Abstract
A series of oxadiazole incorporated ellipticine derivatives were synthesized and evaluated for their anticancer activity on human cancer cell lines Colo-205, MCF-7, A-549, and KB. Five of the newly synthesized target products exhibited potent cytotoxic activities with GI50 values ranging from <0.1 to 23.6 µM, where the positive control etoposide showed GI50 values ranging from 0.13 to 3.08 µM.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The international research agency on cancer research projects declared that the fatality rates due to cancer are higher in less developed countries and an estimated 14 million people developed cancer in 2012 with nearly 8 % increased deaths. These cancer cases may enhance globally up to 19.3 million by 2025 [1]. From worldwide, the most commonly diagnosed cancers in both male and female were lung, breast, and colorectal cancers and the most common cause of cancer deaths are due to lung, liver, and stomach cancers. Chemotherapy was one of the important techniques for the treatment of cancer by using several chemotherapeutic agents and this technique associated with various side effects [2]. Despite of this progress, the discovery of most potent anti-cancer agents is a challenging issue in cancer chemotherapy without any side effects for the future generations.
A plant alkaloid ellipticine (1) was first identified in 1959 as a compound in the leaves of Ochrosia elliptica Labill [3]. It exhibits significant antitumor and anti-HIV activities [4–7]. Ellipticine and most of its derivatives exhibit high efficiency against several types of cancers, complete lack of hematological toxicity and limited side effects was led to main reason for interest towards the research of ellipticine derivatives [8].
It is inhibits the human DNA topoisomerase-II enzyme [9–11]. Many of its derivatives have showed different biological activities, like DNA-binding affinities, breast cancer metastases, brain tumors, kidney sarcomas, and myeloblastic leukemia [12–15].
Many of the heterocyclic ring systems play an important role for the development of novel drugs which enhanced their activities when fused with other ring systems [16, 17] and exhibited antitumor activities against a panel of human tumor cancer cell lines [18–21]. Heterocyclic molecules such as 1,3,4-oxadiazoles constitute an important class of compounds in medicinal and pharmaceutical chemistry because of their remarkable biological and pharmacological properties. They have been displayed many biological activities, such as antibacterial [22], antifungal [23], insecticidal [24], antiviral [25], inflammatory [26], antitubercular [27], and antitumor [28, 29] activity. Zibotentan (ZD4054) (2) was one of the most important drugs with 1,3,4-oxadiazole moiety exhibit the most potent anticancer properties [30].
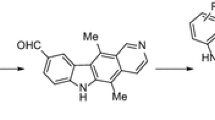
In view of the above pharmacology information of ellipticine and oxadiazole moieties and continuous of these efforts, we have synthesized a new series of oxadiazole incorporated ellipticine derivatives 8a–8j and evaluated for their anticancer activity on human cancer cell lines. Structures of Ellipticine (1), and Zibotentan (ZD4054) (2) was shown in Fig. 1.
Results and discussion
Chemistry
The synthesis of the new 1,3,4-oxadiazole incorporated ellipticine derivatives 8a–8j are shown in Scheme 1. 5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol (3) was undergoes to esterification with ethyl bromoacetate (4) in presence K2CO3, acetone at reflux for 6 h to afford intermediate 5. The intermediate 5 was reacted with hydrazine hydrochloride in ethanol at reflux for 5 h to afford pure acid hydrazide compound 6. This acid hydrazide intermediate 6 was cyclized with different substituted aromatic acids 7a–7j in POCl3 at reflux for 6 h to afford pure compounds 8a–8j. All the structures were characterized by 1H NMR, 13C NMR, and mass spectral data.

In vitro cytotoxicity
The newly synthesized compounds 8a–8j were further evaluated for their anticancer activity against human Colo-205 (colon), MCF-7 (breast), A-549 (lung), and KB (oral carcinoma) cancer cell lines using the standard sulforhodamine B (SRB) [31] assay. The GI50 was calculated for these tested compounds in µM. GI50 may be defined as the drug concentration to cause 50 % reduction in proliferation of cancer cell lines. The test results were shown in the Table 1 in comparison with the positive control such as etoposide. The synthesized compounds 8a–8j were exhibited potent cytotoxic activities giving GI50 values ranging from <0.1 to 34.7 µM, where the positive control etoposide showed GI50 values ranging from 0.13 to 3.08 µM. The compound 8b showed maximum cytotoxic effect on A-549 and MCF-7 cancer cell lines with GI50 values of <0.1 µM. The compound 8i was also exhibited maximum cytotoxic effect on MCF-7 and A-549 cancer cell lines with GI50 values of 1.20 and <0.1 µM, respectively. While the compound 8c showed maximum cytotoxic effect on MCF-7 and A-549 cancer cell lines with GI50 values of 1.45 and 1.22 µM. All the synthesized compounds show less cytotoxic effect on Colo-205 and KB cancer cell lines. The compounds 8a and 8f showed less cytotoxic effect on breast cancer cell line, MCF-7 with GI50 values of 2.56 and 2.81 µM. The compounds 8a, 8e, and 8h showed less cytotoxic effect on lung cancer cell line, A-549 with GI50 values of 4.87, 4.30 and 10.4 µM, respectively. Based on the structure–activity relationship of the synthesized derivatives, it was observed that the compounds 8b, 8c, and 8d has methoxy substituents attached to the aromatic benzene ring which has electron donating property may be contribute the maximum cytotoxic effect and the compound 8i with electron withdrawing trifluoromethyl substituent may also contribute the good cytotoxic effect on breast cancer cell line (MCF-7) and lung cancer cell line (A-549).
Conclusion
In summary, we have described the synthesis of novel ellipticine embedded with 1,3,4-oxadiazole moieties in good yields (8a–8j) and evaluation of their anticancer activity. Most of the compounds showed significant anticancer activity against four different human Colo-205, MCF-7, A-549, and KB cancer cell lines with GI50 values in the range of <0.1–23.6 μM as compared with positive control etoposide. Among the synthesized compounds, compounds 8b, 8c, 8d, 8i, and 8j were showed more potent activity than etoposide.
Experimental
All chemicals and reagents were obtained from Aldrich (Sigma-Aldrich, St. Louis, MO, USA), Lancaster (Alfa Aesar, Johnson Matthey Company, Ward Hill, MA, USA) and were used without further purification. Reactions were monitored by TLC, performed on silica gel glass plates containing 60 F-254, and visualization on TLC was achieved by UV light or iodine indicator. Proton and 13C NMR spectra were recorded on a Gemini Varian-VXR-unity (400 MHz) instrument. Chemical shifts (δ) are reported in ppm downfield from internal TMS standard. Mass ESI spectra were recorded on Micro mass, Quattro LC using ESI+ software with capillary voltage 3.98 kV and ESI mode positive ion trap detector. Melting points were determined with an electro thermal melting point apparatus.
Ethyl 2-(5,11-dimethyl-6H-pyrido[4,3-b]carbazol-9-yloxy)acetate (5, C21H20N2O3)
The compound 5,11-dimethyl-6H-pyrido[4,3-b]carbazol-9-ol (3, 5 g, 19 mmol) was dissolved in 30 cm3 of dried acetone, followed by addition of 2.2 cm3 ethyl bromoacetate (4, 19 mmol) and 2.63 g K2CO3 (57 mmol). The reaction mixture was heated under reflux for 6 h. After completion of the reaction, K2CO3 was removed by filtration and the solvent was evaporated under vacuum to afford crude product. The crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 5, 6.1 g in 92 %. M.p.: 360–362 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 1.38 (t, 3H), 2.81 (s, 3H), 3.21 (s, 3H), 4.32 (q, 2H), 4.74 (s, 2H), 7.09 (d, 1H, J = 8.6 Hz), 7.38 (d, 1H, J = 8.6 Hz), 7.77 (s, 1H), 7.88 (d, 1H, J = 6.40 Hz), 8.40 (d, 1H, J = 6.40 Hz), 9.66 (s, 1H), 11.06 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 171.1, 155.2, 152.4, 143.1, 142.6, 137.0, 134.3, 131.9, 130.8, 126.9, 122.2, 114.3, 112.7, 111.9, 109.6, 108.2, 66.6, 62.3, 15.3, 14.1, 12.8 ppm; MS (ESI): m/z = 348 ([M + H]+).
2-(5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-yloxy)acetohydrazide (6, C19H18N4O2)
A mixture of 5 g ethyl 2-(5,11-dimethyl-6H-pyrido[4,3-b]carbazol-9-yloxy)acetate (5, 14.4 mmol) and 3.94 g hydrazine hydrochloride (57.3 mmol) in ethanol was refluxed for 5 h. The crude product was obtained after distilling off the excess ethanol. Cooling, filtering, then washing with a little cold water, this product 6 was employed in the next step without further purification. Yield 86 % (4.16 g); 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.84 (s, 3H), 3.24 (s, 3H), 4.75 (s, 2H), 7. 10 (d, 1H, J = 8.9 Hz), 7.40 (d, 1H, J = 8.9 Hz), 7.68–7.69 (m, 1H), 7.78 (s, 1H), 7.80 (d, 1H, J = 6.6 Hz), 8.42 (d, 1H, J = 6.6 Hz), 9.67 (s, 1H), 11.07 (s, 1H) ppm; 13C NMR (75 MHz, DMSO-d 6 ): δ = 176.5, 158.1, 154.2, 143.0, 142.8, 136.9, 134.3, 131.7, 126.8, 121.8, 113.6, 111.7, 111.1, 109.8, 108.7, 69.8, 15.3, 12.8 ppm; MS (ESI): m/z = 335 ([M + H]+).
9-[(5-Phenyl-1,3,4-oxadiazol-2-yl)methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8a, C26H20N4O2)
The compound 2-(5,11-dimethyl-6H-pyrido[4,3-b]carbazol-9-yloxy)acetohydrazide (6, 200 mg, 5.98 mmol) was dissolved in 15 cm3 POCl3 and added 73 mg benzoic acid (7a, 5.98 mmol). The reaction mixture was refluxed at 140 °C for 12 h. After completion of reaction, it can be neutralized with aq. NaHCO3 and then workup with ethyl acetate. The organic layer can be evaporated through rotavapor and dried it with Na2SO4. The crude compound was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8a, 181 mg in 72 % yield. M.p.: 367–369 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.85 (s, 3H), 3.25 (s, 3H), 4.76 (s, 2H), 7.11 (d, 1H, J = 8.8 Hz), 7.42–7.60 (m, 5H), 7.76 (s, 1H), 8.21 (d, 2H, J = 8.28 Hz), 8.43 (d, 1H, J = 6.5 Hz), 9.68 (s, 1H), 11.08 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.4, 16.7, 67.4, 110.4, 111.8, 112.5, 112.9, 114.6, 122.5, 128.7, 128.5, 130.3, 131.5, 131.7, 132.7, 133.4, 135.6, 137.4, 142.0, 143.5, 155.8, 159.4, 159.8, 164.5 ppm; MS (ESI): m/z = 421 ([M + H]+).
9-[[5-(3,4,5-Trimethoxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8b, C29H26N4O5)
This compound 8b was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 126 mg 3,4,5-trimethoxybenzoic acid (7b, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8b, 210 mg in 68 % yield. M.p.: 377–379 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.86 (s, 3H), 3.26 (s, 3H), 3.85 (s, 6H), 3.87 (s, 3H), 4.79 (s, 2H), 7.18 (d, 1H, J = 8.4 Hz), 7.43–7.51 (m, 2H), 7.56 (d, 1H, J = 6.5 Hz), 7.60 (s, 2H), 8.44 (d, 1H, J = 6.5 Hz), 9.67 (s, 1H), 11.07 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.3, 16.5, 58.4, 61.3, 68.5, 107.4, 109.7, 112.5, 112.9, 114.7, 122.4, 126.7, 128.5, 132.4, 133.6, 135.6, 137.7, 142.3, 142.4, 142.8, 155.4, 155.8, 159.6, 160.5, 163.4 ppm; MS (ESI): m/z = 511 ([M + H]+).
9-[[5-(4-Methoxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8c, C27H22N4O3)
This compound 8c was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 90 mg 4-methoxybenzoic acid (7c, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8c, 189 mg in 70 % yield. M.p.: 371–373 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.85 (s, 3H), 3.25 (s, 3H), 3.85 (s, 3H), 4.77 (s, 2H), 7.19 (d, 1H, J = 8.5 Hz), 7.14 (d, 2H, J = 7.56 Hz), 7.42–7.50 (m, 2H), 7.55 (d, 1H, J = 6.7 Hz), 7.66 (d, 2H, J = 7.56 Hz), 8.45 (d, 1H, J = 6.9 Hz), 9.66 (s, 1H), 11.08 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.2, 16.4, 56.5, 68.4, 109.4, 111.5, 112.5, 112.8, 114.6, 117.6, 118.5, 122.5, 124.8, 128.6, 132.5, 133.6, 135.3, 137.2, 143.4, 143.8, 154.6, 159.5, 160.3, 163.4, 164.2 ppm; MS (ESI): m/z = 451 ([M + H]+).
9-[[5-(3-Methoxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8d, C27H22N4O3)
This compound 8d was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 90 mg 3-methoxybenzoic acid (7d, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8d, 191 mg in 71 % yield. M.p.: 374–376 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.85 (s, 3H), 3.24 (s, 3H), 3.85 (s, 3H), 4.80 (s, 2H), 7.19 (d, 1H, J = 8.6 Hz), 7.24–7.31 (m, 2H), 7.51–7.65 (m, 4H), 8.12–8.15 (m, 1H), 8.45 (d, 1H, J = 6.9 Hz), 9.65 (s, 1H), 11.09 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.2, 16.4, 58.3, 67.5, 109.6, 111.5, 112.4, 112.7, 114.5, 119.7, 122.6, 126.4, 128.4, 132.5, 132.8, 133.5, 133.8, 135.5, 137.8, 143.2, 143.6, 154.8, 159.5, 160.4, 162.4, 164.4 ppm; MS (ESI): m/z = 451 ([M + H]+).
9-[[5-(4-Chlorophenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8e, C26H19ClN4O2)
This compound 8e was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 93 mg 4-chlorobenzoic acid (7e, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8e, 211 mg in 78 % yield. M.p.: 381–383 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.87 (s, 3H), 3.27 (s, 3H), 4.79 (s, 2H), 7.21 (d, 1H, J = 8.6 Hz), 7.52–7.61 (m, 2H), 7.65 (d, 1H, J = 6.8 Hz), 7.80 (d, 2H, J = 7.89 Hz), 7.84 (d, 2H, J = 7.89 Hz), 8.47 (d, 1H, J = 7.0 Hz), 9.67 (s, 1H), 11.10 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.7, 16.8, 67.5, 109.7, 112.7, 112.9, 114.8, 122.6, 128.5, 128.9, 129.6, 131.5, 132.6, 133.6, 135.7, 137.8, 138.8, 143.6, 143.9, 154.8, 159.8, 160.7, 164.8 ppm; MS (ESI): m/z = 455 ([M + H]+).
9-[[5-(4-Bromophenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8f, C26H19BrN4O2)
This compound 8f was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 120 mg 4-bromobenzoic acid (7f, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8f, 216 mg in 72 % yield. M.p.: 388–390 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.89 (s, 3H), 3.28 (s, 3H), 4.81 (s, 2H), 7.21 (d, 1H, J = 8.7 Hz), 7.47–7.54 (m, 2H), 7.59 (d, 1H, J = 6.9 Hz), 7.70 (d, 2H, J = 7.56 Hz), 7.76 (d, 2H, J = 7.56 Hz), 8.48 (d, 1H, J = 7.02 Hz), 9.68 (s, 1H), 11.10 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.9, 16.8, 67.8, 109.6, 111.6, 112.7, 112.9, 114.7, 122.6, 126.5, 125.8, 128.6, 129.7, 132.6, 133.8, 134.6, 135.8, 137.8, 143.8, 144.3, 154.9, 159.7, 160.9, 165.2 ppm; MS (ESI): m/z = 500 ([M + H]+).
9-[[5-(4-Fluorophenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8g, C26H19FN4O2)
This compound 8g was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 83 mg 4-fluorobenzoic acid (7g, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8g, 210 mg in 80 % yield. M.p.: 385–387 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.86 (s, 3H), 3.25 (s, 3H), 4.75 (s, 2H), 7.19 (d, 1H, J = 8.5 Hz), 7.37 (d, 2H, J = 7.60 Hz), 7.51–7.59 (m, 2H), 7.62 (d, 1H, J = 7.0 Hz), 7.80 (d, 2H, J = 7.60 Hz), 8.45 (d, 1H, J = 6.9 Hz), 9.65 (s, 1H), 11.09 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.6, 16.5, 67.8, 109.7, 111.6, 112.5, 112.9, 114.6, 116.7, 117.8, 122.5, 126.5, 126.8, 126.9, 128.5, 132.6, 133.7, 135.8, 136.8, 143.7, 143.9, 154.7, 159.6, 159.8, 160.5, 164.7 ppm; MS (ESI): m/z = 439 ([M + H]+).
9-[[5-(4-Nitrophenyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8h, C26H19N5O4)
This compound 8h was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 100 mg 4-nitrobenzoic acid (8h, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8h, 221 mg in 80 % yield. M.p.: 393–395 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.90 (s, 3H), 3.30 (s, 3H), 4.82 (s, 2H), 7.22 (d, 1H, J = 8.7 Hz), 7.48–7.55 (m, 2H), 7.59 (d, 1H, J = 7.0 Hz), 7.83 (d, 2H, J = 7.61 Hz), 7.94 (d, 2H, J = 7.61 Hz), 8.49 (d, 1H, J = 7.01 Hz), 9.69 (s, 1H), 11.10 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.9, 16.9, 67.8, 109.6, 111.8, 112.7, 112.9, 114.7, 122.7, 124.8, 126.7, 128.8, 132.6, 133.8, 135.7, 136.6, 137.8, 143.9, 144.3, 149.6, 154.9, 159.8, 160.8, 164.7 ppm; MS (ESI): m/z = 466 ([M + H]+).
9-[[5-[4-(Trifluoromethyl)phenyl]-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8i, C27H19F3N4O2)
This compound 8i was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 113 mg 4-(trifluoromethyl)benzoic acid (7i, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8i, 209 mg in 72 % yield. M.p.: 386–388 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.88 (s, 3H), 3.27 (s, 3H), 4.80 (s, 2H), 7.20 (d, 1H, J = 8.6 Hz), 7.51–7.59 (m, 2H), 7.61 (d, 1H, J = 6.8 Hz), 7.67 (d, 2H, J = 7.80 Hz), 7.82 (d, 2H, J = 7.80 Hz), 8.49 (d, 1H, J = 7.01 Hz), 9.67 (s, 1H), 11.09 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.7, 16.6, 67.8, 109.7, 111.6, 112.6, 112.8, 114.6, 114.9, 122.6, 127.6, 128.6, 128.7, 132.6, 132.8, 133.6, 133.9, 135.7, 137.8, 143.7, 143.9, 154.8, 159.5, 159.8, 164.6 ppm; MS (ESI): m/z = 489 ([M + H]+).
9-[[5-(p-Tolyl)-1,3,4-oxadiazol-2-yl]methoxy]-5,11-dimethyl-6H-pyrido[4,3-b]carbazole (8j, C27H22N4O2)
This compound 8j was prepared following the method described for the preparation of the compound 8a, employing 200 mg 6 (5.98 mmol) with 81 mg 4-methylbenzoic acid (7j, 5.98 mmol), and 15 cm3 POCl3, and the crude product was purified by column chromatography with MeOH/CH2Cl2 (1:9) to afford pure compound 8j, 211 mg in 82 % yield. Mp: 370–372 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ = 2.37 (s, 3H), 2.81 (s, 3H), 3.22 (s, 3H), 4.80 (s, 2H), 7.19 (d, 1H, J = 8.6 Hz), 7.27 (d, 2H, J = 7.34 Hz), 7.50–7.58 (m, 2H), 7.58 (d, 1H, J = 6.5 Hz), 7.63 (d, 2H, J = 7.34 Hz), 8.45 (d, 1H, J = 6.8 Hz), 9.64 (s, 1H), 11.07 (s, 1H) ppm; 13C NMR (100 MHz, DMSO-d 6 ): δ = 13.6, 16.5, 22.7, 67.4, 109.6, 111.4, 112.4, 112.6, 114.6, 122.6, 126.3, 128.2, 128.7, 131.6, 132.6, 133.8, 135.3, 137.4, 141.6, 143.4, 143.6, 154.3, 159.5, 159.8, 164.2 ppm; MS (ESI): m/z = 435 ([M + H]+).
Procedure of the SRB-assay
The synthesized compounds 8a–8j have been evaluated for their in vitro cytotoxicity in human cancer cell lines. A protocol of 48 h continuous drug exposure has been used and a sulforhodamine B (SRB) protein assay has been used to estimate cell viability or growth. The cell lines were grown in DMEM medium containing 10 % fetal bovine serum and 2 mM l-glutamine and were inoculated into 96 well microtiter plates in 90 cm3 at plating densities depending on the doubling time of individual cell lines. The microtiter plates were incubated at 37 °C, 5 % CO2, 95 % air, and 100 % relative humidity for 24 h prior to addition of experimental drugs. Aliquots of 10 cm3 of the drug dilutions were added to the appropriate microtiter wells already containing 90 cm3 of cells, resulting in the required final drug concentrations. For each compound four concentrations (0.1, 1, 10, and 100 µM) were evaluated and each was done in triplicate wells. Plates were incubated further for 48 h and assay was terminated by the addition of 50 cm3 of cold trichloroacetic acid (TCA, final concentration 10 %) and incubated for 60 min at 4 °C. The plates were washed five times with tap water and air dried. B (SRB) solution (50 cm3) at 0.4 % (w/v) in 1 % acetic acid was added to each of the cells, and plates were incubated for 20 min at room temperature. The residual dye was removed by washing five times with 1 % acetic acid. The plates were air dried. Bound stain was subsequently eluted with 10 mM trizma base, and the absorbance was read on an ELISA plate reader at a wavelength of 540 nm with 690 nm reference wavelengths. Percent growth was calculated on a plate by plate basis for test wells relative to control wells. The above determinations were repeated three times. Percentage growth was expressed as the (ratio of average absorbance of the test well to the average absorbance of the control wells) × 100. Growth inhibition of 50 % (GI50) was calculated from [(T i − T z)/(C − T z)] × 100 = 50, which is the drug concentration resulting in a 50 % reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation, where T z = optical density at time zero, C = control growth, and T i = test growth in the presence of drug at the four concentration levels.
References
WHO World Cancer Report 2014 (2013). http://www.nydailynews.com/life-style/health/14-million-people-cancer-2012-article-1.1545738. Accessed 12 Dec 2013
Aydemir N, Bilaloglu R (2003) Mutat Res 537:43
Goodwin S, Smith AF, Horning EC (1959) J Am Chem Soc 81:1903
Stiborova M, Bieler CA, Wiessler M, Frei E (2001) Biochem Pharmacol 62:1675
Stiborova M, Rupertova M, Schmeiser HH, Frei E (2006) Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150:13
Stiborova M, Rupertova M, Frei E (2011) Biochim Biophys Acta 1814:175
Reddy NB, Burra VR, Ravindranath LK, Kumar VN, Sreenivasulu R, Sadanandam P (2016) Monatsh Chem 147:599
Auclair C (1987) Arch Biochem Biophys 259:1
Singh MP, Hill GC, Peoch D, Rayner B, Inabach JL, Lown JW (1994) Biochemistry 33:10271
Chu Y, Hsu MT (1992) Nucleic Acids Res 20:4033
Monnot M, Mauffret O, Simon V, Lescot E, Psaume B, Saucier JM, Charra M, Belehradek J Jr, Fermandjian S (1991) J Biol Chem 25:1820
Fosse P, Rene B, Charra M, Paoletti C, Saucier JM (1992) Mol Pharmacol 42:590
Woodward RB, Iacobucci GA, Hochstein FA (1959) J Am Chem Soc 81:4434
Garbett NC, Graves DE (2004) Curr Med Chem 4:149
Stiborova M, Rupertova MS, Dohalska MB, Weissler M, Frei E (2003) Chem Res Toxicol 16:38
Malleshappa NN, Harun MP (2012) Eur J Med Chem 56:56
Asati V, Mahapatra DK, Bharti SK (2014) Eur J Med Chem 87:814
Hatti I, Sreenivasulu R, Jadav SS, Jayaprakash V, Kumar CG, Raju RR (2015) Med Chem Res 24:3305
Reddy NB, Burra VR, Ravindranath LK, Sreenivasulu R, Kumar VN (2016) Monatsh Chem 147:593
Sreenivasulu R, Sujitha P, Jadav SS, Ahsan MJ, Kumar CG, Raju RR (2016) Monatsh Chem. doi:10.1007/s00706-016-1750-6
Ahsan MJ, Choudhary K, Jadav SS, Yasmin S, Ansari MY, Sreenivasulu R (2015) Med Chem Res 24:4166
Farshori NN, Banday MR, Ahmad A, Khan AU, Rauf A (2010) Bioorg Med Chem Lett 20:1933
Cui ZN, Shi YX, Zhang L, Ling Y, Li BJ, Nishida Y, Yang XL (2012) J Agric Food Chem 60:11649
Li YH, Zhu HJ, Chen K, Liu R, Khallaf A, Zhang XN, Ni JP (2013) Org Biomol Chem 11:3979
El-Emam AA, Al-Deeb OA, Al-Omar M, Lehmann J (2004) Bioorg Med Chem 12:5107
Jayashankar B, Lokanathrai KM, Baskaran N, Sathish HS (2009) Eur J Med Chem 44:3898
Kucukguzel SG, Oruc EE, Rollas S, Sahin F, Ozbek A (2002) Eur J Med Chem 37:197
Bondock S, Adel S, Etman HA, Badria FA (2012) Eur J Med Chem 48:192
Hatti I, Sreenivasulu R, Jadav SS, Ahsan MJ, Raju RR (2015) Monatsh Chem 146:1699
Zibotentan James ND, Growcott JW (2009) Drugs Future 34:624
Skehn P, Storeng R, Scudiero A, Monks J, McMohan D, Vistica D, Jonathan WT, Bokesch H, Kenney S, Boyd RM (1990) J Natl Cancer Inst 82:1107
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Madhavi, S., Sreenivasulu, R. & Raju, R.R. Synthesis and biological evaluation of oxadiazole incorporated ellipticine derivatives as anticancer agents. Monatsh Chem 148, 933–938 (2017). https://doi.org/10.1007/s00706-016-1790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1790-y