Abstract
We propose amperometric biosensors for the determination of aflatoxin M1, based on screen-printed platinum electrodes modified by carbon nanotubes (CNT), graphene oxide (GO), gold nanoparticles (Au-NP) in chitosan, and immobilized tyrosinase. The conditions for obtaining gold nanoparticles are optimized. Aflatoxin M1 exhibits properties of a reversible tyrosinase inhibitor, which makes ensures its determination using biosensors modified by nanomaterials in the concentration range 1 × 10–11–1 × 10–6 M with LOD = 5 × 10–12 M. The kinetic studies of the enzymatic conversion of phenol in the presence of aflatoxin M1 and a tyrosinase biosensor show both two-parameter mismatch inhibition (modification with a CNT/Au-NP composite) and two-parameter coordinated inhibition (modification with GO/Au-NP). Using the proposed enzyme sensors based on tyrosinase, the procedures for determining aflatoxin M1 in dairy products are tested.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Aflatoxin M1 is a hydrolyzed metabolite of aflatoxin B1, a product of microscopic fungi Aspergillus, which naturally contaminates cereals, legumes, and other food products and is capable of transforming into aflatoxin M1 (chemical structure) in animals.

Chemical structure of aflatoxin M1.
This toxin is found in the milk of cows that received feed contaminated with aflatoxin B1 and, therefore, received the name “milk toxin” with the letter symbol M1. Aflatoxins have neither taste nor odor but are highly toxic. A dose of just 2 µg per kilogram of body weight can cause systemic disease, that is, aflatoxicosis [1, 2]. Like its precursor, the M1 toxin represents a severe threat to the health of animals and humans even at low concentrations.
At present, promising, simple, and cost-effective methods for determining mycotoxins are being developed based on a combination of the principles of biocatalytic interactions and voltammetric recording of an analytical signal [3]. Such methods are a worthy alternative to the known chromatographic and optical methods [4–6] and even often significantly surpass them in the selectivity of determinations.
An analysis of the publications has shown that examples of biosensors for the determination of mycotoxins today are few. These are mainly cholinesterase biosensors and aflatoxin-oxidase-based biosensors [7].
The modern approach to the improvement and development of new amperometric biosensors is associated with various ways of modifying the surface of the primary transducers in order to give them the desired properties. Nanomaterials, in particular, carbon nanotubes, are promising for use as a basis for creating miniature biosensor devices due to their unique electronic properties [8]. Graphene oxide has hydrophilic properties because of the presence of oxygen-containing functional groups and is well dispersed in an aqueous medium. This ensures the possibility of grafting and/or entrapment (retention) of the enzyme by the surface of the modifier used. A large number of oxygen-containing groups in combination with a large surface area makes graphene oxide an ideal platform for covalent immobilization of protein [9].
The use of gold nanoparticles is accompanied by the appearance of new physical and electrochemical properties of electrodes and their good conductivity [10]. The use of carbon nanotubes and metal nanoparticles ensures the creation of a required charge density, which makes it possible to control the sensitivity of the sensor directly and to maintain its high electroactivity. Composites based on graphene oxide can be used as an electrode material to improve the electrochemical properties of sensors and biosensors [11]. Graphene oxide can also be deposited on any substrate, thus converting it into a conductor.
Such an approach is very promising for improving the surface of primary converters, which opens up new possibilities in the development of biosensors for the determination of various mycotoxins. It is of interest to develop amperometric biosensors based on immobilized tyrosinase for the determination of mycotoxins.
The goal of this work was to develop amperometric biosensors based on screen-printed platinum electrodes modified with carbon nanotubes, graphene oxide, gold nanoparticles, and immobilized tyrosinase to determine aflatoxin M1, evaluate their analytical capabilities, compare the results of the analysis obtained for modified and unmodified sensors, and use the obtained results to control the content of this mycotoxin in food.
EXPERIMENTAL
Reagents and instruments. The basis of the developed biosensors was a screen-printed system consisting of a working electrode, an auxiliary electrode, and a reference electrode (BVT Technologies, Brno, Czech Republic).
We used platinum-containing paste as the surface material of the working electrode, on which the enzyme was immobilized. An auxiliary electrode is also made of platinum. A silver–silver chloride electrode consisting of silver in a 0.1 M solution of potassium chloride was used as a reference electrode. The volume of the working cell was 200 µL. All measurements with the use of these electrodes were performed using a MEB multipurpose electrochemical detector with digital control [12]. A WiseClean model WUC-A03H ultrasonic bath (DAIHAN Scientific, South Korea) was used at the frequency was 40 kHz to prepare a suspension of carbon nanotubes, graphene oxide, and gold nanoparticles.
Phenol (cp grade) was used as a substrate, the solutions of which were prepared by dissolving an accurately weighed portion in the working buffer solution and used for no more than 3 h. Tyrosinase from fungal tissue (champignons) with a catalytic activity of 165 ± 8 U/mL was used.
A 1% glutaraldehyde solution (ICN Biomedicals) and bovine serum albumin (BSA; Reanal, Hungary) were used. Sodium citrate (cp grade), HCl (cp grade), HAuCl4 ⋅ 4H2O (cp grade), SnCl2 (technical grade), and polyethylene glycol PEG-3000 (Sigma) were used to produce gold nanoparticles.
We used carbon nanotubes with the following geometrical parameters: length of 0.1–10 µm, inner diameter of 2–6 nm, and outer diameter of 10–15 nm (Sigma-Aldrich, United States). Graphene oxide with a predominant size of 75 nm (Sigma-Aldrich, United States) was used.
A chromatographically pure preparation of aflatoxin M1 (solution of aflatoxin M1 in acetonitrile), which is the state standard sample no. 7935-2001 (All-Russian Research Institute of Veterinary Sanitation, Hygiene, and Ecology, Moscow), was used.
The organic solvent (acetonitrile) was distilled off from the standard sample of aflatoxin M1 under vacuum at room temperature. The resulting preparation of mycotoxin was used to prepare working solutions by dissolving it in bidistilled water with the addition of methanol. A phosphate buffer solution with pH 7.05 ± 0.05 was used. The pH values of the aqueous solutions were determined using a pH-150 potentiometer with a glass electrode calibrated by standard buffer solutions.
Preparation of carbon nanotubes for the modification of electrodes. To prepare carbon nanotubes for deposition, they were treated with solutions of nitric and sulfuric acids [8]. Then, carbon nanotubes were centrifuged and repeatedly (5–6 times) washed with water until neutral reaction. The treated nanotubes were dried at 60°C to constant weight. After that, a 0.5% (by weight) solution of chitosan in 0.05 M acetic acid was added to the sample of carbon nanotubes; the mixture was solubilized using ultrasound at room temperature to obtain a homogeneous solution. The final concentration of carbon nanotube was 1 mg/mL. The homogeneity of the CNT solutions used for the modification of the electrode surface was maintained by periodic (at least once a month) ultrasonic treatment.
Preparation of graphene oxide for the modification of the electrode surface. Initial graphene oxide was an aqueous solution with a concentration of 2 mg/mL. For a better fixing of graphene oxide on the electrode surface, a 0.5% chitosan acetate solution was added. The resulting mixture was subjected to ultrasonic treatment at 35°C to obtain a dispersion with a concentration of 1.5 mg/mL [11]. The homogeneity of the graphene oxide solution used to modify the electrode surface was maintained by periodic (at least once a month) ultrasonic treatment.
The dispersion of graphene oxide in chitosan (1 µL) was applied to the electrodes and dried at 20°C for 24 h [12].
Preparation of a homogenate from fungi. We used a homogenate from mushrooms (champignons, Agaricusbisporus) as a source of tyrosinase. A portion of the fungal fruit was finely chopped and ground in a frozen mortar to a pasty texture. Next, a phosphate buffer solution with pH 7.05 was added to the paste, and the suspension was stirred with a magnetic stirrer. The mixture was filtered through a double gauze layer and used as a direct source of tyrosinase [13].
Preparation of the biosensitive part of an amperometric tyrosinase biosensor based on a screen-printed platinum electrode. To obtain the biosensitive part of the biosensor, a mixture containing the enzyme solution (tyrosinase), a BSA solution (50 mg/mL), a phosphate buffer solution (50 mM, pH 7.5), distilled water, and a 1% glutaraldehyde solution were applied to the surface of the working electrode. Glutaraldehyde was added last. After vigorous stirring, 1 µL of the resulting mixture was applied on the surface of the electrodes. The biosensors obtained in this way were left overnight in a closed Petri dish at 4°С. On the next day, the biosensors were washed with water, left to dry in air, and subsequently stored in a refrigerator at 4°C.
RESULTS AND DISCUSSION
An analysis of publications showed that today there are no amperometric tyrosinase biosensors for the determination of aflatoxin M1 in food products. However, the tyrosinase enzyme has an excellent potential for determining the effectors of this enzyme of different nature. For example, the insufficient activity of tyrosinase or its complete absence in the human body leads to some diseases [14]. At the same time, there are simple and affordable ways to obtain an enzyme preparation from the fruit body of fungus Agaricusbisporus. In this regard, we used tyrosinase obtained by the method described above for the development of new amperometric biosensors for the determination of aflatoxin M1.
Under the action of tyrosinase, phenol undergoes biocatalytic hydrolysis with the formation of quinone according to the following mechanisms:
enzymatic reaction [14]

electrochemical reaction

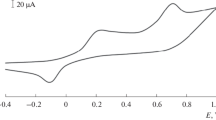
An additional peak is observed for the tyrosinase biosensor at potentials 0.65–0.70 V, which can be attributed to the oxidation of hydrogen peroxide (Fig. 1). According to [15], the electrochemical oxidation of hydrogen peroxide proceeds by reaction
The peak at a potential of 0.2 V is most likely related to the electrochemical oxidation of phenol to the corresponding quinone.
The most substantial catalytic effect for this enzyme is observed in a phosphate buffer solution with pH 7.0 ± 0.05 [16]; therefore, it was used as a supporting electrolyte for measurements with a tyrosinase biosensor. The concentration of the tyrosinase substrate (phenol) used was 1 mM.
Effect of aflatoxin M1 on the catalytic activity of immobilized tyrosinase. Studying the effect of aflatoxin M1 on immobilized tyrosinase included in the biosensitive part of the amperometric biosensor based on screen-printed electrodes showed that in the presence of the toxin in the concentration range of 1 × 10–10–1 × 10–6 M, the analytical signal decreases; that is, aflatoxin M1 inhibits the enzymatic conversion of phenol. This phenomenon was detected for the first time (Fig. 2); previously, a similar effect was observed only for zearalenone [17].
The maximum degree (percentage) of inhibition of the tyrosinase–phenol enzyme–substrate system by aflatoxin M1 is 78 ± 1.0% in the studied concentration range (Table 1).
The accuracy of the determination of aflatoxin M1 in the specified concentration ranges by a biosensor based on immobilized tyrosinase was evaluated by the standard addition method (Table 2).
Effect of carbon nanostructured materials on the analytical characteristics of the developed biosensors. To modify the screen-printed electrodes used, the obtained dispersions of carbon nanotubes or graphene oxide in chitosan were applied to the electrode surface by drop evaporation. Then, a tyrosinase solution was immobilized on the obtained modified surface.
Studying the effect of aflatoxin M1 on tyrosinase immobilized in chitosan on the electrodes modified with carbon nanotubes or graphene oxide showed that the nature of the impact of this compound does not change significantly; that is, the mycotoxin still inhibits tyrosinase. At the same time, electrodes modified with carbon nanotubes or graphene oxide in chitosan enable expansion of the analytical range of mycotoxin and improve the correlation coefficient (Table 1). The maximum degree of inhibition of aflatoxin M1 upon the action of tyrosinase–phenol enzyme–substrate system under these conditions increased and amounted to 85.6 ± 0.8% for carbon nanotubes and 82.0 ± 0.9% for graphene oxide for the studied mycotoxin concentration range.
The accuracy of the determination of aflatoxin M1 in the specified concentration ranges with tyrosinase biosensors was evaluated by the standard addition method (Table 2).
Gold nanoparticles as modifiers of the surface of screen-printed electrodes. Preparation of gold nanoparticles. Modification of the surface of the electrodes serving as the basis of biosensors with carbon nanotubes, graphene oxide, and nanocomposites based on them, including metal nanoparticles is one of the modern methods for changing the properties of biosensors and their analytical characteristics. Gold nanoparticles turned out to be a very useful component for this purpose; for example, nanocomposites carbon nanotubes/metal nanoparticles (CNT/Au-NP) and graphene oxide/metal nanoparticles (GO/Au-NP) are used.
To date, there are quite a few ways to produce gold nanoparticles. We used method [18], because it is quite simple, enables the synthesis of gold nanoparticles from the available reagents under mild conditions, and does not take much time. The best conditions for obtaining gold nanoparticles are presented in Table 3. The presence of gold nanoparticles of a certain size in solutions is confirmed by optical spectra (Fig. 3); they contain plasmon resonance bands of gold nanoparticles with maxima at λ = 580 nm (particle size about 45 nm) and 520 nm (particle size about 25 nm). Based on the shape of the absorption spectra, gold nanoparticles 45 nm in size are more uniform in size than nanoparticles 25 nm in size.
Preliminary studies showed that modification of the electrode surface with suspensions of carbon nanotubes, graphene oxide, and gold nanoparticles leads to a change in the analytical capabilities of biosensors. It was of interest to consider the morphology of the surface of the electrodes in the modification with carbon nanotubes, graphene oxide, and gold nanoparticles. Atomic force microscopy (AFM) is a convenient method for obtaining information; using AFM images, one can observe the surfaces of electrodes at different stages of preparing the biosensitive part of sensors under different conditions (Fig. 4). The AFM images of the electrode surface show that when applying gold nanoparticles of type 2 (Table 3), the surface becomes more developed, the nanoparticles are more uniform, and, moreover, they are more evenly distributed over the electrode surface.
Tyrosinase was immobilized on the obtained modified surface. When modifying the surface of the working electrode, the amount of gold nanoparticles in chitosan applied to the surface of the screen-printed electrode was varied. It is found that the use of 0.5 µL of the solution yields a more reproducible homogeneous surface, providing a fairly intense analytical signal. Subsequently, 0.5 µL of the gold nanoparticle solution was used (Fig. 5).
Effect of electrode surface modification by nanocomposites of CNT/Au-NP and GO/Au-NP on the analytical capabilities of a tyrosinase biosensor. Studying the effects of aflatoxin M1 on the tyrosinase sensors modified with CNT/Au-NP and GO/Au-NP showed that mycotoxin has an inhibitory effect on them. The degree (percentage) of inhibition for aflatoxin M1 upon the action on the tyrosinase–phenol enzyme–substrate system is 90 ± 2% for CNT/Au-NP and 89 ± 1% for GO/Au-NP (Table 1).
The accuracy of the determination of aflatoxin M1 in the specified concentration ranges with tyrosinase biosensors was evaluated by the standard addition method (Table 2).
The use of the tyrosinase biosensors modified with CNT/Au-NP and GO/Au-NP decreases the limit of detection for aflatoxin M1 (Fig. 6).
We performed kinetic studies using the biosensors modified with carbon nanotubes, graphene oxide, and gold nanoparticles in the presence of aflatoxin M1 at different concentrations and a substrate at a concentration of 1 × 10–3 M. Two-parameter mismatch inhibition is observed in the case of a tyrosinase biosensor modified with CNT/Au-NP with the same effect leading to a decrease in the affinity of the substrate and the enzyme (the effector in the presence of phenol at this concentration decreases the rate of its enzymatic conversion). In the case of a tyrosinase biosensor modified with GO/Au-NP, two-parameter coordinated inhibition is observed with the same effect leading to an increase in the affinity of the substrate and the enzyme (the effector in the presence of phenol at this concentration decreases the rate of its enzymatic transformation) (Table 4).
Determination of aflatoxin M1 in food. The proposed biosensors can be used to determine the concentration of aflatoxin M1 in food. We determined this mycotoxin in milk, kefir, and cottage cheese. In the sample preparation stage, the recommendations [19] were used.
Sample preparation. The sample was suspended in a mixture of acetonitrile and water; then, a 1.7 M NaCl solution was added. According to [19], sufficiently complete extraction of the components to be determined should be achieved in this case. The mixture was stirred with a magnetic stirrer for at least 30 min and then centrifuged for 20 min at a speed of 7000 rpm. The supernatant was used to prepare working aqueous solutions by sequential dilution for the subsequent determination of aflatoxin M1 using a tyrosinase biosensor based on CNT/Au-NP-modified electrodes. This tyrosinase biosensor showed the most suitable analytical characteristics, that is, higher sensitivity and correlation coefficient.
It is previously found that there is no analytical signal for a solution obtained after a single extraction of mycotoxin; this solution does not contain components that have an inhibitory effect on immobilized enzymes. Thus, the mycotoxin is completely removed from the sample in a single extraction stage.
Procedure. A specific volume of the substrate solution, a buffer solution, a 0.1 M KCl solution, an analyte solution, and an enzyme sensor were introduced into an electrochemical cell. After 10 min, current value measured at the manifestation potential of the analytical signal. The mycotoxin concentration was determined by an appropriate calibration curve. The results of determining aflatoxin M1 in samples of dairy products are presented in Table 5.
The amount of aflatoxin M1 found in the test food samples is in all cases lower than its maximum permissible concentration (MPC). Thus, the developed tyrosinase biosensor modified with CNT/Au-NP can be used to control the quality of food. A comparison of these results with the data obtained using another biosensor, in particular, cysteine desulfhydrase, the operation of which is based on other principles [3], shows that the results are comparable (equally accurate) and the contribution of the systematic error is insignificant (Table 6).
REFERENCES
Tutel’yan, V.A. and Kravchenko, L.V., Mikotoksiny (Mycotoxins), Moscow: Meditsina, 1985. 321 p.
Alberts, J.F., Engelbrecht, Y., Steyn, P.S., Holzapfel, W.H., and Van Zyl, W.H., Int. J. Food Microbiol., 2006, vol. 109, nos. 1–2, p. 121.
Medyantseva, E.P., Mai Tkhi Tkhan Kh., Varlamova, R.M., Tarasova, E.Yu., Sakhapova, G.R., and Budnikov, G.K., Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki, 2012, vol. 154, vol. 4, p. 92.
Kharandi, N.A., Babri, M., and Azad, J., Food Chem., 2013, vol. 141, no. 3, p. 1619.
Bakar, N.-B., Makahleh, A., and Saad, B., Talanta, 2014, vol. 120, p. 47.
Khayoon, W.S., Saad, B., Salleh, B., Manaf, N.H.A., and Latiff, A.A., Food Chem., 2014, vol. 147, p. 287.
Li, S., Chen, J., Cao, H., Yao, D., and Liu, D., Food Control, 2011, vol. 22, no. 1, p. 43.
Feng, W. and Ji, P., Biotechnol. Adv., 2011, vol. 29, no. 6, p. 889.
Bai, X., Chen, G., and Shiu, K., Electrochim. Acta, 2013, vol. 89, p. 454.
Shapovalova, E.N., Ananieva, I.A., Elfimova, Ya.A., Grineva, L.A., Mazhuga, A.G., and Shpigun, O.A., Moscow Univ. Chem. Bull. (Engl. Transl.), 2012, vol. 67, no. 2, p. 72.
He, L., Wang, H., Xia, G., Sund, J., and Song, R., Appl. Surf. Sci., 2014, vol. 314, p. 510.
Medyantseva, E.P., Varlamova, R.M., Gimaletdinova, D.A., Fattakhova, A.N., and Budnikov, G.K., Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki, 2006, vol. 148, vol. 2, p. 21.
Amjad, A., Suhail, A., and Qayyum, H., Process Biochem., 2005, vol. 40, no. 7, p. 2379.
Berezov, T.T. and Korovkin, B.F., Biologicheskaya khimiya (Biological Chemistry), Moscow: Meditsina, 1998.
Evtyugin, G.A., Budnikov, G.K., and Stoikova, E.E., Osnovy biosensoriki: uchebnoe posobie (Biosensorics: Tutorial), Kazan: Kazan. Gos. Univ., 2007.
Kulis, Yu.Yu., Analiticheskie sistemy na osnove immobilizovannykh fermentov (Analytical Systems Based on Immobilized Enzymes), Vil’nyus: Mokslas, 1981.
Zhao, Q. and Zhuang, Q.K., Electroanalysis, 2005, vol. 17, no. 1, p. 85.
Mai Tkhi Tkhan Kh., Medyantseva, E.P., Varlamova, R.M., Sakhapova, G.R., and Nikovaleva, O.V., Vestn. Kazan. Tekhnol. Univ., 2012, vol. 15, no. 15, p.149.
Shashkanova, O.Yu. and Ermolaeva, T.N., Sorbtsionnye Khromatogr. Protsessy, 2009, vol. 9, no. 5, p. 677.
GOST (State Standard) 30711-2001: Foodstuff. Methods for Detection and Determination of Aflatoxins B 1 and M 1 Content, Moscow: Izd. Standartov, 2001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Zhukova
Rights and permissions
About this article
Cite this article
Varlamova, R.M., Medyantseva, E.P., Khamidullina, R.R. et al. Amperometric Tyrosinase Biosensors Based on Nanomaterial-Modified Electrodes for Aflatoxin M1. J Anal Chem 74 (Suppl 1), 59–67 (2019). https://doi.org/10.1134/S1061934819070189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934819070189