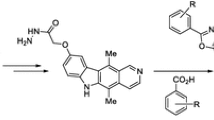
Abstract
A series of ten novel benzimidazole fused ellipticine derivatives have been synthesized and these compounds were evaluated for their antiproliferative activity against four human cancer cell lines (Zr-75-1, MCF-7, HeLa, and A-549). It is observed that all the synthesized compounds showed significant antiproliferative activity with GI50 values ranging from <0.1 to 34.6 μM, while the positive control, etoposide demonstrated the GI50 in the range of 0.2–3.08 μM, respectively. Some of the compounds were distinctly more potent than etoposide, with GI50 concentrations in the submicromolar level on certain cell lines.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of mortality in developed countries. Currently, chemotherapy is the most important treatment for cancer and the ultimate goal is to destroy the cancer cells without any harmful effect on the normal cells. Within the past decade, advances in our understanding of the cell cycle have presented new targets that may allow for the development of more selective chemotherapeutic agents—agents that target only cancer cells. Cancer chemotherapy has achieved significant success through the discovery of various new drugs. Despite this progress, the discovery of most potent anti-cancer agents is a challenging issue in cancer chemotherapy for the future generations.
Ellipticine (1) was first identified in 1959 as a compound in the leaves of a small tropical evergreen Ochrosia elliptica Labill [1]. Ellipticine and its derivatives display potent antitumor and anticancer properties. More recent studies have also indicated that ellipticine and its derivatives are active against HIV [2]. The predominant mechanisms of biological effects of ellipticine were suggested to be intercalation into DNA [3–6] and inhibition of topoisomerase-II [3, 7, 8]. The most clinically successful ellipticinium salt to date is 9-hydroxy-N-methylellipticinium acetate (celiptium, 2), a drug for the treatment of metastatic breast cancer, myeloblastic leukemia and some solid tumors (Fig. 1) [9, 10]. The main reasons for the interest in ellipticine and its derivatives for clinical purposes are their high efficiencies against several types of cancer, their limited toxic side effects, and their lack of haematological toxicity [3].
Similarly, benzimidazoles are an important class of heterocyclic compounds and are promising scaffolds in medicinal chemistry. Benzimidazole derivatives possess a variety of biological activities such as antimicrobial [11], anticancer [12], antifungal [13], and anti-inflammatory [14] activities. Nocadazole (3) has potent antitubulin activity, thus affecting the microtubule component of the cytoskeleton, and it is one of the potent members of the benzimidazole family [15].
In view of pharmacological importance of both ellipticine and benzimidazole moieties, in this article, we synthesized a novel series of benzimidazole fused ellipticine derivatives 6a–6j and evaluated their antiproliferative activity on selected human cancer cell lines.
Results and discussion
Chemistry
The synthetic route for benzimidazole fused ellipticine derivatives 6a–6j is outlined in Scheme 1. Compound 1 was treated with hexamethylenetetramine (HMTA), TFA at reflux for 20 min to afford pure formylated ellipticine compound 4. Further, aldehyde 4 was cyclized with different substituted 1,2-diaminobenzenes 5a–5j in presence of aq. Na2S2O5 in ethanol at reflux for 4 h to afford pure benzimidazole fused ellipticine derivatives 6a–6j.

Antiproliferative activity
The newly synthesized benzimidazole fused ellipticine derivatives 6a–6j were evaluated for their antiproliferative activity in four human cancer cell lines of breast (Zr-75-1, MCF-7), cervical (HeLa), and lung (A-549) origin using sulforhodamine B (SRB) method [16]. Etoposide was used as the positive control and the results are summarized in Table 1. It is observed that all the synthesized compounds showed significant antiproliferative activity with GI50 values ranging from <0.1 to 34.6 μM, while the positive control, etoposide demonstrated the GI50 in the range of 0.2–3.08 μM, respectively. The majority of the tested compounds displayed potent growth inhibition on breast (MCF-7), cervical (HeLa), and Lung (A-549) cancer cell lines as compared to the breast cancer cell lines (Zr-75-1). Some of the compounds like 6b, 6c, 6d, 6g, and 6j were distinctly more potent than etoposide, with GI50 concentrations in the submicromolar level on certain cell lines.
Conclusion
The library of benzimidazole fused ellipticine derivatives 6a–6j was synthesized and evaluated for their antiproliferative activity in four human cancer cell lines of Zr-75-1, MCF-7, HeLa, and A-549. It is observed that all the synthesized compounds showed significant antiproliferative activity with GI50 values ranging from <0.1 to 34.6 μM, while the positive control, etoposide demonstrated the GI50 in the range of 0.2–3.08 μM. Some of the compounds like 6b, 6c, 6d, 6g, and 6j were distinctly more potent than etoposide, with GI50 concentrations in the submicromolar level on certain cell lines.
Experimental
All reagents and solvents used were of commercial grade and were used without any further purification. Progress of reactions was monitored by thin layer chromatography (TLC) performed on silica gel glass plates containing 60 F-254 and visualized on TLC was achieved under UV light or with iodine indicator. Melting points were measured with an Electro thermal melting point apparatus. 1H and 13C NMR spectra were recorded on Bruker XNMR/XWINNMR (300 MHz) instruments. Chemical shifts (δ) are reported in ppm downfield from internal TMS standards. Signal multiplicities are represented by s (singlet), d (doublet), dd (double doublet), and m (multiplet). ESI spectra were recorded on Micro mass, Quattro LC using ESI+ software with capillary voltage 3.98 kV and ESI mode positive ion trap detector.
5,11-Dimethyl-6H-pyrido[4,3-b]carbazole-9-carbaldehyde (4, C18H14N2O)
A reaction flask was charged with 10 g compound 1 (40.62 mmol), 28 g hexamethylenetetramine (HMTA, 203.14 mmol), and 30 cm3 TFA. The reaction mixture was refluxed for 20 min and cooled to room temperature. The resulted reaction mixture was basified with Na2CO3, and extracted with CHCl3. The organic layer was washed with brine and dried over Na2SO4, and then filtered and evaporated. The crude residue was purified by SiO2 column chromatography (CHCl3/MeOH = 9:1) to afford 4 with 9.34 g, in 84 % yield. M.p.: 360–362 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.85 (s, 3H), 7.64 (d, 1H, J = 8.4 Hz), 8.00 (dd, 2H), 8.38 (d, 1H, J = 8.4 Hz), 8.86 (s, 1H), 9.64 (s, 1H), 10.5 (s, 1H) ppm; 13C NMR (125.77 MHz, DMSO-d 6 ): δ = 191.9, 149.8, 146.7, 141.0, 140.7, 132.9, 128.8, 128.5, 128.1, 127.2, 123.2, 122.8, 116.0, 115.7, 111.0, 109.4, 14.3, 11.9 ppm; MS (FAB): m/z = 275 ([M + H]+).
9-(1H-Benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6a, C24H18N4)
A solution of 828 mg Na2S2O5 (4.36 mmol) in 1.6 cm3 H2O was added to 300 mg 5,11-dimethyl-6H-pyrido[4,3-b]carbazole-9-carbaldehyde (4, 1.09 mmol) and added 118.3 mg o-phenylenediamine (5a, 1.09 mmol) in ethanol. After completion of the reaction, the resulting mixture was stirred at reflux for 4 h, the mixture diluted with 50 cm3 of H2O and then extracted with DCM (2 × 40 cm3). The combined extracts were washed with brine, dried over Na2SO4, and evaporated in vacuo. The crude product was purified by column chromatography using MeOH/DCM as the eluent to afford pure compound 6a with 281 mg, in 71 % yield. M.p.: 370–372 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.88 (s, 3H), 3.28 (s, 3H), 7.35 (d, 1H), 7.45–7.51 (m, 2H), 7.56–7.67 (m, 2H), 7.69 (s, 1H), 7.72–7.75 (m, 1H), 8.46–8.59 (m, 2H), 8.86 (s, 1H), 9.23 (s, 1H), 9.45–9.47 (m, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 11.8, 16.5, 110.9, 112.5, 115.8, 118.6, 118.9, 119.4, 121.7, 122.8, 123.5, 123.9, 126.8, 130.6, 131.8, 137.6, 138.5, 139.3, 140.3, 142.5, 149.8, 151.7 ppm; MS (ESI): m/z = 363 ([M + H]+).
9-(5-Methoxy-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6b, C25H20N4O)
The compound 6b was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 0.13 cm3 4-methoxybenzene-1,2-diamine (5b, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6b, 314 mg in 73 % yield. M.p.: 374–376 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.90 (s, 3H), 3.21 (s, 3H), 3.84 (s, 3H), 6.54 (s, 1H), 7.14 (d, 1H, J = 8.2 Hz), 7.34–7.47 (m, 3H), 7.56 (d, 1H, J = 9.3 Hz), 8.57–8.68 (m, 2H), 8.93 (s, 1H), 9.17 (s, 1H), 9.23 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 11.9, 14.7, 54.6, 100.3, 107.6, 111.9, 112.6, 114.6, 117.9, 119.4, 119.8, 121.6, 123.3, 123.9, 126.2, 130.7, 131.4, 133.7, 137.8, 140.5, 141.3, 141.8, 151.8, 152.4, 156.4 ppm; MS (ESI): m/z = 393 ([M + H]+).
9-(5,6-Dimethoxy-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6c, C26H22N4O2)
The compound 6c was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 183 mg 4,5-dimethoxybenzene-1,2-diamine (5c, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6c, 279 mg in 61 % yield. M.p.: 378–380 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.79 (s, 3H), 3.27 (s, 3H), 3.89 (s, 6H), 6.67 (s, 2H), 7.35–7.42 (m, 2H), 7.58 (d, 1H, J = 8.1 Hz), 8.48–8.57 (m, 2H), 8.76 (s, 1H), 8.96 (s, 1H), 9.18 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 11.3, 14.6, 55.7, 97.6, 98.9, 110.6, 112.3, 117.6, 119.5, 119.9, 120.9, 121.7, 122.9, 126.4, 130.5, 131.1, 134.8, 138.6, 140.6, 142.7, 147.3, 150.4, 151.6, 152.8 ppm; MS (ESI): m/z = 423 ([M + H]+).
5,11-Dimethyl-9-[5-(trifluoromethyl)-1H-benzo[d]imidazol-2-yl]-6H-pyrido[4,3-b]carbazole (6d, C25H17F3N4)
The compound 6d was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 191 mg 4-(trifluoromethyl)benzene-1,2-diamine (5d, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6d, 321 mg in 68 % yield. M.p.: 383–385 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.71 (s, 3H), 3.28 (s, 3H), 7.32 (d, 1H, J = 8.2 Hz), 7.43 (s, 1H), 7.53–7.65 (m, 2H), 7.68–7.77 (m, 1H), 7.84–7.89 (m, 1H), 8.34–8.41 (m, 2H), 8.51 (s, 1H), 8.59 (s, 1H), 9.21 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 12.5, 14.8, 110.3, 112.8, 115.7, 117.8, 118.5, 118.9, 120.6, 120.9, 121.8, 122.6, 122.8, 123.5, 126.5, 129.7, 130.9, 137.6, 138.7, 138.8, 140.7, 141.9, 150.6, 152.9 ppm; MS (ESI): m/z = 431 ([M + H]+).
9-(5-Chloro-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6e, C24H17ClN4)
The compound 6e was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 155 mg 4-chlorobenzene-1,2-diamine (5e, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6e, 341 mg in 79 % yield. M.p.: 388–390 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.83 (s, 3H), 3.41 (s, 3H), 7.28 (d, 1H, J = 8.5 Hz), 7.37 (d, 1H, J = 8.1 Hz), 7.47–7.54 (m, 3H), 7.66–7.69 (m, 1H), 8.54–8.59 (m, 2H), 8.63 (s, 1H), 8.67 (s, 1H), 9.28 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 12.6, 15.9, 112.5, 112.9, 118.5, 119.7, 119.9, 120.6, 122.4, 121.9, 123.4, 123.7, 124.6, 125.8, 126.5, 130.5, 134.7, 135.8, 138.6, 140.4, 140.8, 150.5, 152.7 ppm; MS (ESI): m/z = 397 ([M + H]+).
5,11-Dimethyl-9-(5-nitro-1H-benzo[d]imidazol-2-yl)-6H-pyrido[4,3-b]carbazole (6f, C24H17N5O2)
The compound 6f was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 167 mg 4-nitrobenzene-1,2-diamine (5f, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6f, 361 mg in 81 % yield. M.p.: 401–403 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.81 (s, 3H), 3.42 (s, 3H), 7.36 (d, 1H, J = 8.3 Hz), 7.46 (s, 1H), 7.53–7.66 (m, 2H), 8.23 (d, 1H, J = 8.5 Hz), 8.35-8.49 (m, 3H), 8.66 (s, 1H), 8.69 (s, 1H), 9.31 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 12.8, 16.4, 112.3, 114.6, 115.6, 115.9, 118.9, 120.5, 120.7, 121.2, 121.9, 123.5, 123.9, 125.6 130.5, 131.8, 137.6, 137.9, 139.7, 140.9, 141.6, 143.5, 153.8, 154.6 ppm; MS (ESI): m/z = 408 ([M + H]+).
9-(5-Fluoro-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6g, C24H17FN4)
The compound 6g was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 138 mg 4-fluorobenzene-1,2-diamine (5g, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6g, 336 mg in 81 % yield. M.p.: 379–381 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.77 (s, 3H), 3.24 (s, 3H), 7.30–7.38 (m, 2H), 7.40–7.48 (m, 2H), 7.54–7.60 (m, 1H), 7.68 (d, 1H, J = 8.1 Hz), 8.45–8.53 (m, 2H), 8.22 (s, 1H), 8.34 (s, 1H), 9.15 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 11.8, 14.6, 101.4, 110.6, 112.3, 113.9, 114.3, 117.8, 119.7, 120.6, 120.9, 121.7, 122.3, 125.6, 129.8, 134.8, 138.7, 140.7, 141.7, 142.8, 151.6, 152.7, 160.4 ppm; MS (ESI): m/z = 381 ([M + H]+).
9-(5-Bromo-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6h, C24H17BrN4)
The compound 6h was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 149 mg 4-bromobenzene-1,2-diamine (5h, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6h, 348 mg in 72 % yield. M.p.: 409–411 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.81 (s, 3H), 3.37 (s, 3H), 7.34 (d, 1H, J = 8.4 Hz), 7.47–7.76 (m, 5H), 8.42–8.56 (m, 2H), 8.30 (s, 1H), 8.43 (s, 1H), 9.26 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 12.8, 16.8, 112.4, 112.9, 113.6, 119.4, 119.8, 120.4, 121.6, 121.9, 123.5, 123.9, 126.3, 126.8, 128.6, 129.8, 131.4, 137.5, 138.4, 140.7, 142.2, 151.8, 154.5 ppm; MS (ESI): m/z = 442 ([M + H]+).
9-(5,6-Dichloro-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6i, C24H16Cl2N4)
The compound 6i was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 193 mg 4,5-dichlorobenzene-1,2-diamine (5i, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6i, 364 mg in 77 % yield. M.p.: 417–419 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.90 (s, 3H), 3.39 (s, 3H), 7.31 (d, 1H, J = 8.1 Hz), 7.54–7.59 (m, 2H), 7.69 (s, 2H), 8.30–8.43 (m, 2H), 8.49 (s, 1H), 8.53 (s, 1H), 9.34 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 13.4, 16.9, 112.7, 114.9, 118.7, 119.9, 120.5, 120.8, 121.8, 122.7, 123.6, 123.9, 128.5, 128.9, 130.8, 131.5, 131.8, 134.2, 134.8, 140.6, 142.3, 143.5, 154.7, 155.8 ppm; MS (ESI): m/z = 432 ([M + H]+).
9-(5,6-Dinitro-1H-benzo[d]imidazol-2-yl)-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (6j, C24H16N6O4)
The compound 6j was prepared following the method described for the preparation of the compound 6a, employing 300 mg compound 4 (1.09 mmol), 216 mg 4,5-dinitrobenzene-1,2-diamine (5j, 1.09 mmol), 828 mg Na2S2O5 (4.36 mmol) in ethanol, and 1.6 cm3 H2O. The crude product was purified by column chromatography with MeOH/DCM (1:9) to afford pure compound 6j, 374 mg in 76 % yield. M.p.: 421–423 °C; 1H NMR (300 MHz, DMSO-d 6 ): δ = 2.94 (s, 3H), 3.43 (s, 3H), 7.27 (d, 1H, J = 8.1 Hz), 7.47 (s, 1H), 7.55 (d, 1H, J = 9.2 Hz), 8.42 (s, 2H), 8.54–8.61 (m, 2H), 8.67 (s, 1H), 8.69 (s, 1H), 9.39 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 13.8, 16.9, 110.7, 112.9, 114.5, 117.8, 118.8, 120.3, 121.6, 123.5, 123.9, 126.8, 132.5, 132.8, 138.6, 140.6, 140.8, 141.4, 142.6, 143.7, 144,5, 155.4, 156.8 ppm; MS (ESI): m/z = 453 ([M + H]+).
Procedure of the SRB assay
The synthesized compounds 6a–6j have been evaluated for their in vitro cytotoxicity in human cancer cell lines. A protocol of 48 h continuous drug exposure has been used and a sulforhodamine B (SRB) protein assay has been used to estimate cell viability or growth. The cell lines were grown in DMEM medium containing 10 % fetal bovine serum and 2 mM l-glutamine and were inoculated into 96 well microtiter plates in 90 cm3 at plating densities depending on the doubling time of individual cell lines. The microtiter plates were incubated at 37 °C, 5 % CO2, 95 % air, and 100 % relative humidity for 24 h prior to addition of experimental drugs. Aliquots of 10 cm3 of the drug dilutions were added to the appropriate microtiter wells already containing 90 cm3 of cells, resulting in the required final drug concentrations. For each compound, four concentrations (0.1, 1, 10, and 100 µM) were evaluated and each was done in triplicate wells. Plates were incubated further for 48 h and assay was terminated by the addition of 50 cm3 of cold trichloroacetic acid (TCA) (final concentration 10 % TCA) and incubated for 60 min at 4 °C. The plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (50 cm3) at 0.4 % (w/v) in 1 % acetic acid was added to each of the cells, and plates were incubated for 20 min at room temperature. The residual dye was removed by washing five times with 1 % acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on an ELISA plate reader at a wavelength of 540 nm with 690 nm reference wavelengths. Percent growth was calculated on a plate-by-plate basis for test wells relative to control wells. The above determinations were repeated three times. Percentage growth was expressed as the (ratio of average absorbance of the test well to the average absorbance of the control wells) × 100. Growth inhibition of 50 % (GI50) was calculated from [(T i − T z)/(C − T z)] × 100 = 50, which is the drug concentration resulting in a 50 % reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation, where T z = optical density at time zero, C = control growth, and T i = test growth in the presence of drug at the four concentration levels.
References
Goodwin S, Smith AF, Horning EC (1959) J Am Chem Soc 81:1903
Stiborova M, Breuer A, Aimova D, Stiborova-Rupertova M, Wiessler M, Frei E (2003) Int J Cancer 107:885
Auclair C (1987) Arch Biochem Biophys 259:1
Singh MP, Hill GC, Peoch D, Rayner B, Inabach JL, Lown JW (1994) Biochemistry 33:10271
Chu Y, Hsu MT (1992) Nucleic Acids Res 20:4033
Garbett NC, Graves DE (2004) Curr Med Chem 4:149
Monnot M, Mauffret O, Simon V, Lescot E, Psaume B, Saucier JM, Charra M, Belehradek J Jr, Fermandjian S (1991) J Biol Chem 25:1820
Froelich-Ammon SJ, Patchan MW, Osheroff N, Thompson RB (1995) J Biol Chem 270:14998
Rouëssé J, Spielmann M, Turpin F, Le Chevalier T, Azab N, Mondésir JM (1993) Eur J Cancer 29:856
Buzdar AU, Hortobagyi GN, Esparza LT, Holmes FA, Ro JS, Fraschini G, Lichtiger B (1990) Oncology 47:101
Lazer ES, Matteo MR, Possanza GJ (1987) J Med Chem 30:726
Ibrahim ESA, Omar AMME, Mohsen ME, Khalil MA (1980) J Pharma Sci 69:1348
Chen G, Liu Z, Zhang Y, Shan X, Jiang L, Zhao Y, Liang G (2012) ACS Med Chem Lett 4:69
Wang XJ, Xi MY, Fu JH, Zhang FR, Cheng GF, Yin DL, You QD (2012) Chem Lett 23:707
Van Den Bossche H, Rochette F, Hörig C (1982) Advances in pharmacology and chemotherapy. Academic Press, New York
Skehn P, Storeng R, Scudiero A, Monks J, McMohan D, Vistica D, Jonathan WT, Bokesch H, Kenney S, Boyd RM (1990) J Natl Cancer Inst 82:1107
Acknowledgments
N. Bramhananda Reddy thankful to department of Chemistry, Sri Krishnadevaraya University, Anantapur for providing research facilities; Reddymasu Sreenivasulu thankful to Acharya Nagarjuna University, Nagarjuna Nagar for providing HPLC data to test the purity of compounds before doing the cancer activity studies and Dr. Palle Sadanandam thankful to Department of Science & Technology, India for financial assistance in the form of Young Scientist major research project F. No. SB/FT/CS-141/2013.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bramhananda Reddy, N., Burra, V.R., Ravindranath, L.K. et al. Synthesis and biological evaluation of benzimidazole fused ellipticine derivatives as anticancer agents. Monatsh Chem 147, 599–604 (2016). https://doi.org/10.1007/s00706-016-1684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1684-z