Abstract
An amperometric biosensor based on carbon paste electrode coated with a thin layer of carbon nanotubes and Nafion film containing the tyrosinase enzyme was used for direct determination of Trolox equivalent antioxidant capacity (TEAC) in selected Moravian wines. The results were compared with an official spectrophotometric method using 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). Although based on the different principles, results of both the methods were comparable; correlation coefficient 0.9752 was found. TEAC values differ from ca. 50 to ca. 280 mg dm−3 being increased from white wines to red varieties. Optimum conditions for the biosensor application were investigated.
Graphical abstract

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As well known, wine is the fermented juice of a single fruit, the grape. It is probably far older than recorded history. Our age of wine begins with the Phoenicians and Greeks who colonized the Mediterranean. From this area, cultivation of wine spread around the world. In the Czech Republic, the most productive vineyards are in the southern Moravia (18,000 ha), concentrated southeast of the capital Brno. Evidently, the samples selected for this study originated from this area as well.
Concerning composition, wines are alcoholic drinks with high content of polyphenols which have significant antioxidant properties [1]. Polyphenolic compounds include secondary plant metabolites which are released into the wine during processing of grapes [2]. Very low content of interfering ascorbic acid, known as vitamin C, is present in wines because it is destroyed during fermentation [3]. Reductive minerals containing ions of iron, manganese, zinc, and copper could also be present. However, their possible reduction properties do not have significant antioxidant effect when compared with majority of polyphenols [4].
An ability of the substance to eliminate negative presence of free radicals is expressed by antioxidant capacity [5]. To determine the total antioxidants capacity (TAC) in foodstuffs, several spectrometric methods such as ferric reducing antioxidant power (FRAP) [6], 1,1-diphenyl-2-picrylhydrazyl spectrometric assay (DPPH) [7], 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid method (ABTS) [8], and total radical-trapping antioxidant parameter (TRAP) [9] were developed. These methods are usually based on reduction properties of analyzed antioxidants. TAC of wines is represented predominantly with the present polyphenols. Total phenolic content (TPC) of each wine determined using tyrosinase biosensor should be similar with TAC of wines obtained by DPPH assay; this one was, therefore, chosen as a comparative to the procedure utilizing the enzyme biosensor under investigation. Both TAC and TPC values of wines can be expressed in mg dm−3 of Trolox as so-called Trolox equivalent antioxidant capacity (TEAC), usually used to compare food antioxidant capacities [10].
Trolox is a synthetic water-soluble analog of α-tocopherol which is known as vitamin E [11]. As observed, in the presence of molecular oxygen, an oxidation of α-tocopherol to corresponding α-tocoquinone is catalyzed by mushroom tyrosinase enzyme extracted from Agaricus bisporus. This quinone with an alkyl chain can electrochemically be reduced to α-tocohydroquinone at constant working potential [12].
Tyrosinase, a copper-containing metalloenzyme, is usually present in mushrooms [13], bacteria [14], or plant tissues but it can also be found in human body in which it is responsible for the production of melanin and other pigments [15]. The activity of tyrosinase is similar to catechol oxidase from a related class of copper oxidases such as laccase, ascorbate oxidase, etc. [16]. In the presence of tyrosinase, polyphenols are oxidized to corresponding quinones by air oxygen [17]. Oxidations proceed over several steps with the involvement of Cu(II) being converted to Cu(I); in fact, oxygen is the final electron acceptor, which is reduced to water consequently [18]. This specific catalytic reaction can be used to determine TPC.
Behavior of Trolox is significantly influenced by the presence of carbon nanotubes (CNTs). As observed, height of the anodic peak current (at potential of +0.185 V, E pa ) is higher than that of the cathodic peak measured at a bare carbon paste electrode (CPE) at +0.105 V (E pc ). Taking into account this fact, a catalytic effect on oxidation of Trolox can be attributed to CNTs [19].
A similar phenomenon appears in the presence of tyrosinase enzyme. Anodic peak current is very low but corresponding cathodic peak current (at E pc = +0.065 V) increases when compared with a bare CPE. The shift of cathodic peak potential (of about 40 mV closer to the zero value) indicates a catalytic effect of tyrosinase enzyme. This fact also confirms the enzyme activity to Trolox. As shown earlier [12], a significant progress in direct analysis of TEAC is obtained using a conductive copolymer (Nafion) for immobilization the layer of CNTs (CPE/CNTs/Tyrosinase/Nafion).
Lateral alkyl chain with one hydroxyl group on aromatic ring has no fundamental influence on the above-mentioned electrochemical behavior [20]. Similar to the known quinone/hydroquinone couple, two electrons and two hydrogen ions participate in the electrochemical reduction of α-tocoquinone to α-tocohydroquinone in media of neutral supporting electrolytes [21]. This electrochemical reaction simply describes a relationship between molar concentration of the analyte in solution and corresponding signal measured (current response).
Optimal conditions for construction of the tyrosinase biosensor based on carbon nanotubes have been described already. Electrical resistance of the bare CPE should be lower than 10 Ω [22]. The surface density of immobilized CNTs is in the range from 5.7 to 7.1 µg mm−2 [19]. Optimum amount of tyrosinase enzyme present in a Nafion layer is 3.0 µg [12]. Enzyme activity of tyrosinase is the highest at pH 7.0 of the supporting electrolyte [23]. A target of this contribution lies in a search for optimum conditions in amperometric measurements when the biosensor is applied in analysis of wine samples, as well as in the comparison with results of non-electrochemical procedures.
Results and discussion
Effect of working potential
The sensitivity of the biosensor is governed by the redox potential of the electroactive species; in this case by reduction properties of quinones (products of biocatalytic reactions). Electrochemical reduction of α-tocoquinone, as product of biological catalysis, occurs at the searched working potential. The negatively polarized working electrode forces the α-tocoquinone to accept two electrons for its electrochemical reduction. For this reason, a search for the optimum working potential was done in a potential window from 0 to −0.3 V and for stirring speed 200 rpm. Biosensors with both single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) as modifiers were tested. The highest current response was observed using biosensor with MWCNTs at −0.25 V (compare with −0.24 V for hydroquinone [17]). This value was selected and used in further measurements.
Speed of stirring influence
Amperometric measurements in batch system are always carried out at constant speed of stirring. The stirring accelerates a transport of the analyte to biosensor surface. A magnetic stir bar covered with Teflon (1.3 × 0.4 cm) was used. All amperometric measurements were carried out in a voltammetric cell containing corresponding volume of the solution (max. 20 cm3) and at optimal working potential −0.25 V. From stirring speeds 100, 200, 300, 400, and 500 rpm, a value of 400 rpm was selected because at higher speeds, no significant increase of the response was observed.
Response time of CPE/MWCNTs/Tyrosinase/Nafion biosensor
In amperometric techniques, response time belongs to the most important parameters reflecting kinetics of the electrode reactions. As observed in case of Trolox at optimal experimental conditions, the shortest response time (<10 s) was obtained in a pure buffer solution, followed by that obtained during analyses of white wines (>30 s) and/or red wines (>50 s); this can be attributed to the negative influence of sample matrices.
Analysis of wines
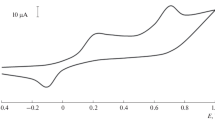
Analysis of white wine (Pálava) by CPE/MWCNTs/Tyrosinase/Nafion biosensor using a multiple standard addition method is shown in Fig. 1. White wines contained less than 100 mg dm−3 and red wines less than 280 mg dm−3 of Trolox.
To compare the results, a spectrophotometric DPPH assay procedure to determine TEAC in selected wine samples was used. In the range of 0.5–12 mmol dm−3 of Trolox, calibration curve described by an equation A = −0.0282 c/mmol dm−3 + 0.5987 with R 2 = 0.9958 was found and used.
On the contrary to white wines, TEACs for red wines increased to nearly 300 mg dm−3. All findings are summarized in Table 1 in which results of both analytical methods are presented. As can be seen, the final results were not exactly the same; however, a satisfactory correlation was found (see Fig. 2). Corresponding correlation coefficient r k = 0.9752 was calculated which is close to the theoretical value 1.
In most cases, results of amperometric measurements (TEAC values) were higher than those of spectrophotometric assessment. To obtain some information about mutual agreement of results of both the analytical procedures, a simple Lord’s u-test [25] was applied as well (see Table 1). Surprisingly, although the amperometric and spectral experiments differ in the principle, statistically insignificant differences of results were observed for two of the wine samples, Pinot Noir and Pinot Gris (u < u crit). In other cases—as expected—these differences were statistically significant (u > u crit) because tyrosinase biosensor represents a specific biological device sensitive only to content of polyphenolic compounds present in wines, whereas spectrophotometric DPPH assay is based on reduction properties of all present species.
Amperometric tyrosinase biosensor based on MWCNTs can successfully be used for direct determination of TEAC in such samples as wines are, and the procedure can be recommended as an alternative method to the official spectrophotometric assays. This biological device has many advantages, e.g., simple application, high sensitivity to polyphenols, etc. In addition, compared to spectrophotometric assay, the procedure does not need any application of solutions containing unstable radicals. But using the biosensor procedure, also some negative problems occurred such as, a short viability of tyrosinase enzyme (3.0 µg in Nafion layer), or shorter week mechanical stability of Nafion layer on the surface of CPE/MWCNTs, caused by a cracking of the membrane due to its drying when used for a longer time. Thus, in the future, an attention will be paid to elimination of such disadvantages.
Experimental
Mushroom tyrosinase (ex. Agaricus bisporus; E.C. 1.14.18.1; 3,130 U mg−1 solid), N,N-dimethylformamide (DMF), radical 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), and 5 % Nafion solution in 55 % ethanol were from Sigma-Aldrich, Vienna, Austria. Both KH2PO4 and Na2HPO4·12H2O for preparation of the 0.1 mol dm−3 phosphate pH 7.0 buffer solution were from PENTA, Prague, Czech Republic. All other chemicals were of the analytical grade purity. Ultrapure water (ρ = 18.3 MΩ cm; Milli-Q system, Millipore) was used for preparing all the solutions.
Wine samples
Seven various Moravian wines such as Pálava, Rulandské šedé (Pinot Gris), Rulandské modré (Pinot Noir), Dornfelder, Zweigeltrebe red, Zweigeltrebe rosé, Veltlínské zelené (Grüner Veltliner) were from wineries in Bošovice, Mutěnice, Velké Němčice, and Popovice.
Preparation of carbon paste electrode
Carbon paste was prepared by mixing 0.5 g of conductive carbon powder CR-2 with average particle size of 2 µm (Maziva Týn nad Vltavou, Czech Republic) with 130 µg of paraffin oil for spectrometry (Merck, Darmstadt, Germany) in a ceramic mortar for 30 min. Fresh paste was pressed into the Teflon holder with electric contact [24].
Immobilization of CNTs on CPE surface
Two different types of carbon nanotubes, multi-walled carbon nanotubes (MWCNTs, diameter 10–30 nm, length 5–15 µm, and specific surface area 40–300 m2 g−1) and single-walled carbon nanotubes (SWCNTs, diameter < 2 nm, length 5–15 µm, and specific surface area >400 m2 g−1) of CNTs were used from Shenzhen Nanotech Port Co. (Shenzhen, China). Suspension (usually 2.0 mg cm−3 in DMF) of CNTs without pretreatment step was homogenized in an ultrasonic wave at room temperature for 1 h. Volume of 20 mm3 of this suspension of CNTs was dropped onto the surface of CPE (diameter of working area 3 mm). The DMF was evaporated in air overnight. Resulting CPEs with immobilized CNTs were used as electric transducer (CPE/CNTs) for preparation of final biological device.
Immobilization of tyrosinase on CPE/CNTs surface
Nafion membrane containing usually 3.0 µg tyrosinase was prepared by following way: 150 mm3 of tyrosinase stock solution in pure water (500 µg cm−3) was mixed with 40 mm3 Nafion solution (neutralized with 8 % ammonia to pH 7) and with 60 mm3 pure water in a small vial; 10 mm3 of this mixture were injected onto the surface of CPE/CNTs and left to dry for 1 h. If not used, the biosensors were stored in a refrigerator at 5 °C.
Amperometry in batch configuration
A three-electrode system consisting of CPE/CNTs/Tyrosinase/Nafion biosensor (working), Ag|AgCl|3.0 mol dm−3 KCl (reference) and platinum wire (counter electrode) was immersed into the cell solution and connected to the potentiostat EmStat (PalmSens Ivium Technologies, The Netherlands). Amperometry as electrochemical technique was used for each of the laboratory experiments. These measurements were carried out in a glass cell containing a buffer solution (10 cm3) with constant speed of stirring (400 rpm). If not stated otherwise, the working potential was −0.25 V. All measurements were realized at 25 ± 1 °C.
Method of multiple standard additions was prioritized to eliminate the negative influence of sample matrices. Usually, 0.5 cm3 of wine was added to 10 cm3 of supporting electrolyte and consequently, 3 or 4 standard additions of 0.01 mol dm−3 Trolox (each of 0.5 cm3) were applied.
Spectrophotometric DPPH assay
As a reference method, the conventional DPPH spectrometric assay was chosen for determination of TEAC in selected wines. Helios Delta UV–VIS spectrometer (Thermo Fisher Scientific, USA) was used for spectrophotometric measurements. The method is based on the measurements of the decreased absorbance values at 517 nm, when the originally violet solution of the DPPH radical changes to yellow after addition of a sample containing antioxidants. In these measurements, volumes of 20 mm3 (white and rosé wines) or 10 mm3 (red wines) were usually pipetted to 4 cm3 DPPH (0.0125 g in 500 cm3) methanolic solution and put in the darkness for 10 min at laboratory conditions. After that, the loss of color was measured in a plastic cuvette.
Statistical analysis
The arithmetic means (\(\overline{x}\)) and ranges (R, difference between the highest and the lowest value in set of data) were calculated for five replications (n = 5). Both precision and mutual agreement of results obtained with the two procedures could be evaluated applying a simple Lord’s u test [25], which is suitable for the comparison of low number of results with the same number of repetitions in each set of data. Values calculated according Eq. (1) were compared with the critical value u crit = 0.306 for number of replications given above and the significance level α = 0.05 (95 % probability). If the calculated u value is higher than u crit, the difference in arithmetic means is statistically significant
References
Šeruga M, Novak I, Jakobek L (2011) Food Chem 124:1208
Netzel N, Strass G, Bitsch I, Könitz R, Christmann M, Bitsch R (2003) J Food Eng 56:223
Sýs M, Pekec B, Kalcher K, Ribitsch V, Feketeföldi B, Vytřas K (2012) In: Kalcher K, Metelka R, Švancara I, Vytřas K (eds) Sensing in Electroanalysis, vol 7. University of Pardubice, Pardubice, p 311
Du B, Zhu FM, Li FY (2012) Adv J Food Sci Technol 4:277
Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K (2013) Pure Appl Chem 85:957
Guo Ch, Yang J, Wei J, Li Y, Xu J, Jiang Y (2003) Nutr Res 23:1719
Paulová H, Bochořáková H, Táborská E (2004) Chem Listy 98:174
Alonso ÁM, Dominguez C, Guillen DA, Barroso CG (2002) J Agric Food Chem 50:3112
Pietta P, Simonetti P, Mauri P (1998) J Agric Food Chem 46:4487
Triantis T, Stelakis A, Dimotikali D, Papadopoulos K (2005) Anal Chim Acta 60:2517
Malyszko J, Karbarz M (2006) J Electroanal Chem 595:136
Sýs M, Metelka R, Mikysek T, Vytřas K (2015) Chem Pap 69:150
Ismaya WT, Rozeboom HJ, Weijn A, Mes JJ, Fusetti F, Wichers HJ, Dijkstra BW (2011) Biochemistry 50:5477
Fairhead M, Thöny-Meyer L (2012) New Biotechnol 29:183
Iozumi K, Hoganson GE, Pennella R, Everett MA, Fuller BB (1993) J Invest Dermatol 100:806
Fontecave M, Pierre JL (1998) Coord Chem Rev 170:125
Sýs M, Pekec B, Kalcher K, Vytřas K (2013) Int J Electrochem Sci 8:9030
Rolff M, Schottenheim J, Decker H, Tuczek F (2011) Chem Soc Rev 40:4077
Sýs M, Pekec B, Kalcher K, Vytřas K (2013) In: Česla P, Fischer J, Vytřas K (eds) Monitorování cizorodých látek v životním prostředí XV. University of Pardubice, Pardubice, p 143
Giacomelli C, Giacomelli FC, Alves LO, Timbola AK, Spinelli A (2004) J Braz Chem Soc 15:748
Zhang Y, Zheng JB (2007) Electrochim Acta 52:7210
Mikysek T, Švancara I, Kalcher K, Bartoš M, Vytřas K (2009) Anal Chem 81:6327
Solná R, Skládal P (2005) Electroanalysis 17:2137
Švancara I, Vytřas K, Metelka R (2010) Casing for carbon paste for electrochemical measurements. In: Czech Patent CZ 301714, 2 Jun 2010 (Chem Abstr 154:220008)
Lord E (1947) Biometrika 34:41
Acknowledgments
Support of the University of Pardubice, Faculty of Chemical Technology (project No. SGFChT06) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sýs, M., Metelka, R. & Vytřas, K. Comparison of tyrosinase biosensor based on carbon nanotubes with DPPH spectrophotometric assay in determination of TEAC in selected Moravian wines. Monatsh Chem 146, 813–817 (2015). https://doi.org/10.1007/s00706-014-1404-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1404-5