Abstract
Background
Moyamoya disease, a progressive occlusive arteriopathy mainly affecting the supraclinoid internal carotid artery, leads to abnormal “Moyamoya vessels” and ischemic events in children due to decreased cerebral blood flow. Surgery, especially indirect revascularization, is suggested for pediatric Moyamoya cases.
Method
We present the Encephalo-Duro-Mio-Synangiosis (EDMS) technique, illustrated with figures and videos, based on 14 years’ experience performing 71 surgeries by the senior author (SGJ) and the Moyamoya Interdisciplinary Workteam at “Prof. Dr. J. P. Garrahan” Pediatric Hospital.
Conclusion
EDMS is a simple and effective treatment for Moyamoya disease, enhancing procedure precision and safety, reducing associated risks, complications, and improving clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Relevant surgical anatomy
The Encephalo-Duro-Mio-Synangiosis (EDMS) is a surgical technique that utilizes tissues irrigated by the External Carotid Artery (ECA) for generating brain collateral blood supply.
-
Superficial Temporal Artery (STA). Located approximately 2 cm anterior to the external auditory canal, the STA bifurcates into frontal and parietal branches. It is crucial to preserve it for maintaining an adequate blood supply for the surgical skin flap.
-
The temporal muscle occupies the temporal fossa and extends beneath the zygomatic arch to the coronoid process. It is innervated by the deep temporal nerve and vascularized mainly by the anterior and posterior deep temporal arteries, branches of the internal maxillary artery (IMA). Preserving these vessels within the deep fascia is essential for the muscle’s postoperative functionality.
-
Middle Meningeal Artery (MMA). An ascending branch of the IMA, it penetrates the skull base through the foramen spinosum and divides into anterior, medial (temporal), and posterior branches. These branches irrigate the external periosteal layer of the dura mater, a layer that has angiogenic activity. Therefore, the dural inversion, a technique in which dural flaps centered around the MMA are inverted, promotes the development of brain collateral blood supply [1].
Accurate understanding and preservation of these anatomical structures are key to the success of the EDMS technique in the treatment of Moyamoya disease in children [9].
Description of the technique
Preoperative Preparation:
-
Preoperative administration of Midazolam and Ketamine is conducted to mitigate hyperventilation, especially associated with crying, prior to entering the operating room (hyperventilation cause cerebral vasoconstriction, and therefore brain ischemia).
-
Cefuroxime is administered 30 min before incision.
Anesthesia and Monitoring:
-
General anesthesia is performed with sevoflurane, propofol, remifentanil and dexmedetomidine, following by orotracheal intubation, aiming to maintain normocapnia (36 to 42 mmHg) and avoiding cerebral vasoconstriction.
-
Stable hemodynamics (normal blood pressure, cerebral perfusion pressure, normocapnia, normothermia, and oxygenation).
-
Body temperature is maintained above 35 °C to balance cerebral blood flow and oxygen consumption.
-
Depth of anesthesia is monitored using bispectral index (BIS) and near infrared spectroscopy (NIRS).
Patient Positioning:
-
The patient is positioned supine with a gel pad under the ipsilateral shoulder for minimal neck twisting and head rotation of about 90° from the midline.
-
A soft horseshoe headrest secures the patient’s head, using fixation tape or a three-pin holder based on age and cranial bone thickness (Fig. 1a).
Surgical Procedure:
-
STA and its branches are located via palpation or ultrasound, marking a curvilinear incision behind the hairline without damaging the STA.
-
Skin preparation and draping are performed following standard protocols. The incision line is then anesthetized using 0.5% lidocaine without vasoconstrictive agents (Fig. 1b).
-
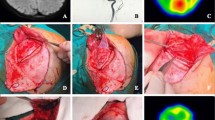
The scalp is incised below the galea aponeurotica, and hemostasis is achieved through bipolar coagulation and hemostatic forceps. The avascular plane between the galea and periosteum is carefully identified and separated with sharp dissection, leaving a thick periosteum-fascia muscular flap (Fig. 2a).
Surgical Procedure. A: Identification and separation of the avascular plane between the galea and periosteum. B: Retrograde dissection technique of the temporal muscle. C and D: Bone Exposure and burr holes. E: Square craniotomy of approximately 7 cm x 7 cm. F: Expansion of temporal squama using high-speed drill and gouge, removing a horizontal section of 1.5 cm
-
The temporal muscle is dissected from the cranium subperiosteally using a retrograde dissection technique to preserve its blood supply and prevent muscular atrophy (Fig. 2b).
-
Preserving the MMA is crucial during craniotomy. Given the variability in the course of the MMA, it is essential to identify its trajectory preoperatively using digital angiography and to remain vigilant during surgery. In some cases, a segment of the MMA may run into the great sphenoid wing before dividing over the dura. To prevent inadvertent injury to the MMA during craniotomy, careful planning and meticulous execution are essential. Four burr holes are strategically placed, as planned according to the patient’s anatomy: the first retrocoronal over the superior temporal line, the second approximately 6–7 cm posterior to it on the same line, covering the motor area. The third and fourth holes are positioned over the temporal squama, at the same level as the first two, creating a square craniotomy (Fig. 2c-e).
-
The temporal squama is meticulously expanded using a high-speed drill and bone rongeur, removing a horizontal portion of approximately 1.5 cm, dissecting previously to avoid rupture of the MMA if it is found intraosseously in the wing segment of the sphenoid. This procedure, combined with the beveling of the inner edge of the lower border of the bone flap, helps alleviate pressure on the temporal muscle utilized in encephalomyosynangiosis (Fig. 2f).
-
The dural opening is tailored based on the MMA’s course, which is assessed through preoperative digital angiography and observed during surgery. The approach is individualized for each patient due to anatomical variations in the MMA’s course, often intersecting the central portion of the intended opening. Depending on the circumstances, one or several dural openings are created, ensuring preservation of the major branches of the MMA (Figs. 3 and 4).
-
To prevent twisting and obstructions during dural inversion, incisions are stopped 2 mm before reaching the MMA. This inversion technique allows contact between the vascular-rich periosteal dura mater and the cortical surface, promoting cortical neovascularization through the MMA (Fig. 3e, f) [3].
-
The arachnoid opening over the brain sulci is performed using a No. 11 scalpel and microscissors under microsurgical magnification, starting at the most cephalic site and avoiding areas with dense pial vasculature (Fig. 3c, d).
-
This procedure exposes the blood vessels on the brain’s surface and the superficially vascularized tissues to the growth factors in the cerebrospinal fluid (CSF). In case of bleeding, it’s important to minimize coagulation as much as possible.
-
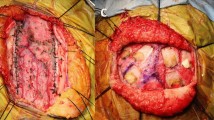
The temporal muscle and periosteum are placed over the exposed cortical surface, suturing them to the dural edges with Silk 4.0 sutures in an watertight manner (Fig. 5a-c).
Surgical Procedure. A, B, and C: Temporal muscle and periosteum repositioned over exposed cortical surface, sutured to dural edges with Silk 4.0 in watertight fashion, efficacy confirmed by Valsalva maneuvers. D: Beveling of the inferior bone edge. E: Horizontal space of the temporal squama previously resected to ensure there is no compression of the temporal muscle. F: Bone flap repositioned and secured with non-absorbable Ethibond 2.0 sutures
-
Dural anchoring points are performed, and hemostasis is achieved with Surgicel and fibrin glue.
-
The bone flap is meticulously repositioned, ensuring there is no compression of the temporal muscle (Fig. 5d, e). It is then secured using either titanium microplates or non-absorbable Ethibond 2.0 sutures (Fig. 5f).
-
Scalp closure involves suturing the galea with absorbable Vicryl 3.0, followed by the closure of the skin with Monocryl 3.0.
-
A sterile dressing with gauze is applied, followed by the placement of a non-compressive cephalic bandage.
-
After awakening, hyperventilation, especially related to crying, is minimized using dexmedetomidine.
-
Intensive care unit with monitoring is mandatory for the pos-operative period.
Indications
Revascularization is recommended for patients diagnosed with Moyamoya disease exhibiting symptoms such as transient ischemic attacks, seizures, strokes, or hemorrhages [7, 8].
Pre-surgical imaging comprises gadolinium-enhanced magnetic resonance imaging (MRI), perfusion studies, and 4-vessel digital angiography, including the ECA, performed within 3 months prior to surgery, with updates for new or persistent symptoms.
Selection of the initial hemisphere for revascularization and the surgical technique, with a preference for the EDMS method in children, hinges on cerebral vascular anatomy, external carotid collateral assessments (on the brain MRI and digital angiography), and symptoms lateralization. EDMS is favored due to lower risk and simplicity in pediatric patients’ smaller cerebral vasculature, unlike the more complex direct anastomosis [2, 4,5,6,7,8,9,10].
An MRI is conducted on postoperative day one, followed by semi-annual and annual scans. A follow-up digital angiography is scheduled one year after the surgery.
For bilateral revascularization, the second procedure is typically planned 3 to 4 months after the first surgery, with an updated MRI.
Limitations
The primary drawbacks of EDMS include cosmetic deformity from temporal muscle transposition and the rare possibility of intracranial mass effect development.
Furthermore, the revascularization process can take up to 6 months, during which the patient remains at risk of stroke. However, clinical improvements are generally observed within the first few weeks post-surgery.
How to avoid complications
Delaying surgery in patients with acute ischemia is recommended due to the risk of postoperative infarction, ideally for 14 days.
Oral antiplatelet therapy (aspirin 100 mg daily) is suspended 2 days before surgery and resumed 24 h afterward. In severe cases, with frequently symptoms, aspirin might not be suspended. To prevent hyperperfusion syndrome, strict control of systolic blood pressure (110–140 mmHg) is maintained.
Maintaining stable hemodynamics, normocapnia, normothermia, and oxygenation is crucial. Experienced neuroanesthesiologists and intensive care physicians are pivotal in managing these parameters and minimizing ischemic risks.
Specific information for the patient
Anesthesia itself poses a risk of ischemic complications in these patients. Revascularization can take up to 6 months, during which the overall risk of stroke and hemorrhage persists. There’s an increased risk of ischemic events in the first 10 days postoperatively. Possible complications include postoperative ischemia, hematoma, CSF fistula, or infection.
10 key point summary
-
1.
It is essential to know the vascular anatomy of external carotid branches involved in the procedure.
-
2.
Prevent hypotension and maintain cerebral perfusion pressure (CPP), normocapnia, normothermia, adequate oxygenation, and correct anemia.
-
3.
Oral antiplatelet therapy is suspended 2 days before surgery and resumed 24 h post-surgery.
-
4.
Careful positioning during surgery is mandatory, assessing bone thickness with a 3-pin head fixation clamp placement and avoiding pressure on the already operated side.
-
5.
Identification of the STA is necessary to mark and avoid it during incision.
-
6.
Infiltrate the skin using anesthetics without vasoconstrictive agents before incision.
-
7.
Carefully dissect the temporal muscle while preserving its deep blood supply.
-
8.
Analyze the middle meningeal artery’s course pre-craniotomy to prevent intraoperative injuries, considering potential intraosseous location within the sphenoid wing.
-
9.
Bevel the lower edge of the bone flap to prevent compression of the temporal muscle.
-
10.
Use absorbable sutures for skin closure to avoid distress during suture removal.
References
Bonasia S, Smajda S, Ciccio G, Robert T (2020) Middle Meningeal artery: anatomy and variations. AJNR Am J Neuroradiol 41(10):1777–1785. https://doi.org/10.3174/ajnr.A6739
Egashira Y, Enomoto Y, Kano K, Iwama T (2023) How I do it: combined bypass for adult moyamoya disease with maximal consideration of cosmetic aspects. Acta Neurochir (Wien) 165(8):2073–2076. https://doi.org/10.1007/s00701-023-05604-8
Gadgil N, Lam S, Pyarali M, Paldino M, Pan IW, Dauser RC (2018) Indirect revascularization with the dural inversion technique for pediatric moyamoya disease: 20-year experience. J Neurosurg Pediatr 22(5):541–549. https://doi.org/10.3171/2018.5.PEDS18163
Lavrysen E, Menovsky T (2019) How I do it: operative nuances of multiple burr hole surgery for moyamoya disease and syndrome. Acta Neurochir (Wien) 161(1):171–175. https://doi.org/10.1007/s00701-018-3743-1
Liao Y, Xu F, Xu B (2022) How I do it: superficial temporal artery to middle cerebral artery bypass for moyamoya disease. Acta Neurochir (Wien) 164(7):1855–1859. https://doi.org/10.1007/s00701-022-05255-1
Nossek E, Langer DJ (2014) How I do it: combined direct (STA-MCA) and indirect (EDAS) EC-IC bypass. Acta Neurochir (Wien) 156(11):2079–2084. https://doi.org/10.1007/s00701-014-2226-2
Shim KW, Park EK, Choi JU, Kim DS (2017) Moya-Moya Disease. In: Di Rocco C, Pang D, Rutka J (eds) Textbook of Pediatric Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-319-31512-6_54-1
Suzuki J (1986) Treatment. Moyamoya Disease. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-95483-2_9
Wanebo JE et al (2014) Moyamoya Disease. II Treatment options: Medical, Endovascular, Perioperative, and Surgical Management. 12 Indirect revascularization procedures for Moyamoya Disease. https://doi.org/10.1055/b-0034-92307
Wang KC, Phi JH, Lee JY, Kim SK, Cho BK (2012) Indirect revascularization surgery for moyamoya disease in children and its special considerations. Korean J Pediatr 55(11):408–413. https://doi.org/10.3345/kjp.2012.55.11.408
Acknowledgements
We would like to express our deepest gratitude to the Moyamoya Interdisciplinary Work Team at Hospital de Pediatría “Prof. Dr. Juan P. Garrahan”, comprised of Buompadre Celeste, Requejo Flavio, Rugilo Carlos, López Germán Darío, Cambaceres Carlos, Muñoz del Toro Sebastián, Sánchez María Luz, Gromadzyn Guido, Cicutti Santiago, Jaimovich Sebastián, and Gonzalez Ramos Javier. Their dedication, professionalism, and teamwork have been fundamental in monitoring and improving the quality of life of patients affected by Moyamoya vascular pathology. Their joint effort and commitment to excellence are an inspiration and have significantly contributed to the success of this work.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were conducted by S.E.C. The first draft of the manuscript was written by S.E.C., and all authors commented on previous versions of the manuscript. Additionally, S.E.C. created the images, and recorded and edited the video, which features a surgery performed by S.G. J. The audio for the video was produced by G.P.G. Language editing and correction were carried out by J.F. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki and adhered to the Good Clinical Practice Guidelines (Resolution 1480/11) of the Argentine Ministry of Health. Furthermore, approval for the protocol was obtained by the Ethics and Research Committee of “Juan P. Garrahan” Pediatric Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cicutti, S.E., Gromadzyn, G.P., Cuello, J.F. et al. How I do it: operative nuances of the Encephalo-Duro-Mio-Synangiosis (EDMS) technique for pediatric moyamoya disease and syndrome. Acta Neurochir 166, 249 (2024). https://doi.org/10.1007/s00701-024-06148-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00701-024-06148-1