep et al. 1999; Norwood et al. 2000). The relative orientation between the CH1 and CH2 subdomains is different in the dystrophin and utrophin structures and it has been proposed that this is associated with the modulation in the sequence of the linker region bridging the CH subdomains. This linker region is folded into an α-helix which could be bent (Fig. 12.2a), and its amino-acid composition (Fig. 12.2b) influences the thermodynamic stability of the whole subdomain, probably through the conformational equilibrium between the open and the closed states of the ABD in solution (Bandi et al. 2015). The solution conformation adopted by the ABD or its conformation after binding to F-actin is still controversial and may vary between dystrophin and utrophin (Sutherland-Smith et al. 2003; Lin et al. 2011; Broderick et al. 2012; Singh and Mallela 2012). Three actin binding sites (ABS) have been distinguished by experimental mapping on the dystrophin ABD1. This mapping indicated that three independent ABS regions are involved in the contact with F-actin. These are from Lys18 to Ala27 (ABS1), from Val89 to Leu116 (ABS2) and from Leu131 to Ser147 (ABS3). These sites mainly involve the CH1 subdomain (residue 9–121) and the N-terminal end of CH2 (Henderson et al. 2010).
Experimental structures of the actin-binding domain composed of two calponin homology (CH) domains obtained for human dystrophin and utrophin and the two CH2 structures of the β spectrin available in the Protein Data Bank (PDB). (a) Backbone representation of the ABD domain emphasizing that the two CH subdomains are mainly composed of alpha-helices. Tridimensional superimposition allows us to establish the very high degree of structural conservation of this domain. (b) Primary sequence alignment (spectrin B, dystrophin, utrophin) shows the highest modulations to be located at both terminal ends and at the linker region bridging the two CH subdomains
The actin binding domain of β spectrin also consists in two CH subdomains, CH1 (residues 51–156) and CH2 (171–282). The CH2 domain was initially supposed to have a key role in the interactions with actin and this is the reason why only the structure of the CH2 domain was determined at high resolution (1.1 Å; PDB ID: 1BKR), but it appears that isolated CH2 domains bind rather weakly to actin as well (Banuelos et al. 1998). Like the corresponding subdomain in dystrophin and utrophin, CH2 is composed of four parallel helices interconnected by longer loops together with three additional helices resulting in a tight and globular structure. The most conservative elements across the various CH domains are the residues forming helices A, C and G, which play a major role in actin binding. Further on in this Chapter, we discuss a phospholipid-sensitive mechanism of actin binding by this domain.
12.2.1.2 Central Domains are Composed of Repeats with Similar Folds in Triple Helical Coiled-Coils
The common feature of these three proteins is the presence of numerous so-called spectrin repeats made of about 110 residues each and folded in triple helical coiled-coils. These coiled-coils are rather unusual since they are not formed by dimerization as in, for example, myosin or tropomyosin. In this case three successive helices (A, B and C) interrupted by two loops fold back on themselves to produce a three strand coiled coil. The interaction between the three alpha-helices is due to the presence of the classical heptad pattern of residues along the sequence (Winder et al. 1995). The heptad is defined as seven residues labelled “a” to “g” where the residues in position “a” and “d” are preferentially hydrophobic while the other are mostly hydrophilic. This pattern allows the folding in coiled-coils with all the “a” and “d” residues constituting the core of the molecule (Fig. 12.3a).
The heptad pattern of the residues in the sequence allows the 3D folding in a triple helical coiled-coil for spectrin and dystrophin repeats. (a) Representation as a helix wheel of the coiled-coil structure: the “a” and “d” residues are situated in the core of the molecule while the other residues are accessible to the solvent and for interactions with partners. A, B and C are the three helices of the triple coiled-coil. (b) The crystal structures of repeat 8 of β-spectrin (1S35) and repeat 1 of dystrophin (3UUN) are superimposed. The rms deviation between the two structures is 1.333 Å. (c) The crystal structure of the R8–9 tandem repeat from β-spectrin showing the common helix constituting the inter-repeat linker joining the repeats
Spectrin repeats are all rather similar in length being constituted by roughly 106 residues each, but there are variations between 99 and 114 amino acid residues. Unlike spectrins, the related dystrophin repeats are largely heterogeneous in length because of variations in length of their alpha-helices. As an example, repeat 14 composed of 94 amino acids (aa) only has a very short A helix (22 residues) and repeat 10 (92 aa) has a very short C helix (25 residues).
Although the sequence homology between the spectrin repeats does not go beyond 30 % (Leluk et al. 2001), and dystrophin and utrophin share a homology of less than 20 % (Nicolas et al. 2014), all spectrin repeats, as well as dystrophin and utrophin repeats, share a common fold roughly 50 Å in length and 20 Å in diameter. This was first described for the α spectrin of D. melanogaster (PDB ID: 2SPC; Yan et al. 1993; Pascual et al. 1997; Muthu et al. 2012;Fig. 12.3b). In a typical spectrin left-handed supercoil segment, helix B is kinked and is slightly longer than the other two helices in the segment (PDB IDs: 1CUN, 1U5P, 1U4Q; Grum et al. 1999; Kusunoki et al. 2004b). However, the positioning of loops interconnecting the helices of individual repeats may vary to some extent (Grum et al. 1999; Kusunoki et al. 2004a). It seems that conservative tryptophan residues are critical for stabilization of the triple-helical structure, although the core of some of the spectrin repeats may deviate from this pattern, as was described for R9 of human β spectrin, where water-mediated hydrogen bonding forms the core (PDB ID: 1S35; Kusunoki et al. 2004a). The tiny dissimilarities among spectrin repeats result in differences in their stabilities. Individual spectrin repeats also have different folding characteristics, as shown for repeats 15–17 of α spectrin (Kwa et al. 2014). Remarkably, helix C and helix A of the next repeat exhibit an uninterrupted helical structure (Fig. 12.3c), thus enabling cooperative propagation of forced unfolding throughout the whole molecule (Kusunoki et al. 2004b; Law et al. 2003). These features, together with a broad array of possible inter-repeat interactions and potential rearrangement of helices within a single repeat, may be keys to understanding the remarkable flexibility of spectrins (Grum et al. 1999; Mirijanian et al. 2007). It is worth underlining that upon full extension the spectrin tetramer is 180–200 nm long, but its physiological rest length is only 55–60 nm. Most recently, a model has been proposed where spectrin is a hollow cylinder and the pitch of each spectrin repeat increases during extension (Brown et al. 2015). Inter-repeat linkers do not all appear as helical in dystrophin or utrophin (Legrand et al. 2011; Nicolas et al. 2014).
12.2.1.3 EF Domains at the C-Terminal End of Dystrophin, Utrophin and α-spectrin
The structures of the EF hands of dystrophin have been determined along with the WW domain in complex with a Pro-rich motif of β-dystroglycan (Huang et al. 2000), establishing that the β-dystroglycan interaction with dystrophin is achieved through both WW and EFH1 subdomains. The recognition mechanism has been revealed to be highly similar to what is observed for Pro-rich sequences binding to SH3 domains. Very little is known about utrophin EFH subdomains. The EFH1 and EFH2 of utrophin share respectively 65 % and 77 % of identity with the corresponding subdomains in dystrophin, probably indicating a high degree of structure similarity. The C-terminus of α spectrins also exhibits structural similarities to calmodulin. The two EF-hands (EFH) present in αII spectrins bind cooperatively two calcium ions with rather low affinities in the 50–100 μM range, unlike the analogous structures in αI that do not bind Ca2+ (Buevich et al. 2004). It seems that EF-hands stabilize interactions of the CH2 domain of the spectrin dimer with actin by binding the linker that comes between the actin binding domain and the first β-spectrin repeat (Korsgren and Lux 2010). Moreover, EF-hands can bind some proteins of the membrane skeleton, such as protein 4.2, in a calcium- and calmodulin-dependent manner (Korsgren et al. 2010).
12.2.2 Dystrophin and Spectrin Structural Dissimilarities
Dystrophin is a monomeric protein which has been found to be difficult to crystallize even as small fragments. The only experimentally defined structure was obtained for repeat 1 (R1) constrained to be a dimer induced by an S-S bridge between two repeats (Muthu et al. 2012). This structure was found to be similar to spectrin repeats, post-validating the reliability of homology models obtained previously based on spectrin structures (Legrand et al. 2011). However, this structure does not show how several repeats are chained together in the full length molecule, especially whether or not the linker region is helical across consecutive repeats, and it does not define their relative positioning. Because of this, in a recent study, we obtained structural models of tandem or multi-repeat fragments in solution using small angle X-ray scattering (SAXS) and molecular modelling. This work showed that the multi-repeat fragments present well-defined kinks at some inter-repeat linkers that give a tortuous and complex topology to the whole dystrophin filament (Delalande et al. to be published; Fig. 12.4).
Structural models of multirepeat fragments of dystrophin obtained by small angle X-ray scattering and molecular modeling. Five of the eight fragments studied are shown here. The N-terminal ends are to the left. They all show kinks placed at inter-repeat linkers with angles as high as 90° between repeats 14 and 15 in the R11–15 fragment (arrow)
These kinks are situated at specific linkers between successive repeats and are not uniformly distributed all along the central domain of dystrophin. Kinks between spectrin repeats are reminiscent of the angle found at the spectrin ankyrin-binding site, one of the determining features of spectrin-ankyrin interactions (see below). Similarly, the angle observed between repeats 16 and 17 shows that region to be one of the key places for the interaction of dystrophin with neuronal nitric oxide synthase (nNOS; see below; Molza et al. 2015).
Utrophin has not yet been studied with such tools, but it is likely that kinks will be present in utrophin as in dystrophin. These kinks would be placed at different sites than in dystrophin since, unlike dystrophin, repeats 10–15 of utrophin do not bind actin (Amann et al. 1999).
In contrast to dystrophin, the functional units of spectrin are tetramers or higher oligomers (Nans et al. 2011). A broad range of oligomeric states of spectrin in erythrocytes reflects the high degree of elasticity and flexibility of the red blood cell membrane. The α/β heterodimer is stabilized mostly by interactions between two C-terminal and two N-terminal repeats of the α and β subunits, respectively (Begg et al. 2000). The next level of organization is achieved by head to head dimer interactions. The key role in spectrin tetramerization is played by the incomplete repeats of both subunits. The roughly 30 residue long N-terminus of α spectrin forms a single helix and the roughly 70 C-terminal residues of β spectrin fold into two helices. These two structural motifs interact to reconstruct a full triple helical structure where hydrophobic interactions are the major players (PDB ID: 3LBX; Ipsaro et al. 2010). The tetramerization regions of αI and αII (PDB IDs: 1OWA and 3F31, respectively) are not equivalent; the linker following the first helix is either unstructured or helical, respectively (Mehboob et al. 2010). However, after binding β spectrin, the conformation of the linker is helical in both cases. This feature may contribute to the differences in flexibility between various isoforms of spectrin. Although the general model presumes spectrin tetramers or higher oligomers as prerequisite elements of the membrane skeleton, tetramerization of nonerythroid spectrin appears to be unnecessary for normal development of D. melanogaster (Khanna et al. 2015).
In all α spectrins, repeat 9 is interrupted by an SH3 domain (Robertsson et al. 2005). The core of this domain consists of antiparallel β strands that form two β-sheets perpendicular to each other (PDB ID: 1SHG; Musacchio et al. 1992). Recently, more and more data point out the role of the SH3 domain of spectrin in numerous processes connected to cell signalling and repair of DNA interstrand cross-links (for review see Machnicka et al. 2014).
Some isoforms of β spectrin have an extended C-terminus where a PH domain can be found (Macias et al. 1994; Zhang et al. 1995). Seven antiparallel β strands that form a barrel and a subsequent conservative α-helix are the core of such structural motifs, which are known to occur in numerous eukaryotic proteins involved in signal transduction, organization of cytoskeleton and many other cellular processes. Although little is known about the physiological role of the PH domain of spectrin, the positively charged lipid-binding surface seems to be sufficient to bind the inositide head group of PIP2 (Hyvonen et al. 1995). Complexation to inositol-1,4,5-triphosphate seems to induce ordering of the binding loops when compared to the structure of the free domain in solution (PDB ID: 1MPH; Nilges et al. 1997). On the other hand, it appears that the presence of the PH domain is necessary for membrane targeting of spectrin in midgut copper cells of D. melanogaster, but the mechanism is not related to PIP2- binding activity (Das et al. 2008).
12.3 The Scaffolding Function of Dystrophin and Spectrins: Binding to Phospholipids and Organizing Protein-Protein Assemblies
The first impression when looking at the structures of spectrins and dystrophin is that their repeating units are just modules that build proteins of elongated shape and are predominantly responsible for the observed structural and mechanical properties. In that sense, dystrophin has long been considered as a rod-shape protein linking the cytoskeletal element actin to the extracellular matrix (Koenig et al. 1988; Ervasti and Campbell 1991). However, more and more data show that such modules are also binding sites for a number of cytoskeletal and signal transduction proteins, as well as for phospholipid membranes (Machnicka et al. 2014; Djinovic-Carugo et al. 2002; Boguslawska et al. 2014b; Le Rumeur et al. 2003; Legardinier et al. 2009; Ervasti and Campbell 1993a; Lai et al. 2009; Prins et al. 2009).
12.3.1 Dystrophin and Spectrin: Two Coiled-Coil Filaments Interacting with Membrane Lipids
Phospholipid binding properties of dystrophin are essential for its main biological function to maintain the cohesion of the sarcolemma during compression and re-extension of the membranes of muscle cells. It is well established that a major part of the dystrophin central domain binds to membrane lipids, but with a heterogeneous interaction pattern all along the filament length (Fig. 12.5). The cartography of the dystrophin central domain emphasizes that, despite a common structural pattern, different repeats show highly different molecular surface properties which define sub-domains involved in specific interactions with various partners (Legrand et al. 2011;Nicolas et al. 2014). For example, repeats R1–R3 strongly interact with anionic lipids, but repeats R20–R24 do not show any similar binding properties (Legardinier et al. 2008). This C-terminal end non-binding feature seems to be a significant difference between dystrophin and spectrin binding properties. Anionic lipids also bind strongly with the subdomain comprising repeats R4–R19 of dystrophin (Legardinier et al. 2009). Another localized binding feature has also been revealed for some repeats of the central domain. For instance, since it binds very weakly to large unilamellar vesicles (LUVs) compared to small unilamellar vesicles (SUVs), except when the LUVs contain PE and/or cholesterol, the binding properties of dystrophin repeat R2 were revealed to be highly sensitive to lipid packing (Le Rumeur et al. 2007). In addition, cholesterol has been shown to increase the insertion of several repeats of dystrophin into the membrane (Ameziane-Le Hir et al. 2014).
For some parts of the central domain, circular dichroism analysis indicated a lower degree of inter-helix interaction in the coiled-coil filament upon binding with phospholipids. This is notably the case in three-repeat fragments like R4–6, R6–9, R12–14 or R17–19. However, coiled-coils should open only partly upon lipid binding, since circular dichroism measurement modulations are not comparable to what can be observed in TFE. Such dynamic behaviour was not observed for longer fragments including R11–15 and R14–17. Among the specific binding profiles encountered along the central domain, the R11–15 fragment seems to be the only subdomain to bind to PC/PE lipids (Legardinier et al. 2009; Sarkis et al. 2011).
Both erythroid and non-erythroid spectrins possess an ability to interact with phospholipids, which has been proven for natural and model membranes (for review see Boguslawska et al. 2014a). Although spectrin repeats exhibit a general lipid binding ability, high affinity sites have recently been recognized (Fig. 12.5). These include PS recognizing repeats 8–10 of the α subunit, and repeats R2–4 and R12–14 of β spectrin (An et al. 2004a, b), as well as a binding site for PE-rich membranes within R14 of β spectrin which partly overlaps with the ankyrin binding domain (described below). Ankyrin-sensitive binding of PE-rich lipids was identified within the N-terminal part of the ankyrin binding domain of both β erythrocytic and β non-erythrocytic spectrin (Hryniewicz-Jankowska et al. 2004; Bok et al. 2007). Further structural studies showed that lipid binding activity is confined to helix C of R14, the highly amphipathic character of which correlates with its mixed 310/α-helical conformation (Czogalla et al. 2007). Remarkably, interaction with PE/PC membranes or detergents provokes partial opening of the coiled-coil structure of the 14th repeat (Czogalla et al. 2008; Fig. 12.6). The current structural model of the ankyrin-dependent lipid binding site of β spectrin suggests that one of the tryptophan residues within the 310/α-helix is slightly shifted outside the hydrophobic core of the helix bundle and thus could initiate interactions with membranes (Wolny et al. 2011). Another spectrin region that interacts with phospholipids is the actin binding domain (ABD). Binding of PIP2 triggers a conformational switch from a closed to an open conformation of the CH1-CH2 tandem. This does not influence binding of actin, but enhances interactions with the second target for ABD, namely protein 4.1 (An et al. 2005). On the other hand, structural studies on the CH domains of α-actinin suggest that opening of the domains influences their affinity for actin (Galkin et al. 2010). Out of the various domains of spectrin, the plecstrin homology domains present in some of the isoforms of β subunits seems to be the best candidates for lipid membrane anchors. However, the PH domain of spectrin binds weakly to phosphatidylinosites without specificity towards these lipids, in contrast to the PH domains from some other proteins (Lemmon et al. 2002). Provided that multiple spectrin tetramers form a network containing many lipid binding domains, interactions with membranes may be very strong due to the resulting multivalent character.
The ankyrin-lipid binding site of spectrin. The triple helical repeats are represented by the ankyrin-sensitive lipid-binding domain within the 14th segment of β-spectrin (ribbon representation of segments 13–15). Membrane binding results in the “open” conformation (amino-acid residues essential for lipid binding in red: W1771, L1775, M1778, W1779, all within helix 14C; the 310 helix is yellow). Alternatively, steric interference upon the interactions of spectrin with the ZU5 domain of ankyrin (gray) blocks the “opening” of the triple helical bundle – PDB ID: 3KBT (amino-acid residues crucial for ankyrin binding in green, residue F917 of ZU5 in yellow) (Adapted from Boguslawska et al. 2014b)
12.3.2 Examples of Protein Partners of Dystrophin and Spectrin
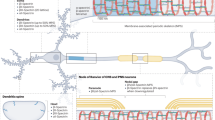
The first protein partner of dystrophin to be deswcribed is β-dystroglycan (Campbell and Kahl 1989), which together with dystrophin and other interacting proteins forms the dystrophin-glycoprotein complex (DGC: Fig. 12.7; Ervasti and Campbell 1991), (b). Among muscle membrane proteins dystrophin only interacts with β-dystroglycan, which in turn interacts with intrinsic membrane proteins such as the sarcoglycans. Sarcoglycans and β-dystroglycan interact with proteins of the extra-cellular matrix, including α-dystroglycan and laminins (Ervasti and Campbell 1993a). Intriguingly, dystrophin interacts with three different types of cellular filaments; filamentous actin (Ervasti et al. 1997), intermediate filaments (Bhosle et al. 2006) and microtubules (Prins et al. 2009), which together form a crucial filamentous network for striated muscles. Other proteins involved in signalling or physiology of the muscle or with other unknown functions are also important partners of dystrophin. These include neuronal Nitric Oxide Synthase (nNOS), the polarity associated protein PAR1-b, dystrobrevin, syntrophin and myosprin (Fig. 12.5). The nNOS–dystrophin interaction has been the most intensively studied since it was established that loss of nNOS localization at the sarcolemma was related to an increase in muscle weakness or damage (Sander et al. 2000; Sato et al. 2008) and to a more severe phenotype in patients with mutated dystrophin (Gentil et al. 2012). The primary nNOS binding site was first localized in the dystrophin central domain, involving both repeats R16 and R17 (Lai et al. 2009, 2013). Work combining biochemical experiments, structural data and molecular modelling revealed the association mode of the nNOS PDZ subdomain with the R16-R17 repeats (Molza et al. 2014, 2015). Since PDZ subdomains form classical homodimers, the non-canonical association of PDZ with a three-helix coiled-coil structure seems at first to be similar to the structural organization of the spectrin-ankyrin complex (a beta sheet lying on a three-helix bundle filament bearing a kink; Fig. 12.8). However, detailed analysis of the two structures reveal as many similarities as differerences. The kink in the coiled-coil axis observed at the linker region between successive repeats involved in the interaction with the partner is present in both structures and seems essential for the stabilization of the association. But topologically, as ankyrin binds the spectrin filament at the linker region between two repeats, its contact with the beta sheet is mainly achieved with the end of the helix C of the first repeat and with the loop bridging helices B and C of the second repeat. In contrast, nNOS-PDZ binds dystrophin on the other side of the linker region, namely with the begining of the helix A of the second repeat and with the loop bridging helices A and B of the first repeat. In addition, whereas a large extended hydrophobic patch is involved at the nNOS recognition interface of dystrophin (Giudice et al. 2013), specific recognition of ankyrin by spectrin is mainly driven through electrostatics (Ipsaro and Mondragon 2010).
Schematic view of the dystrophin associated complex (DGC). Illustration of the scaffolding role of the dystrophin (in red) resulting in the link of sarcolemmal components (in blue) to the cytoskeletal protein network. (MT microtubule, IF intermediate filaments, DG dystroglycan, Syn syntrophin, DB dystrobrevin, SGC sarcoglycans)
Structural model for nNOS-PDZ–dystrophin R16–17 complex based on biochemical mapping of the interface. Three-helix coiled-coil repeats R16 and R17 of dystrophin are shown in backbone representation colored in blue and the nNOS-PDZ subdomain is shown as a grey backbone representation. Principal features established to be involved in the specificity of the interaction are highlighted: the filament kink between successive dystrophin repeats and the β-finger contact with the R17 helix C
Apparently, the spectrin repeats evolved to gain functional specialization within the conserved three-dimensional fold. Here, the most thoroughly explored example is the ankyrin-binding site which includes highly conserved regions within the 14th and 15th repeats of β spectrin (Czogalla and Sikorski 2010). Moreover, this region also includes an ankyrin-sensitive binding site for PE-rich membranes (Wolny et al. 2011). All these features are hidden in a structurally common tandem of triple-helical spectrin repeats, which was confirmed both in crystal structures (PDB IDs: 3EDU, 3F57, 3EDV; Stabach et al. 2009; Ipsaro et al. 2009; Davis et al. 2009) and in solution (Czogalla et al. 2007). The ankyrin-docking site is formed by conservative anionic amino acid residues on one of the facets of helix C of the 14th repeat and some residues within the inter-repeat linker and the loop between helices B and C of the 15th repeat (Fig. 12.6). Formation of the ankyrin-spectrin complex requires shape complementarity rather than induced fit (Ipsaro et al. 2009), and further studies indicated that the inter-repeat kink is one of the determinants of spectrin-ankyrin interactions (LA-Borde et al. 2010). On the other hand, sensitivity of the spectrin repeats to mechanical stress might be transduced into alterations in ligand (e.g. ankyrin) binding, which could provide selective plasticity of the membrane scaffold and a mechanical switch that links membrane deformations and spatial organization of receptors or signal-transducing molecules (Stabach et al. 2009). The ankyrin-dependent lipid-binding site may be involved in preventing the aggregation of the spectrin tetramers that are linked to some transmembrane proteins via ankyrin. As the number of ankyrin molecules and spectrin tetramers in erythrocytes is similar and each spectrin tetramer contains two ankyrin/PE binding sites, it seems that the lipid-binding activity of these sites may play a key role in the appropriate spatial arrangement of individual ankyrin-transmembrane complexes (Chorzalska et al. 2010).
12.4 Dystrophin and Spectrin Mutations Related to Pathologies
The two devastating diseases Duchenne and Becker muscular dystrophies (DMD and BMD) occur after mutation of the DMD gene (Koenig et al. 1989). The main types of mutation resulting in dystrophinopathies are deletions of one or several exon(s), while there are ~10 % of duplications of exons and ~15 % of single point mutations (Tuffery-Giraud et al. 2009; Flanigan et al. 2009). According to the Monaco rule (Monaco et al. 1988), if the deletion in the DMD gene leads to a reading frame shift, there is a total absence of dystrophin in the muscle and severe phenotypes are observed in patients, usually classified as DMD. If the exon deletions do not shift the reading frame, the presence of an internally truncated dystrophin is detected and this is related to a large and variable range in the severity of symptoms as typified by the BMD phenotype. In certain cases, exceptions to the Monaco rule appear where a DMD phenotype with an in-frame mutation is observed. This is particularly prominent when mutations involve the N-terminal actin-binding-domain or the Cys-rich domain affecting the binding of dystrophin to F-actin or β-dystroglycan, respectively.
DMD is characterized by a progressive muscle weakness involving all skeletal and cardiac muscles and is accompanied by highly elevated creatine kinase blood levels (Kohler et al. 2005; Davies and Nowak 2006; Rahimov and Kunkel 2013). The early clinical signs leading to a DMD diagnosis are severe difficulties to walk and to climb stairs in the second or third year of childhood. DMD young patients are never able to run and are confined to a wheelchair before the age of 12 years (main criteria for DMD diagnosis). Progressively, respiratory and cardiac impairments are observed and DMD patients mainly die in their early thirties (Wein et al. 2015). All these specific DMD clinical signs are similarly observed in BMD patients, nevertheless with a very broad range of time courses and severity. Some BMD patients are considered as asymptomatic, while others become wheelchair confined around 16 years of age. Some BMD patients survive to very old ages while others die prematurely from heart failure (Bushby and Gardner-Medwin 1993; Bushby et al. 1993). Histological analyses of DMD muscles show cycles of fiber necrosis and regeneration. The regeneration process is however overtaken by fiber loss mechanisms and fibrosis, as adipose tissue replacement is also increased. All these features are vary markedly during DMD evolution or in different types of BMD. For instance the fibrosis and fatty infiltration increase with the age in DMD patients. Finally, electron microscopy studies have revealed lesions of the plasma membrane in the case of DMD indicating that the maintenance of plasma membrane integrity is the essential role of dystrophin in muscle cells (Petrof et al. 1993).
From a structural point of view, the effect of exon deletion occurring in the central domain of dystrophin and associated with the BMD disease depends on the maintainance of the repeat phasing. These deletions disrupt repeat coding, but this results in main two cases. Indeed, after exon deletion which maintains the reading frame a new junction is created in the shortened dystrophin. This new junction either restores a hybrid repeat comprising a three-helix coiled-coil or it constitutes a new structural motif not in a coiled-coil fold, but leading to the formation of a fractional repeat. The first structural evidence for these rules was obtained through the computation of BMD shortened dystrophin fragments (Nicolas et al. 2012) and has recently been confirmed by the experimental determination of the SAXS-based models for a dystrophin fragment bearing the most frequent BMD deletion of exons 45–47 (∆45–47; Delalande et al. to be published). Analysis of this model based on low-resolution experimental data clearly shows that the structural organization of the coiled-coil filament is strongly disturbed at the newly created junction. It is important to notice that, with the changed exon phasing, this alteration of the structure leads to the loss of the previously characterized binding site of nNOS. In addition, we showed that the two types of structure of the internally deleted dystrophins produced in these BMD patients could partly explain the differences in the clinical severity of the patients (Nicolas et al. 2015).
The most promising strategies for DMD therapy are to transform a DMD patient into a BMD patient with an asymptomatic phenotype by using exon-skipping and gene or cell therapies.
Exon-skipping aims to transform a DMD patient with an out-of-frame deletion into a BMD patient by deleting additional exons to restore the reading frame (Aartsma-Rus and Van Ommen 2007; Goyenvalle et al. 2004). The most valuable deletions may be those restoring a hybrid repeat at the new junction and the studies reporting the correlation between dystrophin structure and clinical outcomes of BMD patients are highly valuable.
Gene therapy aims to deliver DNA sequences expressing the most important parts of the protein into skeletal and heart cells. The DMD gene is the largest human gene and it is not possible to put the entire cDNA sequence into a unique vector that can reach all muscle cells. Therefore, the concept of micro-dystrophin has emerged (Harper et al. 2002). Truncated gene coding sequences (micro-dystrophins), inspired by the truncated dystrophin coding sequences observed in mild BMD patients (England et al. 1990), have been designed (Seto et al. 2012; Fairclough et al. 2013; Jarmin et al. 2014; Mcgreevy et al. 2015). These highly simplified micro-dystrophins only consist of the N- and Cys-rich domains with two hinges and several repeats (Gregorevic et al. 2008; Foster et al. 2008). However, they do not replicate all the functions of dystrophin (Seto et al. 2012) and further improvements are needed based on our knowledge about the structure and function of the central domain of dystrophin (Wilton et al. 2015).
The spectrin-based membrane skeleton is a prerequisite for maintaining the integrity and flexibility of cells. This is particularly important for erythrocytes which experience strong mechanical stresses during high speed flow in the circulation and major deformations due to their passage through capillaries of diameter much smaller than the size of the cells. Thus, defects in the components of the spectrin-based skeleton are reflected in the fragility, fragmentation and premature destruction of red blood cells. Such disorders lead to hemolytic anemias, including hereditary spherocytosis (HS), elliptocytosis (HE), pyropoikylocytosis (HPP) and stomatocytosis (HSt). In the case of the former, most of the molecular defects concern membrane skeleton components involved in vertical interactions, namely spectrin, ankyrin, anion exchanger 1 and protein 4.2. On the other hand, HE and HPP are characterized by molecular defects of proteins involved in horizontal interactions within the membrane skeleton, namely spectrin and protein 4.1 (Gallagher 2004b). Very little is known about the molecular background of HSt, a rare red cell disorder resulting from altered intracellular cation content and some cell volume alterations (Bruce 2009; Boguslawska et al. 2010).
Hereditary spherocytosis (HS) is the most widely spread inherited disease linked to the red blood cell membrane, affecting more than one per two thousand individuals (Da Costa et al. 2013). In three-quarters of cases the inheritance is dominant. The decreased deformability and spheroidal shape of erythrocytes is associated with the loss of membrane surface area in relation to intracellular volume (Eber and Lux 2004). Such cells are sequestered in the spleen and subsequently phagocytosed by macrophages, which results in anaemia and splenomegaly. Manifestation of spherocytosis may vary substantially from patient to patient, starting from very few spherocytes in approx. 35 % of mild cases of HS. Minor to moderate forms of the disease are characteristic of defects in the SPTB gene. These cases comprise 15–30 % of the HS population and the transmission is autosomal dominant, although sporadic mutations have also been described. More than 25 mutations elucidated in this gene include ten nonsense or non-coding sequences, ten null mutations and five missense mutations (Bolton-Maggs et al. 2012). The latter group is represented by spectrin Atlanta and spectrin Kissimmee, where the mutation is located in the conserved region that plays a role in interactions with protein 4.1. More severe forms of HS have their sources in mutations in the SPTA1 gene, although these are relatively rare (<5 % of HS population). In these cases, homozygous or compound heterozygous mutations lead to the development of the disease and can be related to the fact that in erythroid cells α-spectrin is produced in large excess over β-spectrin. An interesting example is the low expression allele αLEPRA (low expression allele of SPTA1 gene, 3.3 %) that contains a C-to-T transition at position −99 of intron 30. The mutation activates an alternative acceptor site 70 nucleotides upstream from the usual site, which leads to mis-splicing and a frameshift in pre-mRNA. αLEPRA is supposed to be broadly involved in recessive or non-dominant HS, although it is silent in normal individuals. Severe hemolytic anaemia is a result of its combination in trans with the α-spectrin Prague mutation (Wichterle et al. 1996). The Prague mutation leads to exon skipping, frameshifts and the production of a truncated protein.
Hereditary elliptocytosis (HE) is another common disease related to mutations in genes encoding membrane skeleton proteins. In general, HE is distributed worldwide, ranging from 1 to 2 cases per ten thousand Caucasians and up to 2 % of the population in malaria endemic regions (Glele-Kakai et al. 1996; Dhermy et al. 2007). HE individuals have a resistance to the parasite P.falciparum, as the absence of protein 4.1 and mutations in the spectrin tetramerization site impede binding of the parasite to the host cells (Waller et al. 2003). The hallmark of HE is a mechanically unstable cell membrane, mostly due to mutations in spectrins (90 % of cases). The shape of erythrocytes becomes elliptic, although the cell morphology and clinical severity is heterogeneous. While the majority of HE cases are asymptomatic, homozygous and compound heterozygous individuals suffer from mild-to-severe anaemia, jaundice and splenomegaly (Gallagher 2004a). Moreover, a few cases of fatal hydrops fetalis were reported (Gallagher et al. 1995). Greater membrane fragility, reflected by the presence of numerous poikilocytes, together with thermal sensitivity of erythrocytes, was initially considered as a distinct type of disorder called hereditary pyropoikylocytosis (HPP), but in fact it corresponds to severe cases of HE (Lecomte et al. 1987). The vast majority of HE cases are related to the disturbed ability of the spectrin dimers to self-aggregate into functional tetramers, which are crucial for mechanical stability of the red blood cell membrane (Lecomte et al. 1993). Indeed, most of the defects are linked to missense mutations located at or in the vicinity of the spectrin tetramerization sites; in the N-terminal helix C and first spectrin repeat of the α subunit or within the two C-terminal helices of the β subunit. Some mutations lead to truncations of the C-terminus of β spectrin. In general, location of the molecular defects determines the clinical phenotype of the disease (Lecomte et al. 1993). When mutations are present within the helices critical to the formation of the spectrin tetramer (Nicolas et al. 1998), and they involve amino acid residues that play a key role in stabilization of the spectrin repeat restored after self-association (Zhang et al. 2001; Gaetani et al. 2008), patients experience a more severe form of HE. It is worth underlining that more than 25 mutations related to HE are located within the SPTA1 gene, while the SPTB gene is less affected. This is related to the issue that another factor that determines the severity of HE is the existence of a low expression α allele in trans. For example, the αLELY (Low Expression Lyon) allele has a C-to-T mutation at nucleotide −12 of intron 45 which leads to deletion of a few amino acid residues within the 20th repeat of α-spectrin. As a consequence, αβ dimer formation is compromised (Wilmotte et al. 1997).
The fact that up to 3 % of the total protein in brain is represented by spectrin reflects how important this protein is in neuronal cells where it interacts with numerous membrane proteins (Davis and Bennett 1983; Machnicka et al. 2014). In neuronal axons spectrin forms a regular network of spectrin tetramers connected to junctional complexes (Xu et al. 2013). Of particular importance is the involvement of non-erythrocytic β spectrins in the docking of synaptic vesicles to the presynaptic membrane, mostly through interactions with synapsin (Sikorski et al. 1991). The latter binds to a roughly 25 amino acid residue segment of the β subunit, and this process is critical for neurotransmission (Sikorski et al. 2000; Zimmer et al. 2000). Deletion mutations of α or β spectrin in D. melanogaster result in a severe distortion of synaptic transmission and disrupt the spatial arrangement of synaptic components (Featherstone et al. 2001).
West syndrome is a relatively rare epileptic disease affecting infants. The basis of the disease apparently relates to distortions in neurotransmitter function. Most recently it was shown that some mutations in αII-spectrin are related to the West syndrome (Saitsu et al. 2010; Writzl et al. 2012). The observed mutations lead to severe cerebral hypomyelination, decreased white matter, brain atrophy and reduced corpus callosum. A deletion within the helix linker between repeats 19 and 20 and duplication within repeat 20 of α-spectrin affect the region of the protein responsible for the formation of spectrin heterodimers. This may disturb β-spectrin-mediated stabilization of membrane proteins and axonal transport (Holleran et al. 2001; Lorenzo et al. 2010). In this context it is also worth mentioning that αII-spectrin plays an important role in the maintenance of the integrity of myelinated axons, and is enriched at the paranodes which flank Ranvier’s node (Ogawa et al. 2006; Voas et al. 2007).
Mutations in the SPTBN2 gene have been connected with spinocerebellar ataxias (SCAs; Ikeda et al. 2006). Such disorders are characterized by degeneration of cerebellum, spinal cord and brainstem. A 39 nucleotide deletion within exon 12 of the the gene coding βIII-spectrin, causing deletion of amino acid residues 532–544, was found in an American family suffering from spinocerebellar ataxia type 5. Similarly, a 15 nucleotide deletion in exon 14, that leads to deletion of amino acid residues 629–634 and introduction of a Trp residue, was found in a French family. Both mutations refer to spectrin repeat 3, which plays a role in the dimerization of α and β subunits. Another example is a T-to-C transition in exon 7 resulting in a substitution (Leu253Pro) in the highly conserved region of the calponin homology domain. βII-spectrin is mainly expressed in Purkinje cells, where it plays a role in the stabilization of membrane proteins including the glutamate transporter EAAT4 (Ohara et al. 1998; Jackson et al. 2001). Mutations of spectrin prevent proper localization of EAAT4 and GluRg2 on the plasma membrane. Loss of these proteins at the cell membrane may lead to glutamate signalling abnormalities that provoke Purkinie cell death and eventually lead to ataxia. βII-spectrin is also associated with Golgi and vesicles (Stankewich et al. 1998) and binds to dynactin, which may suggest a role of spectrin in vesicular transport (Holleran et al. 2001). Mutations in the CH domain of βII-spectrin may alter its ability to bind to the actin skeleton and thus affect the intracellular transport and/or stabilization of membrane proteins.
12.5 Conclusion
As they share a common structural organization – i.e. a multi-domain filament that includes actin-binding, lipid-binding and EF subdomains and a succession of coiled-coil repeats forming a central domain – Dystrophin and Spectrin are two proteins presenting highly similar topologies and interaction networks. All these specificities are closely related to their scaffolding role, which has been revealed to be essential for the maintenance of the architecture of the cell. Nevertheless, in contrast to spectrin, dystrophin is coded by a single gene, it is non-ubiquitous and monomeric in solution. The comparison of these two proteins is also limited by the focus on the very different pathologies that emerge from the mutation of their encoding genes. Recent structural studies dedicated to the filamentous coiled-coil central domain of both dystrophin and spectrin have provided meaningful details in order to highlight their specific role in the cell and are promising in the pursuit towards understanding the organization of the large macromolecular assemblies managed by these two dissimilar sisters of the same family.
References
Aartsma-Rus A, Van Ommen GJ (2007) Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. RNA 13:1609–1624
Amann KJ, Guo AWX, Ervasti JM (1999) Utrophin lacks the rod domain actin binding activity of dystrophin. J Biol Chem 274:35375–35380
Ameziane-Le Hir S, Raguénès-Nicol C, Paboeuf G, Nicolas A, Chéron A, Le Rumeur E, Vié V (2014) Cholesterol favors higher level of insertion and organization of spectrin-like repeat 16–21 of human dystrophin in membrane. Biochim Biophys Acta 1838:1266–1273
An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N (2004a) Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 43:310–315
An X, Guo X, Wu Y, Mohandas N (2004b) Phosphatidylserine binding sites in red cell spectrin. Blood Cells Mol Dis 32:430–432
An X, Debnath G, Guo X, Liu S, Lux SE, Baines A, Gratzer W, Mohandas N (2005) Identification and functional characterization of protein 4.1R and actin-binding sites in erythrocyte beta spectrin: regulation of the interactions by phosphatidylinositol-4,5-bisphosphate. Biochemistry 44:10681–10688
Bandi S, Singh SM, Mallela KM (2015) Interdomain linker determines primarily the structural stability of dystrophin and utrophin tandem calponin-homology domains rather than their actin-binding affinity. Biochemistry 54:5480–5488
Banuelos S, Saraste M, Djinovic Carugo K (1998) Structural comparisons of calponin homology domains: implications for actin binding. Structure 6:1419–1431
Begg GE, Harper SL, Morris MB, Speicher DW (2000) Initiation of spectrin dimerization involves complementary electrostatic interactions between paired triple-helical bundles. J Biol Chem 275:3279–3287
Berghs S, Aggujaro D, Dirkx R JR., Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T & Solimena M (2000) betaIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol, 151, 985–1002.
Bhosle RC, Michele DE, Campbell KP, Li Z, Robson RM (2006) Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem Biophys Res Commun 346:768–777
Boguslawska DM, Machnicka B, Sikorski AF (2010) Hereditary stomatocytoses--diagnostic problems and their molecular basis. Pol Merkur Lekarski 29:119–124
Boguslawska DM, Heger E, Listowski M, Wasinski D, Kuliczkowski K, Machnicka B, Sikorski AF (2014a) A novel L1340P mutation in the ANK1 gene is associated with hereditary spherocytosis? Br J Haematol 167:269–271
Boguslawska DM, Machnicka B, Hryniewicz-Jankowska A, Czogalla A (2014b) Spectrin and phospholipids – the current picture of their fascinating interplay. Cell Mol Biol Lett 19:158–179
Bok E, Plazuk E, Hryniewicz-Jankowska A, Chorzalska A, Szmaj A, Dubielecka PM, Stebelska K, Diakowski W, Lisowski M, Langner M, Sikorski AF (2007) Lipid-binding role of betaII-spectrin ankyrin-binding domain. Cell Biol Int 31:1482–1494
Bolton-Maggs PH, Langer JC, Iolascon A, Tittensor P, King MJ (2012) Guidelines for the diagnosis and management of hereditary spherocytosis–2011 update. Br J Haematol 156:37–49
Broderick MJ, Bobkov A, Winder SJ (2012) Utrophin ABD binds to F-actin in an open conformation. FEBS Open Bio 2:6–11
Brown JW, Bullitt E, Sriswasdi S, Harper S, Speicher DW, Mcknight CJ (2015) The physiological molecular shape of spectrin: a compact supercoil resembling a chinese finger trap. PLoS Comput Biol 11:e1004302
Bruce LJ (2009) Hereditary stomatocytosis and cation-leaky red cells--recent developments. Blood Cells Mol Dis 42:216–222
Buevich AV, Lundberg S, Sethson I, Edlund U, Backman L (2004) NMR studies of calcium-binding to mutant alpha-spectrin EF-hands. Cell Mol Biol Lett 9:167–186
Bushby KM, Gardner-Medwin D (1993) The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy I natural history. J Neurol 240:98–104
Bushby KM, Gardner-Medwin D, Nicholson LV, Johnson MA, Haggerty ID, Cleghorn NJ, Harris JB, Bhattacharya SS (1993) The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy II correlation of phenotype with genetic and protein abnormalities. J Neurol 240:105–112
Byers TJ, Lidov HG, Kunkel LM (1993) An alternative dystrophin transcript specific to peripheral nerve. Nat Genet 4:77–81
Campbell K, Kahl S (1989) Association of dystrophin and an integral membrane glycoprotein. Nature 338:259–262
Chelly J, Hamard G, Koulakoff A, Kaplan JC, Kahn A, Berwald-Netter Y (1990) Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature 344:64–65
Cianci CD, Zhang Z, Pradhan D, Morrow JS (1999) Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry 38:15721–15730
Czogalla A, Sikorski AF (2005) Spectrin and calpain: a ‘target’ and a ‘sniper’ in the pathology of neuronal cells. Cell Mol Life Sci 62:1913–1924
Czogalla A, Sikorski AF (2010) Do we already know how spectrin attracts ankyrin? Cell Mol Life Sci 67:2679–2683
Czogalla A, Jaszewski AR, Diakowski W, Bok E, Jezierski A, Sikorski AF (2007) Structural insight into an ankyrin-sensitive lipid-binding site of erythroid beta-spectrin. Mol Membr Biol 24:215–224
Czogalla A, Grzymajlo K, Jezierski A, Sikorski AF (2008) Phospholipid-induced structural changes to an erythroid beta spectrin ankyrin-dependent lipid-binding site. Biochim Biophys Acta 1778:2612–2620
Chorzalska A, Lach A, Borowik T, Wolny M, Hryniewicz-Jankowska A, Kolondra A, Langner M, Sikorski AF (2010) The effect of the lipid-binding site of the ankyrin-binding domain of erythroid beta-spectrin on the properties of natural membranes and skeletal structures. Cell Mol Biol Lett 15:406–423
D’souza VN, Nguyen TM, Morris GE, Karges W, Pillers DA, Ray PN (1995) A novel dystrophin isoform is required for normal retinal electrophysiology. Hum Mol Genet 4:837–842
Da Costa L, Galimand J, Fenneteau O, Mohandas N (2013) Hereditary spherocytosis, elliptocytosis, and other red cell membrane disorders. Blood Rev 27:167–178
Das A, Base C, Manna D, Cho W, Dubreuil RR (2008) Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. J Biol Chem 283:12643–12653
Davies KE, Nowak KJ (2006) Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol 7:762–773
Davis J, Bennett V (1983) Brain spectrin Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem 258:7757–7766
Davis L, Abdi K, Machius M, Brautigam C, Tomchick DR, Bennett V, Michaely P (2009) Localization and structure of the ankyrin-binding site on beta2-spectrin. J Biol Chem 284:6982–6987
Dhermy D, Schrevel J, Lecomte MC (2007) Spectrin-based skeleton in red blood cells and malaria. Curr Opin Hematol 14:198–202
Djinovic-Carugo K, Gautel M, Ylanne J, Young P (2002) The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett 513:119–123
Dubreuil RR, Grushko T (1998) Genetic studies of spectrin: new life for a ghost protein. BioEssays 20:875–878
Eber S, Lux SE (2004) Hereditary spherocytosis – defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin Hematol 41:118–141
England S, Nicholson L, Johnson M, Forrest S, Love D, Zubrzycka-Gaarn E, Bulman D, Harris J, Davies K (1990) Very mild muscular dystrophy associated with the deletion of 46 % of the dystrophin. Nature 343:180–182
Ervasti J, Campbell K (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121–1131
Ervasti J, Campbell K (1993a) Dystrophin and the membrane skeleton. Curr Opin Cell Biol 5:82–87
Ervasti J, Campbell K (1993b) A Role for the dystrophin-glycoprotein complex as aTransmembrane linker between laminin and actin. J Cell Biol 122:809–823
Ervasti JM, Rybakova IN, Amann KJ (1997) A multiple site side binding model for the interaction of dystrophin with F-actin. Soc Gen Physiol Ser 52:31–44
Fairclough RJ, Wood MJ, Davies KE (2013) Therapy for duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat Rev Genet 14:373–378
Featherstone DE, Davis WS, Dubreuil RR, Broadie K (2001) Drosophila alpha- and beta-spectrin mutations disrupt presynaptic neurotransmitter release. J Neurosci 21:4215–4224
Flanigan KM, Dunn DM, Von Niederhausern A, Soltanzadeh P, Gappmaier E, Howard MT, Sampson JB, Mendell JR, Wall C, King WM, Pestronk A, Florence JM, Connolly AM, Mathews KD, Stephan CM, Laubenthal KS, Wong BL, Morehart PJ, Meyer A, Finkel RS, Bonnemann CG, Medne L, Day JW, Dalton JC, Margolis MK, Hinton VJ, Weiss RB (2009) Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum Mutat 30:1657–1666
Foster H, Sharp PS, Athanasopoulos T, Trollet C, Graham IR, Foster K, Wells DJ, Dickson G (2008) Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol Ther 16:1825–1832
Gaetani M, Mootien S, Harper S, Gallagher PG, Speicher DW (2008) Structural and functional effects of hereditary hemolytic anemia-associated point mutations in the alpha spectrin tetramer site. Blood 111:5712–5720
Galkin VE, Orlova A, Salmazo A, Djinovic-Carugo K, Egelman EH (2010) Opening of tandem calponin homology domains regulates their affinity for F-actin. Nat Struct Mol Biol 17:614–616
Gallagher PG (2004a) Hereditary elliptocytosis: spectrin and protein 4.1R. Semin Hematol 41:142–164
Gallagher PG (2004b) Update on the clinical spectrum and genetics of red blood cell membrane disorders. Curr Hematol Rep 3:85–91
Gallagher PG, Weed SA, Tse WT, Benoit L, Morrow JS, Marchesi SL, Mohandas N, Forget BG (1995) Recurrent fatal hydrops fetalis associated with a nucleotide substitution in the erythrocyte beta-spectrin gene. J Clin Invest 95:1174–1182
Gentil C, Leturcq F, Ben Yaou R, Kaplan JC, Laforet P, Penisson-Besnier I, Espil-Taris C, Voit T, Garcia L, Pietri-Rouxel F (2012) Variable phenotype of del45-55 Becker patients correlated with nNOSmu mislocalization and RYR1 hypernitrosylation. Hum Mol Genet 21:3449–3460
Giudice E, Molza A-E, Laurin Y, Nicolas A, Le Rumeur E, Delalande O (2013) Molecular clues to the dystrophin–nNOS interaction: a theoretical approach. Biochemistry 52:7777–7784
Glele-Kakai C, Garbarz M, Lecomte MC, Leborgne S, Galand C, Bournier O, Devaux I, Gautero H, Zohoun I, Gallagher PG, Forget BG, Dhermy D (1996) Epidemiological studies of spectrin mutations related to hereditary elliptocytosis and spectrin polymorphisms in Benin. Br J Haematol 95:57–66
Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan J, Garcia L, Danos O (2004) Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306:1796–1799
Gregorevic P, Blankinship MJ, Allen JM, Chamberlain JS (2008) Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther 16:657–664
Grum VL, Li D, Macdonald RI, Mondragon A (1999) Structures of two repeats of spectrin suggest models of flexibility. Cell 98:523–535
Harper SQ, Hauser MA, Dellorusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS (2002) Modular flexibility of dystrophin: implications for gene therapy of duchenne muscular dystrophy. Nat Med 8:253–261
Hayes NV, Scott C, Heerkens E, Ohanian V, Maggs AM, Pinder JC, Kordeli E, Baines AJ (2000) Identification of a novel C-terminal variant of beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci 113(Pt 11):2023–2034
Helliwell TR, Man NT, Morris GE, Davies KE (1992) The dystrophin-related protein, utrophin, is expressed on the sarcolemma of regenerating human skeletal muscle fibres in dystrophies and inflammatory myopathies. Neuromuscul Disord 2:177–184
Henderson DM, Lee A, Ervasti JM (2010) Disease-causing missense mutations in actin binding domain 1 of dystrophin induce thermodynamic instability and protein aggregation. Proc Natl Acad Sci U S A 107:9632–9637
Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL (2001) beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem 276:36598–36605
Hryniewicz-Jankowska A, Bok E, Dubielecka P, Chorzalska A, Diakowski W, Jezierski A, Lisowski M, Sikorski AF (2004) Mapping of an ankyrin-sensitive, phosphatidylethanolamine/phosphatidylcholine mono- and bi-layer binding site in erythroid beta-spectrin. Biochem J 382:677–685
Huang X, Poy F, Zhang R, Joachimiak A, Sudol M, Eck MJ (2000) Structure of a WW domain containing fragment of dystrophin in complex with beta-dystroglycan. Nat Struct Biol 7:634–638
Hugnot JP, Gilgenkrantz H, Vincent N, Chafey P, Morris GE, Monaco AP, Berwald-Netter Y, Koulakoff A, Kaplan JC, Kahn A et al (1992) Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc Natl Acad Sci U S A 89:7506–7510
Hyvonen M, Macias MJ, Nilges M, Oschkinat H, Saraste M, Wilmanns M (1995) Structure of the binding site for inositol phosphates in a PH domain. EMBO J 14:4676–4685
Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Durr A, Zuhlke C, Burk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP (2006) Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet 38:184–190
Ipsaro JJ, Mondragon A (2010) Structural basis for spectrin recognition by ankyrin. Blood 115:4093–5101
Ipsaro JJ, Huang L, Mondragon A (2009) Structures of the spectrin-ankyrin interaction binding domains. Blood 113:5385–5393
Ipsaro JJ, Harper SL, Messick TE, Marmorstein R, Mondragon A, Speicher DW (2010) Crystal structure and functional interpretation of the erythrocyte spectrin tetramerization domain complex. Blood 115:4843–4852
Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, Bowers WJ, Federoff HJ, Sternweis PC, Rothstein JD (2001) Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 410:89–93
Jarmin S, Kymalainen H, Popplewell L, Dickson G (2014) New developments in the use of gene therapy to treat Duchenne muscular dystrophy. Expert Opin Biol Ther 14:209–230
Keep NH, Norwood FLM, Moores CA, Winder SJ, Kendrick-Jones J (1999) The 2.0 a structure of the second calponin homology domain from the actin-binding region of the dystrophin homologue utrophin. J Mol Biol 285:1257–1264
Khanna MR, Mattie FJ, Browder KC, Radyk MD, Crilly SE, Bakerink KJ, Harper SL, Speicher DW, Thomas GH (2015) Spectrin tetramer formation is not required for viable development in Drosophila. J Biol Chem 290:706–715
Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM (1987) Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50:509–517
Koenig M, Monaco AP, Kunkel LM (1988) The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53:219–226
Koenig M, Beggs A, Moyer M, Scherpf S, Heindrich K, Bettecken T, Meng G, Muller C, Lindlof M, Kaariainen H (1989) The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet 45:498–506
Kohler M, Clarenbach CF, Boni L, Brack T, Russi EW, Bloch KE (2005) Quality of life, physical disability, and respiratory impairment in Duchenne muscular dystrophy. Am J Respir Crit Care Med 172:1032–1036
Korsgren C, Lux SE (2010) The carboxyterminal EF domain of erythroid alpha-spectrin is necessary for optimal spectrin-actin binding. Blood 116:2600–2607
Korsgren C, Peters LL, Lux SE (2010) Protein 4.2 binds to the carboxyl-terminal EF-hands of erythroid alpha-spectrin in a calcium- and calmodulin-dependent manner. J Biol Chem 285:4757–4770
Kotula L, Desilva TM, Speicher DW, Curtis PJ (1993) Functional characterization of recombinant human red cell alpha-spectrin polypeptides containing the tetramer binding site. J Biol Chem 268:14788–14793
Kusunoki H, Macdonald R, Mondragon A (2004a) Structural insights onto the stability and flexibility of unusual erythroid spectrin repeats. Structure 12:645–656
Kusunoki H, Minasov G, Macdonald R, Mondragon A (2004b) Independent movement, dimerization and stability of tandem repeats of chicken brain alpha-spectrin. J Mol Biol 344:495–511
Kwa LG, Wensley BG, Alexander CG, Browning SJ, Lichman BR, Clarke J (2014) The folding of a family of three-helix bundle proteins: spectrin R15 has a robust folding nucleus, unlike its homologous neighbours. J Mol Biol 426:1600–1610
La-Borde PJ, Stabach PR, Simonovic I, Morrow JS, Simonovic M (2010) Ankyrin recognizes both surface character and shape of the 14-15 di-repeat of beta-spectrin. Biochem Biophys Res Commun 392:490–494
Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D (2009) Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest 119:624–635
Lai Y, Zhao J, Yue Y, Duan D (2013) alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc Natl Acad Sci U S A 110:525–530
Law R, Carl P, Harper S, Dalhaimer P, Speicher DW, Discher DE (2003) Cooperativity in forced unfolding of tandem spectrin repeats. Biophys J 84:533–544
Le Rumeur E, Fichou Y, Pottier S, Gaboriau F, Rondeau-Mouro C, Vincent M, Gallay J, Bondon A (2003) Interaction of dystrophin rod domain with membrane phospholipids: evidence of a close proximity between tryptophan residues and lipids. J Biol Chem 278:5993–6001
Le Rumeur E, Pottier S, DA Costa G, Metzinger L, Mouret L, Rocher C, Fourage M, Rondeau-Mouro C, Bondon A (2007) Binding of the dystrophin second repeat to membrane di-oleyl phospholipids is dependent upon lipid packing. Biochim Biophys Acta 1768:648–654
Le Rumeur E, Winder SJ, Hubert JF (2010) Dystrophin: more than just the sum of its parts. Biochim Biophys Act 1804:1713–1722
Lecomte MC, Dhermy D, Garbarz M, Feo C, Gautero H, Bournier O, Picat C, Chaveroche I, Galand C, Boivin P (1987) Hereditary pyropoikilocytosis and elliptocytosis in a Caucasian family. Transmission of the same molecular defect in spectrin through three generations with different clinical expression. Hum Genet 77:329–334
Lecomte MC, Garbarz M, Gautero H, Bournier O, Galand C, Boivin P, Dhermy D (1993) Molecular basis of clinical and morphological heterogeneity in hereditary elliptocytosis (HE) with spectrin alpha I variants. Br J Haematol 85:584–595
Legardinier S, Hubert J-F, Le Bihan O, Tascon C, Rocher C, Raguénès-Nicol C, Bondon A, Hardy S, Le Rumeur E (2008) Sub-domains of the dystrophin rod domain display contrasting lipid-binding and stability properties. Biochim Biophys Acta 1784:672–682
Legardinier S, Raguénès-Nicol C, Tascon C, Rocher C, Hardy S, Hubert JF, Le Rumeur E (2009) Mapping of the lipid-binding and stability properties of the central rod domain of human dystrophin. J Mol Biol 389:546–558
Legrand B, Giudice E, Nicolas A, Delalande O, Le Rumeur E (2011) Computational study of the human dystrophin repeats: interaction properties and molecular dynamics. PLoS One 6:e23819
Leluk J, Hanus-Lorenz B, Sikorski AF (2001) Application of genetic semihomology algorithm to theoretical studies on various protein families. Acta Biochim Pol 48:21–33
Lemmon MA, Ferguson KM, Abrams CS (2002) Pleckstrin homology domains and the cytoskeleton. FEBS Lett 513:71–76
Lidov HG, Selig S, Kunkel LM (1995) Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Hum Mol Genet 4:329–335
Lin AY, Prochniewicz E, James ZM, Svensson B, Thomas DD (2011) Large-scale opening of utrophin’s tandem calponin homology (CH) domains upon actin binding by an induced-fit mechanism. Proc Natl Acad Sci U S A 108:12729–12733
Lorenzo DN, Li MG, Mische SE, Armbrust KR, Ranum LP, Hays TS (2010) Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J Cell Biol 189:143–158
Machnicka B, Czogalla A, Hryniewicz-Jankowska A, Boguslawska DM, Grochowalska R, Heger E, Sikorski AF (2014) Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim Biophys Acta 1838:620–634
Macias MJ, Musacchio A, Ponstingl H, Nilges M, Saraste M, Oschkinat H (1994) Structure of the pleckstrin homology domain from beta-spectrin. Nature 369:675–677
Mcgreevy JW, Hakim CH, Mcintosh MA, Duan D (2015) Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech 8:195–213
Mehboob S, Song Y, Witek M, Long F, Santarsiero BD, Johnson ME, Fung LW (2010) Crystal structure of the nonerythroid alpha-spectrin tetramerization site reveals differences between erythroid and nonerythroid spectrin tetramer formation. J Biol Chem 285:14572–14584
Mirijanian DT, Chu JW, Ayton GS, Voth GA (2007) Atomistic and coarse-grained analysis of double spectrin repeat units: the molecular origins of flexibility. J Mol Biol 365:523–534
Molza A-E, Férey N, Czjzek M, Le Rumeur E, Hubert J-F, Tek A, Laurent B, Baaden M, Delalande O (2014) Innovative interactive flexible docking method for multi-scale reconstruction elucidates dystrophin molecular assembly. Faraday Discuss 169:45–62
Molza AE, Mangat K, Le Rumeur E, Hubert JF, Menhart N, Delalande O (2015) Structural basis of neuronal nitric-oxide synthase interaction with dystrophin repeats 16 and 17. J Biol Chem 290:29531–29541
Monaco A, Bertelson C, Liechti-Gallati S, Moser H, Kunkel L (1988) An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2:90–95
Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M (1992) Crystal structure of a Src-homology 3 (SH3) domain. Nature 359:851–855
Muthu M, Richardson KA, Sutherland-Smith AJ (2012) The crystal structures of dystrophin and utrophin spectrin repeats: implications for domain boundaries. PLoS One 7:e40066
Nans A, Mohandas N, Stokes DL (2011) Native ultrastructure of the red cell cytoskeleton by cryo-electron tomography. Biophys J 101:2341–2350
Nicolas G, Pedroni S, Fournier C, Gautero H, Craescu C, Dhermy D, Lecomte MC (1998) Spectrin self-association site: characterization and study of beta-spectrin mutations associated with hereditary elliptocytosis. Biochem J 332(Pt 1):81–89
Nicolas A, Lucchetti-Miganeh C, Ben Yaou R, Kaplan JC, Chelly J, Leturcq F, Barloy-Hubler F, Le Rumeur E (2012) Assessment of the structural and functional impact of in-frame mutations of the DMD gene, using the tools included in the eDystrophin online database. Orphanet J Rare Dis 7:45
Nicolas A, Delalande O, Hubert JF, Le Rumeur E (2014) The spectrin family of proteins: a unique coiled-coil fold for various molecular surface properties. J Struct Biol 186:392–401
Nicolas A, Raguenes-Nicol C, Ben Yaou R, Ameziane-Le Hir S, Cheron A, Vie V, Claustres M, Leturcq F, Delalande O, Hubert JF, Tuffery-Giraud S, Giudice E, Le Rumeur E (2015) Becker muscular dystrophy severity is linked to the structure of dystrophin. Hum Mol Genet 24:1267–1279
Nilges M, Macias MJ, O’donoghue SI, Oschkinat H (1997) Automated NOESY interpretation with ambiguous distance restraints: the refined NMR solution structure of the pleckstrin homology domain from beta-spectrin. J Mol Biol 269:408–422
Norwood F, Sutherland-Smith A, Keep N, Kendrick-Jones J (2000) The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure 8:481–491
Nudel U, Zuk D, Einat P, Zeelon E, Levy Z, Neuman S, Yaffe D (1989) Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature 337:76–78
Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband MN (2006) Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci 26:5230–5239
Ohara O, Ohara R, Yamakawa H, Nakajima D, Nakayama M (1998) Characterization of a new beta-spectrin gene which is predominantly expressed in brain. Brain Res Mol Brain Res 57:181–192
Parry DAD, Dixon TW, Cohen C (1992) Analysis of the three-alpha-helix motif in the spectrin superfamily of proteins. Biophys J 61:858–867
Pascual J, Pfuhl M, Walther D, Saraste M, Nilges M (1997) Solution structure of the spectrin repeat: a left-handed antiparallel triple-helical coiled-coil. J Mol Biol 273:740–751
Petrof BJ, Shrager JB, Stedmann HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proceedings of the national academy of sciences, USA, 90, 3710–3714
Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM (2009) Dystrophin is a microtubule-associated protein. J Cell Biol 186:363–369
Rahimov F, Kunkel LM (2013) The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol 201:499–510
Ribiero Ede A Jr, Pinotsis N, Ghisleni A, Salmazo A, Konarev PV, Kostan J, Sjöblum B, Schreiner C, Polyansky AA, Gkougkoulia EA, Holt MR, Aachnann FL, Zagrovic B, Bordignon E, Pirker KF, Svergun DI, Gautel M, Djinovic-Carugo K. (2014) The structure and regulation of human muscle α-actinin. Cell 159:1447–1460
Robertsson J, Petzold K, Löfvenberg L, Backman L (2005) Folding of spectrin’s SH3 domain in the presence of spectrin repeats. Cell Mol Biol Lett 10(4):595–612, PubMed PMID: 16341269
Sahr KE, Laurila P, Kotula L, Scarpa AL, Coupal E, Leto TL, Linnenbach AJ, Winkelmann JC, Speicher DW, VT M et al (1990) The complete cDNA and polypeptide sequences of human erythroid alpha-spectrin. J Biol Chem 265:4434–4443
Saitsu H, Tohyama J, Kumada T, Egawa K, Hamada K, Okada I, Mizuguchi T, Osaka H, Miyata R, Furukawa T, Haginoya K, Hoshino H, Goto T, Hachiya Y, Yamagata T, Saitoh S, Nagai T, Nishiyama K, Nishimura A, Miyake N, Komada M, Hayashi K, Hirai S, Ogata K, Kato M, Fukuda A, Matsumoto N (2010) Dominant-negative mutations in alpha-II spectrin cause west syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am J Hum Genet 86:881–891
Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG (2000) Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 97:13818–13823
Sarkis J, Hubert JF, Legrand B, Robert E, Cheron A, Jardin J, Hitti E, Le Rumeur E, Vie V (2011) Spectrin-like repeats 11–15 of human dystrophin show adaptations to a lipidic environment. J Biol Chem 286:30481–30491
Sato K, Yokota T, Ichioka S, Shibata M, Takeda S (2008) Vasodilation of intramuscular arterioles under shear stress in dystrophin-deficient skeletal muscle is impaired through decreased nNOS expression. Act Myol 27:30–36
Seto JT, Ramos JN, Muir L, Chamberlain JS, Odom GL (2012) Gene replacement therapies for duchenne muscular dystrophy using adeno-associated viral vectors. Curr Gene Ther 12:139–151
Sikorski AF, Terlecki G, Zagon IS, Goodman SR (1991) Synapsin I-mediated interaction of brain spectrin with synaptic vesicles. J Cell Biol 114:313–318
Sikorski AF, Sangerman J, Goodman SR, Critz SD (2000) Spectrin (betaSpIIsigma1) is an essential component of synaptic transmission. Brain Res 852:161–166
Singh SM, Mallela KM (2012) The N-terminal actin-binding tandem calponin-homology (CH) domain of dystrophin is in a closed conformation in solution and when bound to F-actin. Biophys J 103:1970–1978
Stabach PR, Morrow JS (2000) Identification and characterization of beta V spectrin, a mammalian ortholog of Drosophila beta H spectrin. J Biol Chem 275:21385–21395
Stabach PR, Simonovic I, Ranieri MA, Aboodi MS, Steitz TA, Simonovic M, Morrow JS (2009) The structure of the ankyrin-binding site of beta-spectrin reveals how tandem spectrin-repeats generate unique ligand-binding properties. Blood 113:5377–5384
Stankewich MC, Tse WT, Peters LL, Ch’ng Y, John KM, Stabach PR, Devarajan P, Morrow JS, Lux SE (1998) A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci U S A 95:14158–14163
Sutherland-Smith AJ, Moores CA, Norwood FL, Hatch V, Craig R, Kendrick-Jones J, Lehman W (2003) An atomic model for actin binding by the CH domains and spectrin-repeat modules of utrophin and dystrophin. J Mol Biol 329:15–33
Tinsley J, Blake D, Roche A, Fairbrother U, Riss J, Byth B, Knight A, Kendrick-Jones J, Suthers G, Love D, Edwards Y, Davies K (1992) Primary stucture of dystrophin- related protein. Nature 360:591–593
Tuffery-Giraud S, Beroud C, Leturcq F, Yaou RB, Hamroun D, Michel-Calemard L, Moizard MP, Bernard R, Cossee M, Boisseau P, Blayau M, Creveaux I, Guiochon-Mantel A, De Martinville B, Philippe C, Monnier N, Bieth E, Khau Van Kien P, Desmet FO, Humbertclaude V, Kaplan JC, Chelly J, Claustres M (2009) Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum Mutat 30:934–945
Voas MG, Lyons DA, Naylor SG, Arana N, Rasband MN, Talbot WS (2007) alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol 17:562–568
Waller KL, Nunomura W, An X, Cooke BM, Mohandas N, Coppel RL (2003) Mature parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum binds to the 30-kDa domain of protein 4.1 in malaria-infected red blood cells. Blood 102:1911–1914
Wein N, Alfano L, Flanigan KM (2015) Genetics and emerging treatments for Duchenne and Becker muscular dystrophy. Pediatr Clin N Am 62(3):723–742
Wichterle H, Hanspal M, Palek J, Jarolim P (1996) Combination of two mutant alpha spectrin alleles underlies a severe spherocytic hemolytic anemia. J Clin Invest 98:2300–2307
Wilmotte R, Harper SL, Ursitti JA, Marechal J, Delaunay J, Speicher DW (1997) The exon 46-encoded sequence is essential for stability of human erythroid alpha-spectrin and heterodimer formation. Blood 90:4188–4196
Wilton SD, Veedu RN, Fletcher S (2015) The emperor’s new dystrophin: finding sense in the noise. Trends Mol Med 21(7):417–426
Winder SJ, Gibson TJ, Kendrick-Jones J (1995) Dystrophin and utrophin: the missing links! FEBS Lett 369:27–33
Winkelmann JC, Chang JG, Tse WT, Scarpa AL, Marchesi VT, Forget BG (1990) Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem 265:11827–11832
Wolny M, Grzybek M, Bok E, Chorzalska A, Lenoir M, Czogalla A, Adamczyk K, Kolondra A, Diakowski W, Overduin M, Sikorski AF (2011) Key amino acid residues of ankyrin-sensitive phosphatidylethanolamine/phosphatidylcholine-lipid binding site of betaI-spectrin. PLoS One 6:e21538
Writzl K, Primec ZR, Strazisar BG, Osredkar D, Pecaric-Meglic N, Kranjc BS, Nishiyama K, Matsumoto N, Saitsu H (2012) Early onset West syndrome with severe hypomyelination and coloboma-like optic discs in a girl with SPTAN1 mutation. Epilepsia 53:e106–e110
Xu K, Zhong G, Zhuang X (2013) Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339:452–456
Yan Y, Winograd E, Viel A, Cronin T, Harrison SC, Branton D (1993) Crystal structure of the repetitive segments of spectrin. Science 262:2027–2030
Zhang P, Talluri S, Deng H, Branton D, Wagner G (1995) Solution structure of the pleckstrin homology domain of drosophila beta-spectrin. Structure 3:1185–1195
Zhang Z, Weed SA, Gallagher PG, Morrow JS (2001) Dynamic molecular modeling of pathogenic mutations in the spectrin self-association domain. Blood 98:1645–1653
Zimmer WE, Zhao Y, Sikorski AF, Critz SD, Sangerman J, Elferink LA, Xu XS, Goodman SR (2000) The domain of brain beta-spectrin responsible for synaptic vesicle association is essential for synaptic transmission. Brain Res 881:18–27
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Delalande, O., Czogalla, A., Hubert, JF., Sikorski, A., Le Rumeur, E. (2017). Dystrophin and Spectrin, Two Highly Dissimilar Sisters of the Same Family. In: Parry, D., Squire, J. (eds) Fibrous Proteins: Structures and Mechanisms. Subcellular Biochemistry, vol 82. Springer, Cham. https://doi.org/10.1007/978-3-319-49674-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-49674-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49672-6
Online ISBN: 978-3-319-49674-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)