Abstract
Avian leukosis virus subgroup J (ALV-J) is the most prevalent subgroup in chickens and exhibits increased pathogenicity and stronger horizontal and vertical transmission ability among different breeds. Although vertical transmission of ALV-J from infected hens through artificial insemination has been inferred from the detection of the p27 antigen in swabs and serum, there has been no further research on the transmission pattern of ALVs in roosters. In the present study, the positive rate of ALV increased significantly in an indigenous flock after detecting the p27 antigen via enzyme-linked immunosorbent assay (ELISA) and virus isolation in DF-1 cells. Viral sequence comparisons and an indirect fluorescent antibody assay showed that these isolates belonged to the ALV-J subgroup but formed a new branch in a phylogenetic tree when compared to domestic and foreign referential strains. The gp85 gene of the ALV-J isolated from hens and albumen was 94.1–99.7% identical to that in roosters, revealing that these isolates were quite likely transmitted to the hens and their offspring through the semen of ALV-infected roosters by artificial insemination from the Hy-line brown roosters. In addition, we defined four ALV-J infection states in plasma and semen of roosters (P+S+, P-S+, P+S-, and P-S-), which suggests that, in order to eradicate ALV in roosters, it is necessary to perform virus isolation using both semen and plasma. Additionally, ALV detection in semen by ELISA produced false-positive and false-negative results when compared to virus isolation in DF-1 cells. Collectively, our results suggested that an incomplete process of eradication of ALV from ALV-positive roosters led to the sporadic presence of ALV-J in laying hens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian leukosis (AL), which includes both malignant and benign tumorigenic diseases caused by avian leukosis virus (ALV), has caused immense economic losses in the poultry industry worldwide [1, 2]. ALV is divided into 11 subgroups (designated A–K) based on cross-neutralization and variations in the gp85 glycoprotein, which is a group-specific antigen [3]. Avian leukosis virus subgroup J (ALV-J) is the most prevalent subgroup in chickens, and its members exhibit stronger pathogenicity and vertical transmission ability in poultry than those of the other subgroups [4,5,6]. ALV-J infection was a common problem in the poultry industry during 2003–2010, causing myelocytoma in broilers and hemangioma in layers [7,8,9,10,11]. Because of the widespread distribution of ALV-J strains and a lack of organized control measures for local-breed flocks, eradication of ALV in China remains a major challenge. Since 2018, an especially tumorigenic strain of ALV-J has emerged in imported broilers from China, causing severe osteomas of the keel and ribs, leading to massive mortality and morbidity and becoming a major concern for poultry health [3, 11]. Therefore, many domestic farms have started testing for ALV infection [3].
Rubin et al. defined four serological classes of susceptible birds: viremia, no antibody (V + A−); no viremia, with antibody (V − A+); viremia, with antibody (V + A+); and no viremia, no antibody (V − A−) [2, 12]. Another study showed that roosters spread ALV-J to hens by insemination, ultimately resulting in vertical transmission [13]. However, those researchers did not elaborate on the ALV infection status of semen from males or the role of males in the transmission of ALV. ALV virus was isolated using DF-1 cells, and p27 antigens in cloacal swabs or semen were detected directly by enzyme-linked immunosorbent assay (ELISA). However, the accuracy of the ELISA method for detecting the p27 antigen in cloacal swabs or semen has not been determined.
ALV-J can be detected in Hy-line brown roosters, suggesting that roosters are infected by ALV-J and may play a crucial role in viral transmission in poultry. Here, we determined that ALV-J-infected semen can infect hens through artificial insemination. Consequently, ALV-J was transmitted vertically to eggs, leading to an increase in the ALV infection rate on the farm. In addition, we also determined four different ALV-J infection states in roosters and evaluated the reliability of different methods for detecting ALV-J in semen samples. Taken together, our data provide a basis for detecting and eradicating ALV in poultry.
Materials and methods
Ethics statement
All chicken studies were performed according to the Institutional Guidelines of China Agricultural University (approval SKLAB-B-2010-003) and were approved by the Beijing Association for Science and Technology of China (approval SYXK, Beijing, 2007–0023).
Sample origin
Forty-six anticoagulant-containing blood samples were collected aseptically from the wing veins, and 46 semen samples were obtained from 46 30-week-old Hy-line roosters in Hebei Province, China, during November 2020. Ninety-two anticoagulant-containing blood samples from the wing vein and 92 albumen samples from breeding eggs were collected from the same 92 30-week-old indigenous hens on an experimental farm (Supplementary Table S1). The plasma was extracted from the blood samples as reported previously [9]. In addition, 10 albumen samples from breeding eggs of five ALV plasma-positive hens (2 eggs/hen) were collected for virus isolation.
Virus isolation and identification
Semen samples were diluted five times with PBS containing 2% penicillin and streptomycin. After gently mixing upside down, the supernatant was collected by centrifugation to precipitate the cells at 3,000 × g for 2 min. Albumen samples were diluted four times with DMEM in 5-ml syringes. The virus was isolated from the plasma, semen, and albumen samples by inoculating DF-1 cells as described previously, thus confirming ALV infection [9]. The ALV group-specific antigen p27 was detected in the culture supernatant by ELISA (IDEXX, Westbrook, ME, USA; #99–0925).
Indirect immunofluorescence assay (IFA)
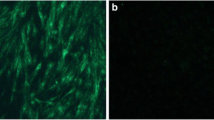
Cells were washed with PBS and fixed in a cold acetone–alcohol mixture (3:2) for 5 min. They were then incubated with the mouse anti-ALV-J monoclonal antibody JE9 at 37°C for 60 min, followed by incubation with goat anti-mouse IgG antibody conjugated with fluorescein isothiocyanate (Sigma, St. Louis, MO, USA) at 37°C for another 60 min. Finally, the cells were observed under a fluorescence microscope.
Primers and polymerase chain reaction (PCR) amplification
DNA was extracted from DF-1 cells according to the instructions of the manufacturer of the DNA Extraction Kit (Tiangen, China; #DP304-03). A pair of universal primers targeting env and LTR, yielding an amplicon of about 2,200 bp, was used to detect exogenous ALVs as reported previously (ALV-F, GATGAGGCGAGCCCTCTCTTTG; ALV-R, TGTGGTGGGAGGTAAAATGGCGT) [11]. The PCR conditions used with the ALV-F/R primers were 95°C for 5 min, 31 cycles of 95°C for 50 s, 55°C for 40 s, and 72°C for 140 s, followed by a final elongation step of 10 min at 72°C. The PCR product was analyzed by 0.8% agarose gel electrophoresis in Tris-acetate-EDTA buffer. The amplicons were cloned, and the positive clones were confirmed by Sanger sequencing. All isolates obtained from positive samples were sequenced. The GenBank accession numbers of the isolates from this study are MW476816-MW476829 (Supplementary Table S2).
Sequence analysis
The sequences were aligned with other ALV-J reference sequences retrieved from the National Center for Biotechnology Information database (Supplementary Table S3). Phylogenetic analysis was performed by the neighbor-joining method with 1,000 bootstrap replicates, using MEGA ver. 6.0 [14].
Results
Increase in the ALV-positive rate of the flock through introduction of ALV-J-infected Hy-line brown roosters
Before introducing Hy-line brown roosters, the positive rate of virus isolation and p27 in albumen for the flock was less than 1% for two consecutive generations, and peak egg production was about 70%. However, after introducing the roosters, the positive rate of virus isolation in plasma and p27 in albumen increased to 5.4% (5/92) and 7.6% (7/92), respectively (Supplementary Table S1). Peak egg production dropped to about 65% without any other clinical signs. Ten albumen samples from five hens with positive plasma (two eggs per hen) were collected for virus isolation, and only one albumen sample was positive. These results indicate that the introduction of Hy-line brown roosters infected with ALV-J increased the positive rate of ALV in the flock.
ALV infection status in semen and plasma from roosters
Out of 46 roosters, 14 tested positive for ALV in their plasma or semen (30.43%). Of those, 12 were positive in plasma (26.09%), eight were positive in semen (17.39%), and six were positive in plasma and semen (Table 1). According to the virus isolation results from semen and plasma of the 46 roosters, there were four different ALV infection states. Six roosters were viremic, with positive semen (P + S+, 13.04%); six were viremic, with negative semen (P + S-, 13.04%); two showed no viremia, but positive semen (P-S+, 4.35%), and the remaining 32 roosters were double-negative (P-S-, 69.57%) (Table 2).
High proportion of false-positives in semen tested directly by ELISA
In addition to virus isolation, the virus was detected directly in the semen by ELISA. The results showed that 11 semen samples were positive, but only six of those semen samples were from roosters whose plasma or semen samples were positive by virus isolation (Supplementary Table S1). These data suggest that ELISA method is not reliable for detection of ALV in semen, possibly due to nonspecific binding of unknown components of the semen to the p27 antigen.
Identification and sequence comparisons of ALV-J isolates from roosters, hens, and albumen
Fourteen positive samples from roosters, hens, and albumen were subjected to virus isolation and sequence analysis. Phylogenetic analysis based on the gp85 gene suggested that all of the isolates belonged to the ALV-J subgroup, but they formed a new branch within subgroup J, and their sequences were 49.6–94.8% identical to domestic and foreign reference sequences (Fig. 1). The sequence differences were mainly concentrated in the hr1, hr2, and vr2 regions. In addition, IFA was performed, and the results were consistent with the phylogenetic analysis, as clear green fluorescence was observed using monoclonal antibody JE9 (Fig. 2).
Phylogenetic analysis based on gp85 gene sequences of the isolates and referential strains. The phylogenetic tree was generated using MEGA5.1 software (bootstrap method with 1,000 replicates). ALV-A to E, K, and J are divided and indicated at the right. The source of each ALV-J reference strain is shown in parentheses. A green diamond indicates that the strain was isolated from rooster semen. A red triangle indicates that the strain was isolated from rooster plasma. A blue square indicates that the strain was isolated from hen plasma and albumen. The sources of the reference sequences and isolates are indicated. Abbreviations: USA, United States; UK, United Kingdom; CHN, China; RUS, Russia; EGY, Egypt; NGA, Nigeria
Comparison of the gp85 nucleotide sequences in semen and plasma samples from the same rooster showed that they were > 98.6% identical in five of the six roosters, but those of the remaining rooster were only 96% identical to each other and 94.1% identical to those of the other five (Fig. 3). The virus isolates from the roosters, hens, and albumen samples showed 94.1–100% sequence identity in the gp85 gene. The sequence identity between the isolates from albumen and plasma from the same hen was 99.3%, and it was 98.7–99.8% between the isolates in the above five semen and plasma samples from the hen, suggesting that the virus was likely transmitted to the hens and their offspring through insemination by the roosters.
Discussion
Since the first strain of ALV-J, HPRS-103, was isolated from meat-type breeder chickens in the UK in 1988, it has rapidly spread around the world, causing severe economic losses in the poultry industry [4, 15]. As the most pathogenic ALV subgroup, ALV-J induces immunosuppression and malignant or benign tumorigenic diseases in chickens, including hemangiomas, myelomas, and fibrosarcomas [2, 16,17,18]. An outbreak of hemangiomas associated with ALV-J was reported in commercial layer chickens between 2006 and 2010 in China [7, 19]. However, the virus has been successfully eradicated at the pedigree and multiplier levels by implementing an ALV purification project by a few major breeding companies. Therefore, there have been few reports of ALV-J infections in layers in recent years. The outbreak of ALV-J in broiler breeders during 2018–2019 was caused by introduction of broiler breeders from overseas [20]. Sun et al. discovered that coinfection with avian hepatitis E virus and avian leukosis virus subgroup J was the cause of an outbreak of hepatitis and liver hemorrhagic syndrome in a Hy-line brown layer chicken flock in China in 2017 [21]. In this study, we found subclinical infection of ALV-J in the roosters of Hy-line layers, indicating the importance of continued monitoring of roosters for ALV using the random sampling method.
Different methods have been used to detect the virus in various rooster and hen samples from the same flock. ELISA is used to detect viruses directly in semen, but in this study, only 50% of the ELISA results were consistent with the virus isolation results. Virus isolation is the only reliable method of confirming an ALV-J infection, whereas ELISA can produce both false-positive and false-negative results. At the same time, different infection states were identified in semen and plasma samples from the same rooster, namely, viremia but semen positive (V + S+, 13.04%), no viremia, but semen positive (V-S+, 4.35%), viremia, but semen negative (V + S-, 13.04%), and double-negative (V-S-, 69.56%). The positive rate of virus isolation from plasma was higher than that from semen, suggesting that both semen and plasma samples should be tested for the virus when attempting to eliminate ALV from roosters.
In experiments with specific-pathogen-free chickens, Li reported that ALV-J can be transmitted in semen to hens by insemination [13], but this resulted in ALV-J antibody production without viremia in the hens and their offspring. This can be explained by the low virulence of the ALV-J strain used in this experiment. The current study was based on infected clinical flocks. ALV-J was isolated from hens and breeding eggs. Although the positive rate of virus isolation in the albumen samples was low, one ALV-J strain was detected and sequenced. The isolates from albumen and the plasma from the same hen showed 99.3% sequence identity to each other and 98.7–99.8% identity to isolates from five semen samples and to isolates from plasma samples from the hen, demonstrating that they may have been derived from the same source. Thus, ALV-J from roosters can be transmitted to hens and their offspring through insemination.
Previous studies have demonstrated that ALV-J displays a high level of genetic variation and recombination [22, 23], which allows for the development of new variants with changes in antigenicity, tissue tropism, host range, and pathopoiesis [24]. Gao et al. reported that ALV-J is subjected to greater selection pressure in the ovarian follicles of hens, which promotes the evolution of the virus [25]. In this study, the ALV-J in the semen of roosters had only 96% sequence identity to that in the plasma, indicating that the rooster semen may have been under heavy selection pressure that promoted the evolution of ALV-J, which is why the isolates were located in a new branch in the phylogenetic tree when compared with the reference strains.
In summary, we isolated ALV-J from Hy-line brown roosters and determined the complete chain of transmission of ALV-J from roosters to hens through insemination and then to the offspring by vertical transmission. The use of ELISA to detect the ALV in semen did not provide reliable results. There were four ALV-J infection states in the plasma and semen of roosters, and it is therefore necessary to perform virus isolation from both semen and plasma. We speculate that the reason why sporadic cases of ALV-J infection in laying hens still occurs is incomplete elimination of the virus from roosters.
References
Weiss RA, Vogt PK (2011) 100 years of Rous sarcoma virus. J Exp Med 208(12):2351–2355
Payne LN, Nair V (2012) The long view: 40 years of avian leukosis research. Avian Pathol 41(1):11–19
Dong X, Zhao P, Xu B, Fan J, Meng F, Sun P, Ju SD, Li Y, Chang S, Shi WF, Cui Z (2015) Avian leukosis virus in indigenous chicken breeds. China Emerg Microbes Infect 4(12):e76
Payne LN, Howes K, Gillespie AM, Smith LM (1992) Host range of Rous sarcoma virus pseudotype RSV(HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup. designated J J Gen Virol 73(Pt 11):2995–2997
Venugopal K, Howes K, Flannery DM, Payne LN (2000) Isolation of acutely transforming subgroup J avian leukosis viruses that induce erythroblastosis and myelocytomatosis. Avian Pathol 29(4):327–332
Li XJ, Lin WC, Chang S, Zhao P, Zhang XH, Liu Y, Chen WG, Li BH, Shu DM, Zhang HM, Chen F, Xie Q (2016) Isolation, identification and evolution analysis of a novel subgroup of avian leukosis virus isolated from a local Chinese yellow broiler in South China. Arch Virol 161(10):2717–2725
Lai HZ, Zhang HN, Ning ZY, Chen RL, Zhang WY, Qing AJ, Xin CA, Yu KZ, Cao WS, Liao M (2011) Isolation and characterization of emerging subgroup J avian leukosis virus associated with hemangioma in egg-type chickens. Vet Microbiol 151(3–4):275–283
Pan W, Gao YL, Qin LT, Ni W, Liu ZS, Yun BL, Wang YQ, Qi XL, Gao HL, Wang X (2012) Genetic diversity and phylogenetic analysis of glycoprotein GP85 of ALV-J isolates from Mainland China between 1999 and 2010: coexistence of two extremely different subgroups in layers. Vet Microbiol 156(1–2):205–212
Li Y, Liu XM, Liu HX, Xu CG, Liao YL, Wu XC, Cao WS, Liao M (2013) Isolation, identification, and phylogenetic analysis of two avian leukosis virus subgroup J strains associated with hemangioma and myeloid leukosis. Vet Microbiol 166(3–4):356–364
Cui Z, Du Y, Zhang Z, Silva RF (2003) Comparison of Chinese field strains of avian leukosis subgroup J viruses with prototype strain HPRS-103 and United States strains. Avian Dis 47(4):1321–1330
Li J, Meng F, Li W, Wang Y, Chang S, Zhao P, Cui Z (2018) Characterization of avian leukosis virus subgroup J isolated between 1999 and 2013 in China. Poult Sci 97(10):3532–3539
Rubin H, Fanshier L, Cornelius A, Hughes WF (1962) Tolerance and immunity in chickens after congenital and contact infection with an avian leukosis virus. Virology 17:143–156
Li Y, Cui S, Li W, Wang Y, Cui Z, Zhao P, Chang S (2017) Vertical transmission of avian leukosis virus subgroup J (ALV-J) from hens infected through artificial insemination with ALV-J infected semen. BMC Vet Res 13(1):204
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Zhang YW, Su Q, Zhang ZH, Cui ZZ, Chang S, Zhao P (2020) Molecular characteristics of the re-emerged avian leukosis virus in China, 2018–2019. Transbound Emerg Dis 67(3):1141–1151
Sun Y, Du T, Liu B, Syed SF, Chen Y, Li H, Zhao Q (2016) Seroprevalence of avian hepatitis E virus and avian leucosis virus subgroup J in chicken flocks with hepatitis syndrome. China BMC Vet Res 12(1):261
Wang YX, Li J, Li Y, Fang LC, Sun XL, Chang S, Zhao P, Cui ZZ (2016b) Identification of avian leukosis virus subgroup J-associated acutely transforming viruses carrying the v-src oncogene in layer chickens. J Gen Virol 97(5):1240–1248
Wang YX, Li J, Li Y, Fang LC, Sun XL, Chang S, Zhao P, Cui ZZ (2016a) Identification of ALV-J associated acutely transforming virus Fu-J carrying complete v-fps oncogene. Virus Genes 52(3):365–371
Cheng Z, Liu J, Cui Z, Zhang L (2010) Tumors associated with avian leukosis virus subgroup J in layer hens during 2007 to 2009 in China. J Vet Med Sci 72(8):1027–1033
Zhang Y, Su Q, Zhang Z, Cui Z, Chang S, Zhao P (2020) Molecular characteristics of the re-emerged avian leukosis virus in China, 2018–2019. Transbound Emerg Dis 67(3):1141–1151
Sun Y, Lu QZ, Zhang JF, Li XX, Zhao JK, Fan WQ, Ji PP, Wang K, Zhou EM, Zhao Q (2020) Co-infection with avian hepatitis E virus and avian leukosis virus subgroup J as the cause of an outbreak of hepatitis and liver hemorrhagic syndromes in a brown layer chicken flock in. China Poult Sci 99(3):1287–1296
Meng F, Dong X, Hu T, Chang S, Fan J, Zhao P, Cui Z (2016) A deep sequencing reveals significant diversity among dominant variants and evolutionary dynamics of avian leukosis viruses in two infectious ecosystems. BMC Vet Res 12(1):287
Dong X, Meng F, Hu T, Ju SD, Li Y, Sun P, Wang YX, Chen WQ, Zhang FS, Su HQ, Li SF, Cui H, Chen JX, Xu SZ, Fang LC, Luan HB, Zhang ZJ, Chang S, Li JL, Wang L, Zhao P, Shi WF, Cui Z (2017) Dynamic co-evolution and interaction of avian leukosis virus genetic variants and host immune responses. Front Microbiol 8:1168
Lupiani B, Hunt H, Silva R, Fadly A (2000) Identification and characterization of recombinant subgroup J avian leukosis viruses (ALV) expressing subgroup A ALV envelope. Virology 276(1):37–43
Gao X, Li Q, Fang L, Song H, Cui Z, Meng F, Zhang Z (2020) The follicle promotes the evolution of variants of avian leukosis virus subgroup J in vertical transmission. Biochem Biophys Res Commun 521:1089–1094
Funding
This work was supported by the National Natural Science Foundation Youth Fund (31802201).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This article does not include experiments with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Hugo Soudeyns.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
About this article
Cite this article
Meng, F., Li, Q., Han, R. et al. A study on the infection status and transmission of avian leukosis virus subgroup J in Hy-line brown roosters. Arch Virol 167, 1521–1527 (2022). https://doi.org/10.1007/s00705-022-05452-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-022-05452-4