Abstract
The present communication elicits the use of copper nanoparticles on aluminium oxide derived from Cu–Al hydrotalcite as a heterogeneous catalyst in the green and operationally simple approach for the synthesis of selective 1,2-disubstituted benzimidazoles and quinoxaline. Wide ranges of substituted o-phenylenediamines and aldehydes or α-bromo ketones were used to achieve the desired products using water as the reaction medium. The recoverability and reusability of the catalyst are the significant features in this eco-friendly green protocol.
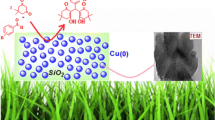
Graphical Abstract
A simple and efficient process for the synthesis of benzimidazoles and quinoxaline in presence of Cu(0)/Al2O3 catalyst at room temperature is described.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Benzimidazole and quinoxaline are important N-heterocyclic units in pharmaceutical industry due to their broad biological functions such as antiviral, antiulcer, antihypertensive and anticancer activities [1,2,3,4,5]. Additionally, benzimidazole moieties frequently find applications in materials science, especially in organic light-emitting diodes and membranes for fuel cells [6, 7]. Similarly, quinoxalines are interesting class of compounds due to the presence of its core structure in many biocides [8], various biofunctional molecules [9] and pharmaceuticals [10].
In general, the synthesis of benzimidazoles moieties comprises the condensation of 1,2-phenylenediamines with aldehydes [11, 12] or carboxylic acid derivatives [13] in presence of strong acids such as polyphosphoric acid or mineral acids. Recently, transition-metal-catalyzed C–N cross-coupling reactions for the synthesis of benzimidazole derivatives have been reported [14,15,16,17,18,19,20,21]. Palladium- and copper-catalyzed aryl amination/condensation protocols have been utilized for the synthesis of 1,2-disubstituted benzimidazoles [22]. Brasche and Buchwald reported the copper catalyzed synthesis of benzimidazoles from amidines through C–H functionalization/C–N bond formation reaction [23]. Shi and co-workers have used the palladium catalyzed C–H activation reaction for the formation of benzimidazoles [24]. The CuI/N,N′-dimethylethylenediamine (DMEDA)-mediated regiospecific reaction of 1,2-dihaloarenes with N-substituted amidines or guanidines for the formation of benzimidazoles was reported by Deng and co-workers [25]. Later, You and co-workers have described the regio-specific synthesis of 1,2-disubstituted benzimidazoles [26].
Other synthetic methodologies, such as reductive cyclization reaction of o-nitroaniline with aldehydes [27], rhodium catalyzed hydroformylation of N-alkenyl-1,2-diaminobenzenes [28], tandem carbonylation–cyclization reaction of o-phenylenediamines [29] and tandem dehydration–coupling reaction of 2-bromoaniline catalyzed by palladium have been reported [30]. However, the main limitation of these methodologies is the formation of both 2-substituted and 1,2-disubstituted benzimidazoles. The selective synthesis of 1,2-disubstituted benzimidazoles have been reported by very few research groups [31,32,33,34]. Among the methods reported, some of the processes suffer from long reaction times, use of toxic catalysts/solvent and a tedious work-up process.
Further, the synthesis of quinoxaline is generally accomplished by the oxidative cascade reactions of o-phenylenediamine with α-hydroxy ketones [35, 36], epoxides [37] and diols [38] in the presence of either noble metal or additional oxidants. Reaction of o-phenylenediamine with dicarbonyl compounds also produces quinoxaline as reported by Lindsley and co-workers [39]. Additionally, multicomponent reactions have recently been introduced for the formation of quinoxaline [40]. Progress has also been made in the synthesis of quinoxaline derivatives by using Brønsted acid [41], and molecular iodine [42, 43]. However, It is intriguing to note that rarely α-bromoketones have been used as reaction partners of o-phenylenediamine for the formation of quinoxalines [33, 44] and most of the reported methodologies suffer from serious draw backs such as use of stoichiometric amount of catalyst, long reaction time, use of toxic solvents, unsatisfactory yields and inability to recover and reuse the catalyst.
Recently, we have focused our efforts on the synthesis of mono dispersed and highly stable Cu(0) from copper–aluminium hydrotalcite [45,46,47]. One of the important characteristics of nano-Cu(0) on alumina is that it is prepared from single precursor, Cu–Al HT Brucite like structures and, upon reduction, Cu(II) gets selectively reduced to Cu(0) with high dispersion and stability. Herein, we report the activity of this nano-Cu(0) on alumina catalyst towards the synthesis of benzimidazole and quinoxaline derivatives (Scheme 1).
2 Results and Discussion
Copper–aluminum hydrotalcites [(Cu–Al HT) Cu:Al 3:1] was prepared by co-precipitation of Cu- and Al nitrates. The thermal reduction of copper aluminiumhydrotalcite to nano copper(0) on alumina catalyst in presence of hydrogen flow is performed according to the procedure reported earlier [45]. Copper:aluminium ratio (Cu:Al) in nano Cu(0)/Al2O3 catalysts was found to be 2.49:1 by inductively coupled plasma-atomic emission spectroscopy (ICPMS). The Cu content of Cu(0)/Al2O3 was obtained 8 wt% by atomic absorption analysis (AAS). The average particle size of Cu(0)/Al2O3 was estimated to be 8.0 nm which is well matched with transmission electron microscopy.
The XRD pattern was used to investigate both the crystal structure and the average crystalline size of Cu nano particles.
As shown in Fig. 1, the clear and strong peaks corresponding to (111), (200), and (220) indicate the formation of highly crystalline Cu nanoparticles [48].
There is no change in the crystal structure of Cu nano particles phases in both fresh and used form of catalysts (Fig. 1a, c). Transmission electron microscopic (TEM) studies of both fresh and used catalysts were carried out to understand the shape and size of the particles. Figure 2 a and b shows the TEM images of the fresh and the used catalyst after the fifth cycle. Interestingly it is observed that the shape and size of the particles remain unchanged. This supports that the morphology of the catalyst remains the same even after recycling.
The X-ray photoelectron spectroscopic (XPS) investigation of Cu–Al HT, Fig. 3a and fresh Cu(0)/Al2O3, Fig. 3b shows that there is a change in oxidation state of copper from +2 to 0. The XPS analysis of Cu(0)/Al2O3 and Cu–Al HT catalyst at the Cu 2p level shows 2p3/2 line at 932.3, and 934.7 eV and 952.2 and 954.6 eV for 2p1/2, which corresponds to Cu in 0 and +2 oxidation states, respectively.
2.1 Optimization Conditions
In the optimization study for the benzimidazole synthesis, various solvents such as dichloromethane, acetonitrile, acetone, methanol, toluene and DMF were tested to check their activity and selectivity (Table 1). Surprisingly, dichloromethane produced poor yield of the product (Table 1, entry 1), while acetonitrile gave good yields. However, increase in reaction temperature had no effect on the isolated yield of the product (Table 1, entries 2 and 3). Only moderate yields of the product was obtained at room temperature and in 60–110 °C with the other organic solvents used (Table 1, entries 4–7). Importantly, Cu(0)/Al2O3 catalyst showed excellent yield and chemoselectivity by furnishing 3a as the sole product in water at room temperature (Table 1, entry 8).

The model reaction was also optimized for catalyst concentration and reaction time (Table 1, entries 9–11). 8 wt% of nano Cu(0)/Al2O3 was sufficient for maximum product yields (Table 1, entry 8) while 4 wt% catalyst loading gave moderate yields of the product (Table 1, entry 9). Interestingly, an increment in catalyst concentration more than 8 wt% did not show effective increase in product yields (Table 1, entry 11).
We also screened a variety of other copper catalysts such as commercially available CuO, Cu2O, copper bronze, CuCl, CuSO4 and Cu–Al HT with the model substrates and water as solvent (Table 2). The reaction proceeded even without the presence of any catalyst. However, nearly equal quantities of product 3a and 4 were obtained (Table 2, entry 1). The Cu(0)/Al2O3 catalyst was found to be more active and selective than the other copper catalysts tested (Table 2, entries 2–8).
The excellent yield and chemoselectivity mediated by Cu(0)/Al2O3 catalyst in water inspired us to further investigate this transformation. Under the optimized reaction conditions, the scope of this reaction was extended by subjecting different diamines and aldehydes. The aromatic aldehydes carrying either electron-donating or electron-withdrawing substituents reacted efficiently to produce the corresponding products in good to excellent yields.
Methyl, isopropyl and tertiary butyl groups on the aldehydes were well tolerated and the corresponding products were obtained in good yields (Table 3, products 3b–3d). The aromatic mono-, di- and tri-sustituted benzaldehydes containing electron donating groups (4-methoxy, 2,3-dimethoxy and 3,4,5-trimethoxy benzaldehyde) produced good yields of the corresponding products (Table 3, products 3e–3h). Furthermore, the reactivity of the substituent groups varies with their position on the aromatic ring of the aldehyde. Introduction of an electron-withdrawing group at the para and meta-position of benzaldehyde compared to ortho-position gave better product yields (Table 3, products 3i–3k). This variation in product yields with position of the substituent group may be due to resonating, inductive or steric effects. Substituted benzaldehydes containing electron-withdrawing fluoro and trifluoromethyl group also furnished good yields of product (Table 3, products 3l and 3m) Interestingly, heteroaromatic aldehydes such as pyridine-2-aldehyde, pyridine-3-aldehyde and furfuraldehyde produced moderate to good yields of the products under similar reaction conditions (Table 3, products 3n-3r).
The proposed mechanism of the Cu(0)/Al2O3 catalyzed synthesis of 1,2-disubstituted benzimidazoles, may tentatively be visualized to occur via the formation of an N,N′-dibenzylidene-o-phenylenediamine. The aldehyde firstly reacted with diamine to form the intermediate 1 (Scheme2). Then in the presence of electrophilic catalyst, the intramolecular 1,3-hydride migration was induced to produce the 1,2-disubstituted benzimidazole. This kind of mechanism has been previously proposed in literature [12, 49] while Yuanjiang Pan and co-workers where the first ones to validate it with isotope labelling reaction using benzaldehyde-d 1 [33].
Encouraged by the high catalytic activity of Cu(0)/Al2O3 in the reaction of o-phenylenediamines with aldehydes, we thought that functional ketones may also react with o-phenylenediamine to give functional heterocyclic compounds. As illustrated in Table 4, the catalyst, Cu(0)/Al2O3 (Cu 8 wt%, 25 mg, 2.69 mol%) and water as solvent at room temperature with a variety of α-bromoketones and o-phenylenediamines proved to be efficient catalytic protocol to produce functionalized quinoxalines. The minimal effect of the substituent groups on the course of the reaction was observed when both substituted ketones as well as amines were used (Table 4, entries 4b–4e).
The recyclability study of recovered catalyst Cu(0)/Al2O3 in the quinoxaline synthesis was also performed. Figure 4 presents the results of recycling of the catalyst using 1,2-phenylenediamine and α-bromoketone as the model substrate. Cu(0)/Al2O3 catalyst shows nearly consistent activity up to five reaction cycles. After each cycle, the Cu(0)/Al2O3 catalyst was recovered by simple centrifugation, washed with water and diethyl ether, oven-dried and used directly for the next cycle without any further purifications. Only a slight loss of catalytic activity was observed. Moreover, the leaching of the copper metal after each cycle was determined by AAS and was found to be negligible. Interestingly, the recovered catalyst’s diminished catalytic activity could be regenerated using thermal reduction in presence of hydrogen flow (Fig. 4 cycle 6).
We envisaged a plausible reaction mechanism of the formation of quinoxaline derivatives from o-phenylenediamine and phenacyl bromide (Scheme 3). Initially the electrophilic catalyst activates the phenacyl bromide making it susceptible for a nucleophilic attack by the amino group of phenylenediamine to afford the intermediate (1) intermediate 1 then cyclises to form the intermediate (2) the charged water group in intermediate 3 is expelled out by the nucleophilic attack from the adjacent amino group which results in the formation of intermediate 4 which readily undergoes aromatization under air oxidation to afford 2-phenylquinoxaline as the final product.
3 Conclusion
In summary, a practical and green catalytic synthetic method has been developed for the facile synthesis of 1,2-disubstituted benzimidazoles and quinoxalines with excellent yields. The broad ranges of functional heterocyclic compounds have been synthesized in water in the presence of copper nanoparticles stabilised on alumina without using any additional surfactant or oxidant. The catalyst can be used for five cycles with almost consistent activity.
4 Experimental Section
4.1 General Experimental Procedure for the Synthesis of 1,2-Disubstituted Benzimidazoles in Water
A 10 mL Schlenk flask was charged with aldehyde (2 mmol), 1,2-diamine (1 mmol), Cu(0)/Al2O3 catalyst (Cu 8 wt%, 25 mg) in water (2 mL). The reaction mixture was stirred at room temperature under vigorous stirring for appropriate time. After the completion of the reaction, as monitored by TLC, 5 mL of ethyl acetate was added in the reaction mixture. The catalyst was separated by simple centrifugation and the reaction mixture was treated with brine (10 mL). The organic layer was separated and the aqueous layer was back extracted with ethyl acetate (3 × 5 mL). The combined ethyl acetate extract was dried with anhydrous Na2SO4 (50 g) and was concentrated under reduced pressure. The pure product was isolated by flash column chromatography on silica gel using ethyl acetate/hexane (10%) as an eluent.
4.1.1 1-Benzyl-2-phenyl-1H-benzo[d]imidazole (3a) [49]
White solid; 1H NMR (400 MHz, CDCl3) δ 7.87 (d, 1H), 7.71–7.65 (m, 2H), 7.48–7.40 (m, 3H), 7.36–7.25 (m, 4H), 7.22 (q, 2H), 7.09 (d, 2H), 5.44 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 154.2, 143.2, 136.4, 136.1, 130.0, 129.3, 129.2, 128.7, 127.8, 126.0, 123.0, 122.7, 120.0, 48.4. ESI-MS [M + H]+: m/z = 285; HRMS (ESI orbitrap): calc. for C20H16N2 [M + H]+: 285.1386, found: 285.1386.
4.1.2 1-(2-Methylbenzyl)-2-(o-tolyl)-1H-benzo[d]imidazole (3b) [32]
White solid; 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 7.9 Hz, 1H), 7.38–7.26 (m, 3H), 7.24–7.16 (m, 2H), 7.16–7.10 (m, 2H), 7.05–6.99 (m, 1H), 6.64 (d, J = 7.8 Hz, 2H), 5.18 (s, 2H), 2.23 (s, 3H), 2.15 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 153.9, 143.1, 138.3, 134.9, 134.0, 130.4 25.7, 130.3, 129.8, 127.5, 126.3, 126.0, 125.6, 122.8, 122.3, 120.0, 110.5, 45.7, 19.8, 19.0. ESI-MS [M + H]+: m/z = 313; HRMS (ESI orbitrap): calc. for C22H20N2 [M + H]+: 313.16993, found: 313.16824.
4.1.3 1-(4-Isopropylbenzyl)-2-(4-isopropylphenyl)-1H-benzo[d] imidazole (3c) [50]
White solid; 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 7.9 Hz, 1H), 7.64 (d, J = 8.2 Hz, 2H), 7.32–7.26 (m, 3H), 7.22–7.14 (m, 4H), 7.02 (d, J = 8.1 Hz, 2H), 5.42 (s, 2H), 2.99–2.83 (m, 2H), 1.27 (s, 3H), 1.26 (s, 3H), 1.23 (d, J = 3.8 Hz, 3H), 1.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 154.3, 150.9, 148.4, 143.2, 136.1, 133.8, 129.3, 127.5, 127.1, 126.8, 125.9, 122.8, 122.5, 119.8, 110.6, 48.2, 34.1, 33.7, 23.9. ESI-MS [M + H]+: m/z = 369; HRMS (ESI orbitrap): calc. for C26H28N2 [M + H]+: 369.23064 found: 369.23064.
4.1.4 1-(4-(Tert-butyl)benzyl)-2-(4-(tert-butyl)phenyl)-1H-benzo[d]imidazole (3d) [51]
White solid; 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 7.9 Hz, 1H), 7.66 (d, J = 8.3 Hz, 2H), 7.47 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.3 Hz, 2H), 7.32–7.27 (m, 1H), 7.21 (dd, J = 6.4, 3.5 Hz, 2H), 7.05 (d, J = 8.3 Hz, 2H), 5.44 (s, 2H), 1.34 (s, 8H), 1.30 (s, 8H); 13C NMR (101 MHz, CDCl3) δ 154.3, 153.2, 150.7, 143.1, 136.0, 133.4, 129, 127.0, 126.0, 126.0, 122.8, 122.7, 119.8, 110.6, 48.2, 34.8, 34.5, 31.3; ESI-MS [M + H]+: m/z = 397; HRMS (ESI orbitrap): calc. for C28H32N2 [M + H]+: 397.2638, found: 397.2637.
4.1.5 1-(4-Methoxybenzyl)-2-(4-methoxyphenyl)-1H-benzo[d]imidazole (3e) [29]
White solid; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 7.9 Hz, 1H), 7.64 (d, J = 8.8 Hz, 2H), 7.29 (ddd, J = 7.3, 5.3, 2.6 Hz, 1H), 7.21 (dd, J = 6.1, 3.1 Hz, 2H), 7.03 (d, J = 8.7 Hz, 2H), 6.97 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.7 Hz, 2H), 5.38 (s, 2H), 3.85 (s, 3H), 3.78 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 160.9, 159.1, 154.1, 143.1, 136.1, 130.7, 128.5, 127.2, 122.7, 122.5, 119.7, 114.33, 114.0, 110.4, 55.3, 47.9 ; ESI-MS [M + H+]+: m/z = 345; HRMS (ESI orbitrap): calc. for C22H20N2O2 [M + H]+: 345.1597, found: 345.1611.
4.1.6 1-(2,3-Dimethoxybenzyl)-2-(2,3-dimethoxyphenyl)-1H-benzo[d]imidazole (3f)
Yellow solid (MP: 86–90 °C); 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.8 Hz, 2H), 7.30–7.23 (m, 5H), 7.22–7.17 (m, 2H), 7.16–7.13 (m, 4H), 7.07–7.02 (m, 2H), 6.82 (t, J = 7.9 Hz, 2H), 6.76 (dd, J = 8.2, 1.3 Hz, 2H), 6.28 (dd, J = 7.6, 1.3 Hz, 2H), 5.34 (s, 4H), 3.91 (s, 6H), 3.82 (s, 6H), 3.65 (t, J = 5.7 Hz, 12H); 13C NMR (126 MHz, CDCl3) δ 152.7, 152.5, 151.7, 147.5, 146.4, 143.2, 135.3, 130.0, 125.4, 124.5, 124.1, 123.8, 122.6, 122.0, 119.8, 114.0, 111.7, 110.9, 61.4, 60.4, 55.9, 55.72, 43.4; ESI-MS [M + H]+: m/z = 405; HRMS (ESI orbitrap): calc. for C24H24N2O4 [M + H]+: 405.1808, found: 405.1809.
4.1.7 1-(3,4,5-Trimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (3g) [49]
White solid; 1H NMR (400 MHz, CDCl3) δ 7.91–7.84 (m, 1H), 7.37–7.34 (m, 1H), 7.34–7.28 (m, 2H), 6.92 (s, 2H), 6.36 (s, 2H), 5.40 (s, 2H), 3.89 (s, 3H), 3.82 (s, 3H), 3.72 (d, J = 1.2 Hz, 6H), 3.71 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 154.1, 154.0, 153.3, 142.8, 139.4, 137.4, 136.4, 132.5, 125.1, 123.2, 122.8, 119.9, 110.0, 106.3, 102.7, 60.89, 56.2, 56.0, 48.5; ESI-MS [M + H]+: m/z = 465; HRMS (ESI orbitrap): calc. for C26H28N2O6 [M + H]+: 465.2020, found: 465.2033, calc. for C26H28N2O6 [M + Na]+: 487.1839, found: 487.1852.
4.1.8 6-Nitro-1-(3,4,5-trimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)-1H-benzo[d]imidazole (3h)
Orange Solid (MP: 144–148 °C); 1H NMR (500 MHz, CDCl3) δ 8.33–8.26 (m, 2H), 7.92 (d, J = 9.0 Hz, 1H), 6.96 (s, 2H), 6.32 (s, 2H), 5.47 (d, J = 17.1 Hz, 2H), 3.91 (s, 3H), 3.83 (s, 3H), 3.74 (s, 6H), 3.73 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 158.8, 154.1, 153.6, 147.4, 143.8, 140.4, 137.9, 135.8, 131.2, 123.8, 120.0, 118.9, 106.9, 106.5, 102.8, 60.9, 56.3, 56.0, 48.9. ESI-MS [M + H]+: m/z = 510; HRMS (ESI orbitrap): calc. for C26H27N3O8 [M + H]+ : 310.08223, found: 310.08375.
4.1.9 1-(2-Nitrobenzyl)-2-(2-nitrophenyl)-1H-benzo[d]imidazole (3i) [49]
Yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.15 (ddd, J = 13.0, 7.2, 2.6 Hz, 2H), 7.86 (d, J = 7.9 Hz, 1H), 7.74–7.60 (m, 2H), 7.56–7.42 (m, 3H), 7.39–7.33 (m, 1H), 7.33–7.27 (m, 1H), 7.15 (d, J = 7.9 Hz, 1H), 6.95 (d, J = 7.1 Hz, 1H), 5.69 (s, 2H); 13C NMR (126 MHz, CDCl3) δ 149.8, 149.0, 147.0, 143.0, 134.8, 134.3, 133.1, 132.1, 131.5, 128.9, 128.4, 125.5, 125.27, 123.9, 123.2, 110.2, 45.7. ESI-MS [M + H]+: m/z = 375; HRMS (ESI orbitrap): calc. for C20H14N4O4 [M + H]+: 375.10878, found: 375.10951.
4.1.10 1-(3-Nitrobenzyl)-2-(3-nitrophenyl)-1H-benzo[d]imidazole (3j) [33]
Pale yellow solid; 1H NMR(300 MHz, CDCl3 + DMSO) δ 8.52 (d, J = 1.9 Hz, 1H), 8.39–8.32 (m, 1H), 8.19 (d, J = 8.1 Hz, 1H), 8.11–8.00 (m, 2H), 7.89 (d, J = 6.8 Hz, 1H), 7.72 (t, J = 8.0 Hz, 1H), 7.58 (t, J = 7.9 Hz, 1H), 7.45–7.29 (m, 4H), 5.65 (s, 2H); 13C NMR (75 MHz, CDCl3 + DMSO) δ 151.0, 148.4, 142.8, 138.0, 135.8, 134.8, 131.8, 131.4, 130.3, 130.0, 124.6, 124.1, 124.0, 123.5, 123.2, 121.0, 120.4, 110.2, 47.6; ESI-MS [M + H]+: m/z = 375; HRMS (ESI orbitrap): calc. for C20H14N4O4 [M + H]+: 375.10878, found: 375.10954.
4.1.11 1-(4-Nitrobenzyl)-2-(4-nitrophenyl)-1H-benzo[d]imidazole (3k) [49]
White solid; 1H NMR (400 MHz, CDCl3) δ 8.33 (d, J = 8.9 Hz, 2H), 8.24 (d, J = 8.8 Hz, 2H), 7.93 (d, J = 7.9 Hz, 1H), 7.85 (d, J = 8.8 Hz, 2H), 7.44–7.38 (m, 1H), 7.38–7.32 (m, 1H), 7.27 (d, J = 9.3 Hz, 3H), 7.21 (d, J = 7.9 Hz, 1H), 5.58 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 148.5, 147.9, 143.1, 142.8, 135.7, 130.0, 126.7, 124.6, 124.1, 123.8, 120.8, 110.2, 48.0; ESI-MS [M + H]+: m/z = 375; HRMS (ESI orbitrap): calc. for C20H14N4O4 [M + H]+: 375.10878, found: 375.10947.
4.1.12 1-(4-Fluorobenzyl)-2-(4-fluorophenyl)-1H-benzo[d]imidazole (3l) [33]
White solid; 1H NMR (500 MHz, CDCl3) δ 7.87 (d, J = 7.9 Hz, 1H), 7.64 (dd, J = 7.9, 5.4 Hz, 2H), 7.32 (t, J = 7.5 Hz, 1H), 7.26 (t, J = 7.4 Hz, 1H), 7.21 (d, J = 7.9 Hz, 1H), 7.14 (t, J = 8.4 Hz, 2H), 7.02 (dd, J = 15.7, 7.2 Hz, 4H), 5.39 (s, 2H).; 13C NMR (101 MHz, CDCl3) δ 152.4, 143.1, 140.0, 135.9, 133.3, 131.8, 130.6, 129.5, 126.2, 125.9, 125.1, 123.9, 123.3, 122.4, 120.5, 110.3, 48.0; ESI-MS [M + H]+: m/z = 321; HRMS (ESI orbitrap): calc. for C20H14F2N2 [M + H]+: 321.1197, found: 320.1198.
4.1.13 1-(4-(Trifluoromethyl)benzyl)-2-(4-(trifluoromethyl)phenyl)-1H-benzo[d]imidazole (3m) [52]
Red solid; 1H NMR (500 MHz, CDCl3) δ 7.91 (d, J = 8.1 Hz, 1H), 7.78 (d, J = 8.2 Hz, 2H), 7.73 (d, J = 8.4 Hz, 2H), 7.62 (d, J = 8.2 Hz, 2H), 7.40–7.35 (m, 1H), 7.33–7.28 (m, 1H), 7.21 (t, J = 7.1 Hz, 3H), 5.51 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 152.4, 143.17, 140.0, 135.9. 133.3, 129.5, 126.2, 125.8, 123.9, 123.3, 120.5, 110.3, 48.06. ESI-MS [M + H]+: m/z = 421; HRMS (ESI orbitrap): calc. for C22H14F6N2 [M + H]+: 286.1133, found: 286.1128.
4.1.14 2-(Pyridin-2-yl)-1-(pyridin-2-ylmethyl)-1H-benzo[d]imidazole (3n ) [49]
White solid; 1H NMR (400 MHz, CDCl3) δ 8.58 (tdd, J = 2.6, 1.7, 0.8 Hz, 2H), 8.49 (d, J = 8.1 Hz, 1H), 7.89–7.86 (m, 1H), 7.84 (dd, J = 7.9, 1.8 Hz, 1H), 7.49 (td, J = 7.8, 1.8 Hz, 1H), 7.38 (dd, J = 7.2, 0.9 Hz, 1H), 7.35–7.29 (m, 2H), 7.29–7.25 (m, 2H), 7.15 (dd, J = 6.7, 5.0 Hz, 1H), 6.91 (d, J = 7.9 Hz, 1H), 6.30 (s, 2H).13C NMR (101 MHz, CDCl3) δ 157.4, 149.8, 149.1, 148.7, 137.2, 124.6, 123.9, 123.1, 122.3, 120.0, 110.8, 51.1. ESI-MS [M + H]+: m/z = 286; HRMS (ESI orbitrap): calc. for C18H14N4 [M + H]+: 287.12912 found: 287.12764.
4.1.15 3-(Pyridin-3-yl)-1-(pyridin-3-ylmethyl)-1H-benzo[d]imidazole (3o) [32]
Yellow gummy liquid; 1H NMR (400 MHz, CDCl3) δ 8.91 (s, 1H), 8.72 (t, J = 12.7 Hz, 1H), 8.60 (dd, J = 25.7, 10.1 Hz, 1H), 8.47 (s, 1H), 8.01 (d, J = 7.9 Hz, 1H), 7.90 (d, J = 7.9 Hz, 1H), 7.43 (dd, J = 7.7, 4.9 Hz, 1H), 7.40–7.33 (m, 1H), 7.30 (t, J = 6.2 Hz, 2H), 7.28–7.22 (m, 2H), 5.50 (s, 2H); 13C NMR (126 MHz, CDCl3) δ 150.9, 149.5, 147.8, 143.2, 136.7, 135.7, 133.6, 131.5, 126.3, 123.9, 123.8, 123.3, 120.4, 110.2, 46.16; ESI-MS [M + H]+: m/z = 286; HRMS (ESI orbitrap): calc. for C18H14N4[M + H]+: 286.1291, found: 286.1292.
4.1.16 2-(Furan-2-yl)-1-(furan-2-ylmethyl)-1H-benzo[d]imidazole (3p) [49]
Brown solid; 1H NMR (400 MHz, CDCl3) δ 7.83–7.74 (m, 1H), 7.62 (d, J = 19.4 Hz, 1H), 7.49 (dd, J = 5.9, 2.9 Hz, 1H), 7.35–7.27 (m, 3H), 7.22 (d, J = 3.3 Hz, 1H), 6.63–6.59 (m, 1H), 6.31–6.20 (m, 2H), 5.64 (s, 2H).13C NMR (126 MHz, CDCl3) δ 149.6, 145.4, 143.9, 142.9, 142.6, 135.5, 123.2, 122.9, 119.8, 112.9, 112.1, 110.5, 110.0, 108.3, 41.7; ESI-MS [M + H]+: m/z = 265; HRMS (ESI orbitrap): calc. for C16H12N2O2[M + H]+: 265.0971, found: 265.0979.
4.1.17 2-(Furan-2-yl)-1-(furan-2-ylmethyl)-5, 6-dimethyl-1H-benzo[d]imidazole (3q) [53]
Brown Solid; 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 31.2 Hz, 2H), 7.33 (s, 1H), 7.24 (d, J = 11.4 Hz, 1H), 7.14 (s, 1H), 6.58 (s, 1H), 6.23 (d, J = 30.9 Hz, 2H), 5.57 (s, 2H), 2.39 (s, 3H), 2.37 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.9, 145.6, 143.6, 143.2, 142.5, 141.6, 134.0, 132.5, 131.8, 119.8, 112.2, 111.9, 110.5, 110.1, 108.1, 41.6, 20.7, 20.3. ESI-MS [M + H]+: m/z = 293; HRMS (ESI orbitrap): calc. for C18H16N2O2 [M + H]+: 293.12845, found: 293.12657.
4.1.18 2-(Furan-2-yl)-1-(furan-2-ylmethyl)-6-nitro-1H-benzo[d]imidazole (3r)
Brown solid; (MP: 146–150 °C) 1H NMR (500 MHz, CDCl3) δ 8.49 (d, J = 2.0 Hz, 1H), 8.23 (dd, J = 8.9, 2.1 Hz, 1H), 7.80 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 1.1 Hz, 1H), 7.39 (d, J = 3.5 Hz, 1H), 7.36 (t, J = 3.1 Hz, 1H), 6.69 (dd, J = 3.5, 1.7 Hz, 1H), 6.37 (d, J = 3.1 Hz, 1H), 6.33 (dd, J = 3.1, 1.8 Hz, 1H), 5.76 (s, 2H); 13C NMR (126 MHz, CDCl3) δ 155.6, 145.1, 143.2, 119.6, 119.0, 115.2, 112.6, 110.7, 109.2, 107.1, 42.1; ESI-MS [M + H]+: m/z = 310; HRMS (ESI orbitrap): calc. for C16H11N3O4 [M+H]+: 310.0837, found: 310.0822.
4.2 General Experimental Procedure for the Synthesis of Quinoxalines from Substituted 1, 2-Diamines and α-Bromo Ketones
A 10 mL Schlenk flask was charged with diamines (1 mmol), α-bromoketone (1 mmol), Cu(0)/Al2O3 catalyst (Cu 8 wt%, 25 mg) in water (2 mL). The reaction mixture was stirred at room temperature for appropriate time. After the completion of the reaction, as monitored by TLC, 5 mL of diethyl ether was added and the catalyst was separated by simple centrifugation. The reaction mixture was treated with brine (10 mL) and the organic layer was separated. The aqueous layer was back extracted with diethyl ether (3 × 5 mL). The combined organic extract was dried with anhydrous Na2SO4 (50 g) and was concentrated under reduced pressure. The pure product was isolated by flash column chromatography on silica gel using ethyl acetate/hexane (10%) as an eluent.
4.2.1 2-Phenylquinoxaline (4a) [51]
White solid; 1H NMR (400 MHz, CDCl3) δ 9.34 (s, 1H), 8.23–8.18 (m, 2H), 8.18–8.10 (m, 2H), 7.82–7.72 (m, 2H), 7.61–7.50 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 151.90 (s), 143.40 (s), 142.35 (s), 141.63 (s), 136.83 (s), 130.27 (d, J = 9.7 Hz), 129.62 (d, J = 9.8 Hz), 129.19 (s), 127.59 (s).ESI-MS [M + H]+: m/z = 207.
4.2.2 6-Nitro-2-phenylquinoxaline (4b) [53]
Yellow solid; 1H NMR (400 MHz, CDCl3) δ 9.50 (s, 1H), 9.03 (d, J = 2.4 Hz, 1H), 8.56 (dd, J = 9.2, 2.4 Hz, 1H), 8.33–8.23 (m, 3H), 7.66–7.56 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 154.3, 147.4, 145.5, 144.9, 140.3, 135.6, 131.4, 131.2, 129.4, 127.9, 125.6, 123.8.ESI-MS [M + H]+: m/z = 252.
4.2.3 6-Chloro-2-phenylquinoxaline (4c) [54]
White solid; 1H NMR (400 MHz, CDCl3) δ 9.32 (s, 1H), 8.25–8.13 (m, 3H), 8.05 (d, J = 8.9 Hz, 1H), 7.68 (dd, J = 17.2, 10.1 Hz, 1H), 7.62–7.50 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 150.6, 142.8, 142.2, 141.6, 136.6, 135.2, 130.5, 129.8, 129.5, 129.1, 128.8. ESI-MS [M + H]+: m/z = 241.
4.2.4 5-Methyl-2-phenylquinoxaline (4d) [55]
White solid; 1H NMR (400 MHz, CDCl3) δ 9.31 (s, 1H), 8.16 (t, J = 14.6 Hz, 2H), 8.10–7.82 (m, 2H), 7.66–7.45 (m, 4H), 2.61 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 140.8, 140.1, 137.0, 132.6, 131.8, 130.0, 129.1, 127.4, 21.8. ESI-MS [M + H]+: m/z = 221.
4.2.5 2-(4-Chlorophenyl)quinoxaline (4e) [51]
White solid; 1H NMR (400 MHz, CDCl3) δ 9.31 (s, 1H), 8.20–8.10 (m, 4H), 7.84–7.72 (m, 2H), 7.55 (d, J = 8.6 Hz, 2H).13C NMR (101 MHz, CDCl3) δ 150.6, 142.8, 142.2, 141.6, 136.6, 135.2, 130.5, 129.8, 129.5, 129.1, 128.8. ESI-MS [M + H]+: m/z = 241.
References
Gravatt GL, Baguley BC, Wilson WR, Denny WA (1994) J Med Chem 37:4338–4345
Kim JS, Gatto B, Yu C, Liu A, Liu LF, LaVoie EJ (1996) J Med Chem 39:992–998
Roth T, Morningstar ML, Boyer PL, Hughes SH, Buckheit Jr RW, Michejda CJ (1997) J Med Chem 40:4199–4207
Horton DA, Bourne GT, Smythe ML (2003) Chem Rev 103:893–930
Bai Y, Lu J, Shi Z, Yang B (2001) Synlett 4:544–546
Meuthen B, Jandel AS (2008) Coil Coating, Auflage, Friedr. Vieweg & Sohn Verlag, Wiesbaden, p 65
Scherer GG (2008) Fuell cells II. Springer, Berlin, pp 65–120
Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK (1990) J Med Chem 33:2240–2254
Gazit A, App H, McMahon G, Chen J, Levitzki A, Bohmer FD (1996) J Med Chem 39:2170–2177
Seitz LE, Suling WJ, Reynolds RC (2002) J Med Chem 45:5604–5606
Preston PN (1981) Chemistry of heterocyclic compounds. Wiley, New York
Kokare ND, Sangshetti JN, Shinde DB (2007) Synthesis 18:2829–2834
Dudd LM, Venardou E, Garcia-Verdugo E, Licence P, Blake AJ, Wilson C, Poliakoff M (2003) Green Chem 5:187–192
Saha P, Ali MA, Ghosh P, Punniyamurthy T (2010) Org Biomol Chem 8:5692–5699
Mahesh D, Sadhu P, Punniyamurthy T (2015) J Org Chem 80:1644–1650
Song XR, Qiu YF, Song B, Hao XH, Han YP, Gao P, Liu XY, Liang YM (2015) J Org Chem 80:2263–2271
Rasheed Sk, Rao DN, Das P (2015) J Org Chem 80:9321–9327
Huang J, Chan J, Chen Y, Borths CJ, Baucom KD, Larsen RD, Faul MM (2010) J Am Chem Soc 132:3674–3675
Ueda S, Buchwald SL (2012) Angew Chem Int Ed 51:10364–10367
Chen C, Shang G, Zhou J, Yu Y, Li B, Peng J (2014) Org Lett 16:1872–1875
Shargi H, Sarvari MH, Moeini F (2008) Can J Chem 86:1044–1051
Zou B, Yuan Q, Ma D (2007) Angew Chem Int Ed 46:2598–2601
Brasche G, Buchwald SL (2008) Angew Chem Int Ed 47:1932–1934
Xiao Q, Wang W-H, Liu G, Meng F-K, Chen J-H, Yang Z, Shi Z-J (2009) Chem Eur J 15:7292–7296
Deng X, McAllister H, Mani NS (2009) J Org Chem 74:5742–5745
Zhao D, Hu J, Wu N, Huang X, Qin X, Lan J, You J (2011) Org Lett 13:6516–6519
Yang D, Fokas D, Li J, Yu L, Baldino CM (2005) Synthesis 1:47–56
Anastasiou D, Campi EM, Chaouk H, Jackson WR (1992) Tetrahedron 48:7467–7478
Perry RJ, Wilson BD (1993) J Org Chem 58:7016–7021
Brain CT, Brunton SA (2002) Tetrahedron Lett 43:1893–1895
Chebolu R, Kommi DN, Kumar D, Bollineni N, Chakraborti AK (2012) J Org Chem 77:10158–10167
Shelkar R, Sarode S, Nagarkar J (2013) Tetrahedron Lett 54:6986–6990
Wan J-P, Gan S-F, Wu J-M, Pan Y (2009) Green Chem 11:1633–1637
Bahrami K, Khodaei MM, Nejati A (2010) Green Chem 12:1237–1241
Sithambaram S, Ding Y, Li W, Shen X, Gaenzler F, Suib SL (2008) Green Chem 10:1029–1032
Cho CS, Oh SG (2007) J Mol Catal A 276:205–210
Antoniotti S, Dunach E (2002) Tetrahedron Lett 43:3971–3973
Cho CS, Oh SG (2006) Tetrahedron Lett 47:5633–5636
Zhao Z, Wisnoski DD, Wolkenberg SE, Leister WH, Wang Y, Lindsley CW (2004) Tetrahedron Lett 45:4873–4876
Neochoritis C, Stephanidou-Stephanatou J, Tsoleridis CA (2009) Synlett 20:302–305
Brown DJ (2004) In: Taylor EC, Wipf P (eds) The chemistry of heterocyclic compounds, vol 61. John Wiley Sons, Hoboken, NJ, pp 1–510
Bhosale RS, Sarda SR, Ardhapure SS, Jadhav WN, Bhusare SR, Pawar RP (2005) Tetrahedron Lett 46:7183–7186
More SV, Sastry MNV, Wang CC, Yao C-F (2005) Tetrahedron Lett 46:6345–6348
Das B, Venkateswarlu K, Suneel K, Majhi A (2007) Tetrahedron Lett 48:5371–5374
Tiwari DK, Jaya P, Sridhar B, Tiwari DK, Likhar PR (2015) Chem Commun 51:13646–13649
Arundhathi R, Damodara D, Mohan VK, Kantam ML, Likhar PR (2013) Adv Synth Catal 355:751–756
Damodara D, Arundhathi R, Likhar PR (2014) Adv Synth Catal 356:189–198
Mott D, Galkowski J, Wang L, Luo J, Zhong C–J (2007) Langmuir 23:5740–5745
Ravi V, Ramu E, Vijay K, Rao AS (2007) Chem Pharm Bull 55:1254–1257
Mohammadizadeh MR, Taghavi Z (2011) E-J Chem 8:101–106
Ma Z-H, Lin S, Nie J (2012) Synth Commun 42:506–515
Chebolu R, Kommi DN, Kumar D, Bollineni N, Chakraborti AK (2012) J Org Chem 77:10158 – 10167
Mukhopadhyaya C, Dattaa A, Butcherb RJ, Paula BK, Guchhait N, Singha R (2009) Water mediated expeditious and highly selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles by Dowex 50W: fluorescence properties of some representative compounds, vol 13. ARKIVOC, Gainesville, FL, pp 1–22
Vadagaonkar KS, Hanuman P, Kalmode HP, Murugan K, Chaskar AC (2015) RSC Adv 5:5580–5590
Kapil Kumar K, Mudshinge SR, Goyal S, Gangar M, Nair VA (2015) Tetrahedron Lett 56:1266–1271
Acknowledgements
The authors wish to thank the CSIR and industry sponsored project SSP-0670 for financial support. S.L thanks DST, govt of India for financial support (GAP-0566). The authors are grateful to the Director, CSIR-IICT for providing the necessary infrastructure. J.P is grateful to Dr. Dharmendra Kumar Tiwari for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pogula, J., Laha, S. & Likhar, P.R. Nano Copper(0)-Stabilized on Alumina: Efficient and Recyclable Heterogeneous Catalyst for Chemoselective Synthesis of 1,2-Disubstituted Benzimidazoles and Quinoxalines in Aqueous Medium. Catal Lett 147, 2724–2735 (2017). https://doi.org/10.1007/s10562-017-2166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2166-6