Abstract
It is well known that Parkinson’s disease is characterized by a variety of non-motor symptoms. A gustatory deficit is hypothesized to be one of them although few and only cross-sectional studies are available. The aim of our pilot study was to prospectively investigate the taste function in Parkinson’s disease patients after some years from the first evaluation (mean follow-up 4.35 ± 0.49 years; time range 3.5–5.6 years). A group of 26 patients was re-examined (16 males and 10 females; mean age 70.9 ± 8.4 years, range 54–88 years). Taste function was assessed in one session, by means of the Whole Mouth Test (WMT) and Taste Strips Test (TST). Olfaction was also evaluated with the Sniffin’ Sticks Identification Test (SST). All these tests are commercially available (Burghart Company, Germany). All patients were able to understand and complete the procedure. Although scores decreased over time, no significant difference was found between global taste scores of first and second evaluation, neither comparing every single taste quality (WMT: p = 0.234, Mann–Whitney U test; TST: p = 0.747, Mann–Whitney U test; McNemar chi-square in the range of 0–1.455). These results confirm a persistent but slight and stable taste impairment, in patients with Parkinson’s disease. Future studies on a much larger sample of patients are certainly required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among various non-motor symptoms typical of Parkinson’s disease (PD), a chemosensory olfactory impairment is notoriously one of the main signs of disease since early stages (Haehner et al. 2014). Smell and taste disorders are common in the general population, and the majority of patients reporting an alteration of smell function have some degree of taste dysfunction as well (Deems et al. 1991). As reported in the recent review of the literature (Cecchini et al. 2015), there are few cross-sectional studies regarding taste perception in PD (Sienkiewicz-Jarosz et al. 2005; Lang et al. 2006; Shah et al. 2009; Deeb et al. 2010; Kim et al. 2011; Cecchini et al. 2014). Despite the different gustatory tests adopted by the research groups, it is generally reported that also taste could be affected in PD. Moberg et al. (2007) investigated in particular phenylthiocarbamide perception in PD and they found a higher proportion of PD non-tasters in comparison with healthy controls. In addition, in contrast with smell dysfunction, taste impairment has been less frequently reported on clinical interviews of PD patients (Kashihara et al. 2011). Another recent study also found that taste function in PD is not influenced by dopaminergic processes and that its dysfunction seems to rely on the specific tongue regions assessed (Doty et al. 2015). To date, no study has prospectively evaluated the progression of taste impairment. The purpose of this pilot work was, therefore, to re-evaluate the gustatory function of a subgroup of the same PD patients analyzed in our previous study (Cecchini et al. 2014). To this aim, the taste of 26 PD patients was evaluated by means of liquid solutions and dried filter paper strips, in the whole oral cavity. In addition, to keep the same approach adopted in our previous work, we also assessed olfaction.
Patients and methods
The entire cohort of PD patients enrolled in our previous study was considered after a mean follow-up of 4.35 ± 0.49 years (range 3.5–5.6 years). All patients were followed at the Neurology Unit of Verona Hospitals, Italy, and were re-evaluated by an expert neurologist and selected for the present study. They were all able to understand and complete the procedure and no cognitive decline was found (defined as MMSE <24 and impact on activity of daily life). Among the 61 PD outpatients initially evaluated (PD1-total cohort), it was possible to enroll 26 subjects for a second evaluation (PD2: 16 men, 10 women; mean age 70.9 ± 8.4 years, range 54–88 years). The recruited patients were ranked within stages I–III according to the Modified Hoehn and Yahr scale (H&Y) (Goetz et al. 2004). The demographic and clinical features of PD patients together with the taste test scores are summarized in Table 1. A total of 35 patients could not be re-evaluated due to the following reasons: 13 patients developed other comorbidities potentially interfering with chemosensory performance or it was impossible to contact them; nine patients developed dementia; seven patients refused to collaborate; three patients deceased; two patients moved away; one patient suffered an important head trauma. All patients were under treatment with levodopa or other medications for PD. All investigations were performed in accordance with the Helsinki Declaration and with the ethical approval of the local IRB, and patients’ informed consent was obtained.
In keeping with our previous work, prior to olfactory testing all participants were asked to rate their olfactory and taste functions and the answer (very good, good, normal, less than normal, very less than normal) was registered. Thereafter, olfaction and taste performances were evaluated in a well-ventilated and quiet room. The whole session lasted 30–40 min per patient and comprised a set of validated tests based on a forced-choice paradigm. Smell was investigated with the Sniffin’ Sticks Identification test (SST, 16-stick version, Burghart Company, Germany). It consists of 16 pen-like odor dispensing devices containing different common odors that have to be named reading a paper list of four response options for every odor (Hummel et al. 2007). The score range is 0–16 points.
Taste was assessed with the Whole Mouth Test (WMT, Burghart Company, Germany) and the Taste Strips Test (TST, Burghart Company, Germany) in the whole oral cavity (Hummel et al. 2007; Landis et al. 2009). The WMT is a rapid screening taste test that uses four spray taste solutions at suprathreshold concentration in 10 g of water (sucrose 10% for sweet, citric acid 5% for sour, sodium chloride 7.5% for salty, quinine 0.05% for bitter). Each solution has to be sprayed two or three times into the oral cavity. TST is a validated and detailed taste test for determination of gustatory sensitivity. It uses spoon-shaped filter papers impregnated with four concentrations of the four basic tastes (sweet, sour, salty, bitter). Strips are placed on the tongue and the patient is asked to move it around with the mouth closed. The concentrations of reagents used for the taste strips are detailed in the Table (supplementary). For both TST and WMT, each correct answer was rated as one point and no feedback was given to the patients. The score range for WMT is 0–4 points while for TST is 0–16 points. The higher the score, the better the subject’s identification performance. Before each paper strip administration, the mouth was rinsed with water. Then, a global gustatory sensitivity was evaluated, giving the patient the possibility to move the tongue for each paper strip. Each correct answer was rated one point. The maximum score for the whole test was globally 16 with 4 maximum points for each taste quality.
Statistical analysis
Statistical analysis was performed by routines written in Matlab 7.1, by means of Mann–Whitney U test and Spearman rank correlation test. To evaluate the taste deficit trend across time, McNemar test and ANOVA were performed. Moreover, two-way ANOVA analysis of variance with the factors “status” (PD1 and PD2 evaluation) and gender (female or male) was carried out to unravel gender-related effects. The significance level was set at p ≤ 0.050. Unless otherwise defined, data are given as mean ± standard deviation (SD).
Results
Smell
Concerning the SST score result across time, no significant differences between PD1 and PD2 were found (p = 0.450, Mann–Whitney U test). Self-rating assessment of smell performance correlates with the objective evaluation (ρ = 0.400; p = 0.043, Spearman Correlation test).
Taste
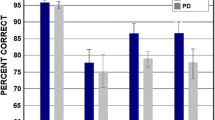
WMT test showed no significant over time differences (p = 0.234, Mann–Whitney U test), as 92.3% of the PD1 and 80.8% of PD2 participants were able to recognize the 4 suprathreshold taste solutions. Concerning the TST score result across time, no significant differences between PD1 and PD2 were found (p = 0.747, Mann–Whitney U test). The percentage of correct and incorrect answers, for every taste quality, and a chromatic representation of them are reported in Table 2 and Fig. 1, respectively. In addition, no significant between-gender difference was found (p = 0.233). Likewise, we found no significant difference between PD1 and PD2 when analyzing the different taste qualities separately (TST sweet: p = 0.394; sour: p = 0.879; salty: p = 0.314; bitter: p = 0.640 by ANOVA test).
The radar chart depicts the total percentage of correct answers for each taste quality and concentration tested using the Taste Strips Test (TST) during the first and second evaluation (Panel a: PD1, Panel b: PD2). Each quadrant represents one tastant (left top sour, right top sweet, right bottom bitter, left bottom salty) where the four concentrations are showed in four concentrical areas. The smallest and central area corresponds to the strip 1 (lowest concentration) while the largest and outlying represents the strip 4 (highest concentration). The bar shows a chromatic representation of the TST percentage of correct answers in the total pool of 26 patients
The correlation between concentrations and the ability to correctly perceive taste was also statistically analyzed in PD1 and PD2 by means of the Spearman correlation test, showing that the ability to correctly perceive taste generally improved as the concentrations increase (Table 3, panel A); considering each taste quality, salty taste showed the minor value of correlation for both PD1 and PD2.
Regarding the strip concentration for each taste quality, and the percentage of correct answers per strip, no significant difference was found between PD1 and PD2 (McNemar test) (Table 3 panel B).
With respect to the self-rating assessment of the PD2 taste performance, we found no significant correlation with TST results (ρ = 0.371; p = 0.063).
Discussion
In the present study, we investigated the taste performance in a group of 26 patients with PD by means of the WMT and the TST after a time range of 3.5–5.6 years from the first evaluation. To the best of our knowledge, this is the first prospective study addressing the taste perception in well-characterized population of PD patients. The second assessment of the taste performance did not show significantly different results in comparison with the first one. These data suggest that PD patients, over time, can recognize efficiently all the four evaluated tastes with spray solutions (WMT), while by means of TST test (that is based on different concentrations) the taste impairment is confirmed, even if the global score remains within the normal range. This feature is opposite to what we see in olfactory scores, where also suprathreshold evaluation gives results strongly impaired. The olfactory deficit is clearly perceived by the patients, as shown by the significant correlation between the smell self-rating assessment and the objective evaluation. On the other hand, PD patients did not notice any change in taste perception in their everyday life and this notion can be also supported by the result of the absence of correlation between the taste self-rating assessment and the objective evaluation, as reported previously (Cecchini et al. 2014). This is probably due to a too modest impairment to be subjectively perceived thus meaning that it is only detected by more sensitive tests. This supports the notion that it is always important to test both smell and taste function with validated methods to obtain an accurate evaluation of the chemosensory status of the patient.
Our study also found that sweet perception was the most conserved among all the taste qualities in both evaluations, with a percentage of correct answers over 60% even at the lowest concentration strip. This is not surprising since the sweet perception is phylogenetically related with the recognition of high-caloric food source, having an evolutionary role for energy intake as well as for the hedonic reward system. On the other hand, this might also account for the propensity for sweet food in PD patients for more advanced disease (Sienkiewicz-Jarosz et al. 2013).
Moreover, from our results it is possible to see that sour and salty taste were mostly confused confirming data reported by previous works on healthy subjects (Landis et al. 2009; Ahne et al. 2000; Mueller et al. 2003). In fact, it is reported that these two taste qualities are very similar in terms of produced sensation (irritation, tingling, stinging) and in the daily life they are often assembled in food, favoring the possible confusion between them (Mueller et al. 2003; Gilmore and Green. 1993). In fact, for sour perception, at all concentrations, there is the minor percentage of correct answers both for PD1 and PD2. Nevertheless, it is important to mention that the lowest concentration of each taste quality is not correctly identified by roughly half of the healthy subjects as reported by Mueller and collaborators (2003).
Our findings confirm that gustatory function, even when compromised, is preserved in its more basic physiology. We believe that it is still too early to draw definitive conclusions about the clinical importance of the taste deficit generally reported by PD studies. In fact, even if a chemosensory interaction with olfaction could be also considered (Landis et al. 2010), the decreased taste function over time seems to be quite independent from olfaction (marginal correlation between olfactory and taste performance) particularly because olfaction impairment does not progress any further. In fact, olfaction is deeply and precociously impaired in PD, so making easier to consider a disease-trait rather than a state-trait. By contrast, taste impairment does not show any ceiling effect and might be a candidate to be potentially seen as a state-trait. This could be in support of the hypothesis reported by various studies that taste dysfunction can be linked to the advanced phases of the disease associated with cortical involvement. Indeed, according to Braak’s staging theory, the first- and second-order gustatory neurons are spared in PD (Braak et al. 2002). Hence, it could be hypothesized that the further spreading of neurodegeneration would be followed by taste impairment even at suprathreshold concentrations, thus meaning that taste will be deeply impaired probably only in advanced stages. On the other hand, this represents a research limit, because in advanced disease with cortical involvement it is not possible to assess taste with common validated taste tests, since patient’s collaboration is requested.
In addition, the results of our study have the limit that obtained on a relatively small sample of patients, since more than half of patients were excluded due to different clinical reasons. In conclusion, these results have to be considered a first step toward a new type of studies, which will include longitudinal assessment of taste function in PD and other neurodegenerative diseases. It would certainly be instrumental for future research to plan multicenter studies to obtain a larger pool of patients and finally unravel the taste function trend in PD patients over time.
References
Ahne G, Erras A, Hummel T, Kobal G (2000) Assessment of gustatory function by means of tasting tablets. Laryngoscope 110:1396–1401
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249:1–5
Cecchini MP, Osculati F, Ottaviani S, Boschi F, Fasano A, Tinazzi M (2014) Taste performance in Parkinson’s disease. J Neural Transm 121:119–122
Cecchini MP, Fasano A, Boschi F, Osculati F, Tinazzi M (2015) Taste in Parkinson’s disease. J Neurol 262:806–813
Deeb J, Shah M, Muhammed N et al (2010) A basic smell test is a sensitive as dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJMed 103:941–952
Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB Jr (1991) Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg 117:519–528
Doty RL, Nsoesie MT, Chung I, Osman A, Pawasarat I, Caulfield J, Hurtig H, Silas J, Dubroff J, Duda JE, Ying JS, Tekeli H, Leon-Sarmiento FE (2015) Taste function in early stage treated and untreated Parkinson’s disease. J Neurol 262:547–557
Gilmore MM, Green BG (1993) Sensory irritation and taste produced by NaCl and citric acid: effects of capsaicin desensitization. Chem Senses 18:257–272
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway R, Moore CG, Wenning GK, Yahr MD, Seidl L (2004) Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
Haehner A, Hummel T, Reichmann H (2014) A clinical approach towards smell loss in Parkinson’s disease. J Parkinsons Dis 4:189–195
Hummel T, Kobal G, Gudziol H, Mackay-Sim A (2007) Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3000 subjects. Eur Arch Otorhinolaryngol 264:237–243
Kashihara K, Hanaoka A, Imamura T (2011) Frequency and characteristics of taste impairment in patients with Parkinson’s disease: results of a clinical interview. Intern Med 50:2311–2315
Kim H-J, Jeon BS, Lee J-Y et al (2011) Taste function in patients with Parkinson’s disease. J Neurol 258:1076–1079
Landis BN, Welge-Luessen A, Brämerson A, Bende M, Mueller CA, Nordin S, Hummel T (2009) “Taste Strips”—a rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J Neurol 256:242–248
Landis BN, Scheibe M, Weber C, Berger R, Brämerson A, Bende M, Nordin S, Hummel T (2010) Chemosensory interaction: acquired olfactory impairment is associated with decreased taste function. J Neurol 257:1303–1308
Lang CJG, Leuschner T, Ulrich K et al (2006) Taste in dementing diseases and parkinsonism. J Neurol Sci 248:177–184
Moberg PJ, Balderston CC, Rick JH, Roalf DR, Weintraub D, Kleiner-Fisman G, Stern MB, Duda JE (2007) Phenylthiocarbamide (PTC) perception in Parkinson disease. Cogn Behav Neurol 20:145–148
Mueller C, Kallert S, Renner B, Stiassny K, Temmel AF, Hummel T, Kobal G (2003) Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 41:2–6
Shah M, Deeb J, Fernando M et al (2009) Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 15:232–237
Sienkiewicz-Jarosz H, Scinska A, Kuran W et al (2005) Taste responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 76:40–46
Sienkiewicz-Jarosz H, Scinska A, Swiecicki L, Lipczynska-Lojkowska W, Kuran W, Ryglewicz D, Kolaczkowski M, Samochowiec J, Bienkowski P (2013) Sweet liking in patients with Parkinson’s disease. J Neurol Sci 329:17–22
Acknowledgements
We gratefully acknowledge the participants in this trial, as well as their family members and caregivers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ricatti, M.J., Ottaviani, S., Boschi, F. et al. A prospective evaluation of taste in Parkinson’s disease. J Neural Transm 124, 347–352 (2017). https://doi.org/10.1007/s00702-016-1638-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1638-y