Abstract
The potential genotoxicity of Cu2+ was investigated in Vicia faba and Pisum sativum seedlings in hydroponic culture conditions. Cu2+ caused a dose-dependent increase in micronuclei frequencies in both plant models. Cytological analysis of root tips cells showed clastogenic and aneugenic effects of this heavy metal on V. faba root meristems. Cu2+ induced chromosomal alterations at the lowest concentration used (2.5 mM) when incubated for 42 h, indicating the potent mutagenic effect of this ion. A spectrum of chromosomal abnormalities was observed in V. faba root meristems, illustrating the genotoxic events leading to micronuclei formation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal deposition has increased dramatically over the last decades as a result of anthropogenic activities leading to heavily polluted areas worldwide. One of the major problems of this environmental pollution is copper over-accumulation in soils. Its concentration can reach 500 mg kg–1 in vineyard soils (Deluisa et al. 1996; Brun et al. 2001) or even more in the vicinity of copper-nickel smelters in the northern hemisphere, with 5800 mg kg–1 being measured in the organic layer of the strongest polluted places (Helmisaari et al. 1995). Copper is an essential micronutrient for higher plant growth and metabolism (Maksymiec 1997). However, its high bioavailability in soils make it a potentially toxic substance causing inhibition of growth and oxidative injuries (Hall 2002; Schützendübel and Polle 2002; Yruela 2005).
The formation of micronuclei (MCN) in root tips has been widely described and used as a bioassay for the evaluation of in vivo mutagenic effects of environmental pollutants on plants (Grant and Owens 1998; Knasmüller et al. 1998; Türkoglu 2007). MCN can be composed of small chromosome fragments resulting from chromosome breaks caused by clastogenic activity. The failure of entire chromosomes to migrate during anaphase as a result of the aneugenic effects of genotoxic agents can also lead to MCN formation (Krishna and Hayashi 2000; Çava and Ergene-Gözükara 2003).
In this study, we analysed and compared the potentially genotoxic effects of high Cu2+ concentrations on two closely related Fabaceae species, Vicia faba and Pisum sativum. Cytological analysis of V. faba meristematic root cells showed the potential clastogenic and aneugenic effects of Cu2+ on V. faba chromosomes. The different types of mitotic chromosomal abnormalities could be grouped into two classes, according to the structural and/or numerical disturbances that might lead to MCN formation.
Materials and methods
The genotoxicity of an essential heavy metal for meristematic root cells of two related Fabaceae species, V. faba and P. sativum, was analysed. Both species have a low chromosome number (2n = 12 and 2n = 14, respectively), making them suitable for cytogenetic studies. Seeds of V. faba var. Aguadulce and P. sativum var. Douce de Provence were germinated on saturated paper at 25°C for 4 to 5 days, and transferred to a hydroponic support in the following nutrient solution (pH 7): 3.9 mM Ca(NO3)2, 6.5 mM KNO3, 2 mM MgSO4, 0.9 mM KH2PO4 plus micronutrients: 90 μM Fe-ethylene diamine tetra-acetic acid, 2.7 μM MnSO4, 0.8 μM ZnSO4, 4.5 μM H3BO3, 4 μM CuSO4 and 2.0 μM Mo7O24(NH4)6. Once roots had reached a length of 2–3 cm, additional concentrations of CuSO4 (1 to 50 mM) were added to the hydroponic solution for 42 h at 25°C with a light/dark photoperiod of 16:8 h. All tests were repeated three times, using a negative control (containing no additional Cu2+), and nutrient solution containing 4 × 10–3 M maleic hydrazide (MH) as a positive control. MH is a herbicide known to be a mutagenic and clastogenic agent (Marcano et al. 2004).
Root tips (meristem zones) were cut and placed overnight in the dark in the Carnoy fixation solution containing ethanol and glacial acetic acid (3:1) at 4°C, and then stored in 70% ethanol. Root tips were rinsed with distilled water and hydrolysed with 1 N HCl for 10 min. The root cap was removed before squashing root tissues, and samples were stained with orcein. The slides were examined under a Zeiss microscope. At least three slides were stained per replica and at least 1000 cells were scored from each slide. Therefore, the analysis was conducted on an average of 9000 cells per treatment. MCN frequency was calculated from the number of MCN scored divided by the total cells scored, and expressed in terms of MCN/1000 cells.
Results and discussion
Micronuclei induction
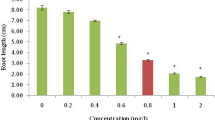
In order to assess the genotoxic effects of Cu2+, different concentrations were applied for a transient period of 42 h in hydroponic cultures. The frequencies of cells with Cu2+-induced MCN in meristematic root tips of both plant species are presented in Fig. 1. Low MCN frequencies were detected in root tips of control plants. However, as expected, MH dramatically increased the frequency of MCN in root meristems as compared to control plants, showing that this herbicide can be used as a positive control for genotoxicity studies.
Cu2+ significantly enhanced the frequency of MCN formation in the root tips of V. faba and P. sativum (p ≧ 0.05). MCN induction was significant (p < 0.05) when 2.5 mM Cu2+ was used. In both plant species, the frequency of MCN formation was proportional to the Cu2+ concentration added to the hydroponic solution. In our experiments, the highest frequency of cells with MCN was detected in the presence of 50 mM Cu2+. At this high Cu2+ concentration, the MCN frequency in root tips was dramatically higher in V. faba than in P. sativum (20 times higher), suggesting that V. faba is more sensitive than P. sativum. The potential tolerance of P. sativum to chemicals has been demonstrated with MH treatment and other chemicals (Jain and Sarbhoy 1987a, b). As previously suggested (Ma et al. 1995), a greater total length of the diploid complement and higher number of metacentric chromosomes may result in greater sensitivity to DNA damage-inducing agents like heavy metals. Vicia contains long and thin chromosomes suitable for genotoxicity analysis, while Pisum contains shorter chromosomes (Blixt 1972). Further investigation is required to determine whether metacentric chromosomes present a potential target for heavy metal action.
Induction of chromosomal aberrations
We investigated chromosomal aberrations in V. faba root cells, since this species displayed higher sensitivity to Cu2+ in terms of MCN induction. In addition to MCN formation, cytological analysis of root meristems revealed drastic changes in the organization and morphology of chromosomes (Fig. 2). We could group the chromosomal abnormalities detected in meristematic cells into two classes: structural and numerical aberrations. Our results corroborate the documented MCN formation in animal cells containing chromosomal aberrations induced by exposure to genotoxic agents (Krishna and Hayashi 2000; Iarmarcovai et al. 2006). Chromosome stickiness should be the initial event that occurs in prophase cells. This has been described as chromosome agglutinations displaying a sticky appearance (Rieger et al. 1976) and a winding of chromosomal fibres created by chromatid bridges (Jiang et al. 2000). This phenomenon is regarded as a physiological effect exerted by chemical agents that may affect peripheral proteins such as DNA topoisomerase II (Gaulden 1987). According to Gömürgen (2005), this chromosomal abnormality leads to chromosome bridges, a result of the failure of free anaphase separation, unequal translocation or inversion of chromosome segments. Since stickiness holds the two chromosomes together, the separation process can lead to chromosome rupture, i.e. chromosome break formation. Therefore, chromosome stickiness, chromosome bridges and chromosome breaks are closely related. These chromosome aberrations, sticky metaphase, anaphase bridges and fragments of chromosomes in telophase (Fig. 2a–d), were detected during the mitotic phases when V. faba seedlings were incubated with Cu2+. Since Cu2+ induced chromosome fragmentation, this heavy metal should be considered as a clastogenic agent.
Cytological analysis of chromosomal aberrations in Vicia faba meristematic root cells, illustrating the different origins of MCN formation. The mitotic chromosomal abnormalities can be grouped into two classes: structural and numerical aberrations. Both structural and numerical aberrations may be observed in mitotic cells. a: stickiness in metaphase, b and c: chromosome bridges in anaphase, d: chromosome breaks in telophase, e and f: laggard chromosomes in telophase, g: isolated chromosome in telophase, h: acentric chromosome and anaphase bridge, i and j: micronuclei in telophase and interphase, respectively, k: micronuclei in prophase. Arrows indicate the chromosomal aberrations and Asterisks the micronuclei
Besides structural aberrations, Cu2+ also induced numerical aberrations in V. faba root meristems. Numerical aberrations resulting from acentric fragments or lagging chromosomes lead to aneuploidy, i.e. loss of genetic material and MCN formation. Aneugenic effects are represented by laggard chromosomes (Fig. 2e) and acentric chromosomes in telophase (Fig. 2f,g). Figure 2h illustrates that anaphase bridges and acentric chromosomes, which lead to structural and numerical aberrations, respectively, can occur simultaneously in mitotic cells. Earlier reports have demonstrated that heavy metals can cause spindle perturbance. Several authors have described the alteration of chromosome numbers by environmental aneuploidy-inducing agents that induce microtubule and kinetochore disorganization in mitotic cells (Borboa and De la Torre 1996; Voutsinas et al. 1997; Seoane and Dulout 2001; Qian 2004). Dysfunction of the spindle mechanism is mainly due to the reactivity of metal ions with thiol groups of tubulin, a multi-cysteine protein in which the cysteine residues are actively involved in regulating microtubule-assembly dynamics (Chaudhuri et al. 2001). Since Cu2+ has a high affinity for sulphydryl groups (Maksymiec 1997), the aneuploidy phenomenon observed in Vicia root meristems might be due to the high sensitivity of the microtubular apparatus to this ion.
The structural and numerical disturbances of chromosomes induced by the heavy metal during mitosis are expected to lead to MCN formation. Chromosome breaks or anaphase bridges, grouped into structural aberrations, result in chromosomal fragments segregating independently to daughter nuclei. Chromosome laggards or non-separated chromosomes, grouped into numerical aberrations, fail to migrate towards either of the daughter nuclei during telophase of the mitotic cells (Krishna and Hayashi 2000; Iarmarcovai et al. 2006). MCN formation was also obvious in V. faba root cells during telophase or interphase (Fig. 2i,j). Cells containing abnormal chromosomes could enter a new mitotic cycle (Fig. 2k).
To conclude, our work illustrates the genotoxic events leading to MCN formation through different pathways in the studied model plant. This is obvious even when an essential element like Cu2+ is used at a high concentration (>2.5 mM), causing chromosome breaks and chromosome migration impairment. This heavy metal, therefore, acts as a potent genotoxic agent.
In an environmental perspective, the bioavailability of Cu2+ might become an increasing concern for a range of living organisms including cultivated plants. The necessity to elucidate Cu2+ toxicity results from its systematic use as a fungicide, algicide or bacteriocide in agriculture. The evaluation of Cu2+ bioavailability is highly complex and plant species specific, since it is related not only to the physical-chemical properties of the soil but also to processes governed by plants (Hinsinger et al. 2006). Plant bioassay systems allowing the detection of cellular genotoxic events in meristems provide the best approach for assessing metal bioavailability, especially for Cu2+ which accumulates substantially in the roots of plants. Another indicator of Cu2+ bioavailability has also been developed, in which Cu2+ uptake is estimated by quantifying the Cu2+ content of the plant (Brun et al. 2001). Combining plant bioassays and root analyses of Cu2+ content would allow us to define phytotoxicity thresholds in plants.
Abbreviations
- MCN:
-
micronuclei
- MH:
-
maleic hydrazide
References
Blixt S (1972) Mutation genetic in Pisum. Agric Hort Genet 30:1–293
Borboa L, De la Torre C (1996) The genotoxicity of Zn(II) and Cd(II) in Allium cepa root meristematic cells. New Phytol 134:481–486
Brun LA, Maillet J, Hinsinger P, Pépin M (2001) Evaluation of copper availability to plants in copper-contaminated vineyard soils. Environ Pollut 111:293–302
Çava T, Ergene-Gözükara S (2003) Evaluation of the genotoxic potential of lambda-cyhalothrin using nuclear and nucleolar biomarkers on fish cells. Mutat Res/Genet Toxicol Environ Mutagen 534:93–99
Chaudhuri AR, Khan IA, Ludueña RF (2001) Detection of disulfide bonds in bovine brain tubulin and their role in protein folding and microtubule assembly in vitro: a novel disulfide detection approach. Biochemistry 40:8834–8841
Deluisa A, Giandon P, Aichner M, Bortolami P, Bruna L, Lupetti A, Nardelli F, Stringari G (1996) Copper pollution in Italian vineyard soils. Commun Soil Sci Plant Anal 27:1537–1548
Gaulden ME (1987) Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosomal aberrations. Mutagenesis 2:357–365
Gömürgen AN (2005) Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia 70:119–128
Grant WF, Owens E-T (1998) Chromosome aberration assays in Crepis for study of environmental mutagens. Mutat Res 410:291–307
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Helmisaari H-S, Derome J, Fritze H, Nieminen T, Palmgren K, Salemaa M, Vanha-Majamaa I (1995) Copper in Scots pine forests around a heavy-metal smelter in South-Western Finland. Water Air Soil Pollut 85:1727–1732
Hinsinger P, Plassard C, Jaillard B (2006) Rhizosphere: a new frontier for soil biogeochemistry. J Geochem Explor 88:210–213
Iarmarcovai G, Botta A, Orsière T (2006) Number of centromeric signals in micronuclei and mechanisms of aneuploidy. Toxicol Lett 166:1–10
Jain AK, Sarbhoy RK (1987a) Cytogenetical studies on the effects of some chlorinated pesticides. I. Effect on somatic chromosomes of Lens and Pisum. Cytologia 52:47–53
Jain AK, Sarbhoy RK (1987b) Cytogenetical studies on the effects of some chlorinated pesticides. I. Effect on meiotic chromosomes of Lens and Pisum. Cytologia 52:55–61
Jiang W, Liu D, Li A (2000) Effects of Cu2+ on root growth, cell division and nucleolus of Helianthus annuus L. Sci Tot Environ 256:59–65
Knasmüller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, Paschke A, Göd R, Kundi M (1998) Detection of genotoxic effects of heavy metal contaminated soils with plant bioassays. Mutat Res 420:37–48
Krishna G, Hayashi M (2000) In vitro rodent micronucleus assay: protocol, conduct and data interpretation. Mutat Res 455:155–166
Ma TH, Xu Z, Xu C, McConnell H, Rabago EV, Arreola GA, Zhang H (1995) The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat Res 334:185–195
Maksymiec W (1997) Effect of copper on cellular processes in higher plants. Photosynthetica 34(3):321–342
Marcano L, Carruyo MH, Del Campo A, Montiel A (2004) Cytotoxicity and mode of action of maleic hydrazide in root tips of Allium cepa. Environ Res 94:221–226
Qian X-W (2004) Mutagenic effect of chromium trioxide on root tip cells of Vicia faba. J Zhejiang Univ Sci 5:1570–1576
Rieger R, Michaelis A, Green MM (1976) Glossary of genetics and cytogenetics: classical and molecular. Springer, Berlin, p 139
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Seoane AI, Dulout FN (2001) Genotoxicity ability of cadmium, chromium and nickel salts studied by kinetochore staining in the cytokinesis-blocked micronucleus assay. Mutat Res 490:99–106
Türkoglu S (2007) Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res 626:4–14
Voutsinas G, Zarani F, Kappas A (1997) The effect of environmental aneuploidy-inducing agents on the microtubule architecture of mitotic meristematic root cells in Hordeum vulgare. Cell Biol Int 21:411–418
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17(1):145–156
Acknowledgment
This work was supported by a grant from ADEME “Bioindicateurs de Qualité des Sols” (PNETOX 2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souguir, D., Ferjani, E., Ledoigt, G. et al. Exposure of Vicia faba and Pisum sativum to copper-induced genotoxicity. Protoplasma 233, 203–207 (2008). https://doi.org/10.1007/s00709-008-0004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-008-0004-9