Abstract
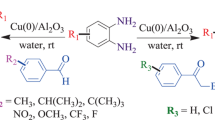
o-Aminoaryl ketones undergo smooth condensation with α-methylene ketones in the presence of nanocrystalline aluminium oxide under mild reaction conditions to afford the corresponding poly-substituted quinolines in excellent yields. The catalyst can be recovered by simple filtration and can be recycled in subsequent reactions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quinoline derivatives are well known not only in medicinal chemistry, because of their wide occurrence in natural products [1, 2] and drugs [3, 4], but also in polymer chemistry, electronics, and optoelectronics for their excellent mechanical properties [5]. Diblock and triblock copolymers incorporating polyquinoline blocks have been found to undergo hierarchical self-assembly into a variety of nano and meso structures with interesting electronic and photonic functions [6, 7]. Friedlander quinoline synthesis is one of the most straightforward approaches practiced for the synthesis of quinolines.
The Friedlander quinoline synthesis consists of the reaction between an aromatic ortho-amino aldehyde and an aldehyde or ketone having an α-methylene functionality. Friedlander reactions are generally carried out either by heating an aqueous or alcoholic solution of the reactants under reflux in the presence of a base or by heating a mixture of the reactants at high temperatures ranging from 150 to 220 °C in the absence of a catalyst [8, 9]. Under thermal or basic catalysis conditions, o-aminobenzophenone fails to react with simple ketones such as cyclohexanone, deoxybenzoin, and β-keto esters [8, 9]. Subsequent work showed that acidic catalysts are more effective than basic catalysts for the Friedlander annulation [8, 9]. Acidic catalysts such as hydrochloric acid, sulfuric acid, p-toluenesulfonic acid, and polyphosphoric acid have been widely employed for this conversion [8–10]. A few recent procedures reported for the synthesis of quinolines involve the use of catalysts such as SnCl2, BiCl3, I2 [11, 12], montmorillonite KSF clay [13], ionic liquids [14], Bi(OTf)3 [15], Y(OTf)3 [16], silver dodecatungstophosphate (Ag3PW12O40) [17], silica sulfuric acid, sulfamic acid, neodymium nitrate [18, 19], dodecylphosphonic acid (DPA) [20], proline [21], silica supported phosphomolybdic acid [22], and sodium ethoxide [23], etc. However, these methods suffer from the serious drawbacks of harsh reaction conditions, use of expensive catalysts, long reaction times, etc. Thus, a simple and environmentally benign procedure involving the use of inexpensive and readily available reagents is desirable.
Continuing our research into the development of new synthetic methods and the use of heterogeneous catalysts, the recent surge in the development of environmentally benign procedures prompted us to test the feasibility of using nano Al2O3 for the synthesis of quinolines without using any co-catalyst or additive. We report herein a highly efficient, cost effective, and environmentally benign procedure for the synthesis of multi-substituted quinolines (Scheme 1).
Results and discussion
Al2O3 is usually prepared by decomposition of a variety of aluminium salts or aluminium hydroxide. However, formed by this method it usually has relatively large grain size, inhomogeneous morphology, and small surface area. These structural and textural features limit its application as a catalyst. Several novel methods such as mechanical synthesis, vapor phase reaction, precipitation, combustion, and sol–gel methods have been reported in literature for preparation of nanosized Al2O3 particles [24]. These methods are highly advantageous in terms of the crystallite size and shape, surface area, and surface basic characteristics of the synthesized Al2O3 particles.
The particle size and surface morphology of Al2O3 synthesized by wet chemical procedures depend upon several factors such as the rate of hydrolysis of the aluminium salt, temperature, type of base, concentration of the salt, and drying and calcination steps [25]. Proper choice of these conditions can lead to particles of uniform morphology and size. The scanning electron micrograph (SEM) of the synthesized Al2O3 particles is shown in Fig. 1. The average size of the obtained Al2O3 particles was 50 nm.
Taking advantage of nanosized Al2O3 particles in terms of their surface basic characteristics, and considering the limitations of Friedlander reactions of o-aminobenzophenone with simple ketones and β-keto esters under basic catalysis conditions, we decided to investigate the behavior of 2-aminoaryl ketones with various simple ketones and β-keto esters in the presence of these nanoparticles as catalyst.
Interestingly, cyclic ketones such as cyclohexanone also underwent smooth condensation with 2-aminoaryl ketones to afford the respective quinolines. In most cases, the products were isolated by simple filtration. The crude products were purified either by recrystallization from 1:2 diethyl ether–n-hexane or by silica gel column chromatography.
In addition, this method is equally effective for both cyclic and acyclic ketones (Table 1). Various substituted 2-aminoaryl ketones such as 2-aminoacetophenone, 2-aminobenzophenone, and 2-amino-5-chlorobenzophenone reacted smoothly with α-methylene ketones to produce a range of quinoline derivatives.
The use of 3 mol% of the catalyst was sufficient to promote the reaction. Larger amounts of the catalyst did not improve the yields. The best result was obtained with 3 mol% Al2O3 nanoparticles under reflux in chloroform. However, in the absence of Al2O3, the reaction did not proceed even after long reaction times (12–24 h).
Unlike previous methods, the present procedure does not require either strong acids or high temperatures (190–250 °C) to afford quinoline derivatives. This method has several advantages for example higher yields, shorter reaction times, cleaner reaction profiles, and simple experimental and work up procedures. In addition, polymer-like condensation products resulting from reactions between o-aminoaryl ketones were not found.
Finally, the efficacy of the Al2O3 as a catalyst for the synthesis of 3a as a model compound was compared with that of other reported catalysts such as Ag3PW12O40, sulfamic acid, Bi(OTf)3, Y(OTf)3, silica gel-supported NaHSO4 (NaHSO4.SiO2), and KSF clay (Table 2). These experiments revealed that Al2O3 nanoparticles are an equally efficient, cost-effective, and environmentally benign catalyst useful in the synthesis of quinoline derivatives. Practically, the catalyst is easier to prepare from readily available reagents and except for the isolation step wherein the solvent was essential, the reaction proceeded under solvent-free conditions.
The insolubility of the Al2O3 catalyst in different organic solvents provided an easy method for its separation from the product. The catalyst was separated by filtration and reused after activation with only a gradual decrease in its activity. For example, the reaction of 2-amino-5-chlorobenzophenone and ethyl acetoacetate afforded the corresponding quinoline derivative in 98, 94, and 89% yields over three successive runs. IR spectra of the resulting solids indicate that the catalyst can be recovered without structural degradation (Fig. 2).
In conclusion, we have described a highly efficient, cost-effective and environmentally benign procedure for synthesis of 2,4,6-trisubstituted quinolines using Al2O3 nanoparticles as a heterogeneous and reusable catalyst. This operationally simple method can be used as a useful alternative to existing procedures for the synthesis of trisubstituted quinolines.
Experimental
Synthesis of nanosized Al2O3
The Al2O3 nanoparticles were synthesized by precipitation of aluminium hydroxide gels in aqueous solution using Al(NO3)3 as salt and aqueous ammonia as the precipitating agent. Initially, the pH of 200 cm3 distilled water was adjusted to 7 by addition of aqueous ammonia. To this solution 0.1 M aluminium nitrate solution was added dropwise with continuous stirring. The rate of addition of the salt solution was kept at 20 cm3/h during the addition; the pH was maintained at 7 by controlled addition of aqueous ammonia solution. After completion of the precipitation procedure, the mixture was stirred at room temperature for 12 h, filtered, repeatedly washed with distilled water, dried at 120 °C, and calcined at 500 °C for 2 h. The temperature of the furnace was increased linearly from room temperature to 500 °C at 10 °/min. At 500 °C, the temperature was maintained for 2 h to yield the final material.
Synthesis of quinolines: general procedure
A mixture of the o-amino ketone (1 mmol), ketone (1 mmol), and Al2O3 nanoparticles (0.03 mmol) was heated under reflux in chloroform (10 cm3) until completion of reaction (TLC). The catalyst was isolated by filtration and washed with chloroform (10 cm3). From the combined filtrate the solvent was removed under vacuum and the pure products were obtained by column chromatography.
Spectral data of the quinolines described in this article are identical with those reported previously in the literature [15–19, 26].
Reusing of the catalyst
We investigated the reusability of the catalyst. For this purpose after completing the model reaction, the catalyst was isolated by filtration and washed three times with 10 cm3 portions of methanol, dried at 150 °C overnight, and subjected to a second run of the reaction process with the same substrate.
References
Morimoto Y, Matusuda F, Shirahama H (1991) Synlett 202
Michael JP (1997) Nat Prod Rep 14:605
Markees DG, Dewey VC, Kidder GW (1970) J Med Chem 13:324
Campbell SF, Hardstone JD, Palmer MJ (1998) J Med Chem 31:1031
Agrawal AK, Jenekhe SA (1993) Chem Mater 5:633
Jenekhe SA, Chen XL (1998) Science 279:1903
Jenekhe SA, Chen XL (1999) Science 283:372
Thummel RP (1992) Synlett 1
Glaldiali S, Chelucci G, Mudadu MS, Gastaut MA, Thummel RP (2001) J Org Chem 66:400
Sterkowski L, Czamy A (2000) J Fluor Chem 104:281
Jia CS, Wang GW (2006) Tetrahedron Lett 47:289
Wu J, Xia HG, Gao K (2006) Org Biomol Chem 4:126
Yadav JS, Subba Reddy BV, Sunitha V, Srinivasa Reddy K, Ramakrishna KVS (2004) Tetrahedron Lett 45:7947
Palimkar SS, Siddiqui SA, Daniel T, Lahoti RJ, Srinivasan KV (2003) J Org Chem 68:9371
Yadav JS, Reddy BVS, Premalatha K (2004) Synlett 963
De SK, Gibbs RA (2005) Tetrahedron Lett 46:1647
Yadav JS, Reddy BVS, Sreedhar P, Srinivasa Rao R, Nagaiah K (2004) Synthesis 2381
Yadav JS, Purushottam Rao P, Sreenu D, Srinivasa Rao R, Naveen Kumar V, Nagaiah K, Prasad AR (2005) Tetrahedron Lett 46:7249
Varala R, Enugala R, Adapa SR (2006) Synthesis 3825
Ghassamipour S, Sardarian AR (2009) Tetrahedron Lett 50:514
Jiang B, Dong J, Jin Y, Du XL, Xu M (2008) Eur J Org Chem 2693
Das B, Krishnalah M, Laxminarayana K, Nandankumar D (2008) Chem Pharm Bull 56:1049
Yang D, Jiang K, Li J, Xu F (2007) Tetrahedron 63:7654
Shojaie-Bahaabad M, Taheri-Nassaj E (2008) Mater Lett 62:3364
Kumar D, Reddy VB, Mishra BG, Rana RK, Nadagouda MN, Varma RS (2007) Tetrahedron 63:3093
Desai UV, Mitragotri SD, Thopate TS, Pore DM, Wadgaonkar PP (2006) Arkivoc xv:198
Acknowledgments
The authors are thankful to Alzahra Research Council for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadjadi, S., Shiri, S., Hekmatshoar, R. et al. Nanocrystalline aluminium oxide: a mild and efficient reusable catalyst for the one-pot synthesis of poly-substituted quinolines via Friedlander hetero-annulation. Monatsh Chem 140, 1343–1347 (2009). https://doi.org/10.1007/s00706-009-0191-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-009-0191-x