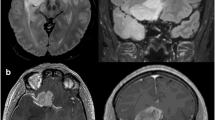
Abstract
Background
High-field intraoperative MRI (IoMRI) is part of the neurosurgical armamentarium to improve the extent of glioma resection (EOR).
Objective
To report our oncological and functional outcomes using IoMRI for neuronavigated glioma surgery.
Methods
In this prospective monocentric study, we reported 100 consecutive adult patients operated on for glioma using IoMRI with neuronavigation, under general anesthesia without intraoperative stimulation, from July 2014 to April 2017. The volumetric evaluation was based on the FLAIR hypersignal for non-enhancing tumors after Gadolinium infusion and on the T1 hypersignal after Gadolinium infusion for enhancing tumors. We evaluated the surgical workflow, the EOR and the clinical outcomes (using Karnofsky performance score (KPS)).
Results
Sixty-nine patients underwent one IoMRI, and 31 from two IoMRI controls. At first IoMRI, the median tumor residue was higher in the FLAIR group than in the T1G+ group whereas no more difference was reported after the second IoMRI between the 2 groups (p = 0.56). Additional resection was performed 6 times more frequently in the FLAIR group (OR = 5.7, CI (1.9–17)). The median EOR was 100% (IQR, 93.6–100) whatever the tumor type (first surgery or recurrence) and location. Higher residues were reported only in the insula area (p = 0.004). The median KPS was 90 (IQR, 80–100) at discharge, 3, 6 and 12 months after surgery, with no statistical difference between low- and high-grade gliomas (p = 0.34).
Conclusion
IoMRI neuronavigated surgery provided maximal EOR whatever the type of glioma and location. IoMRI was all the more useful for non- or minimally enhancing tumors. The step by step surgical resection, introducing the concept of “staged volume” surgery, ensured a high security for the surgeon and low permanent morbidity for the patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extent of resection (EOR) in glioma surgery plays a crucial role in prolonging the survival of patients, either for de novo or recurrent tumors [14, 18, 24, 26]. Optimal resection allows a better response to adjuvant treatments and an improved and prolonged quality of life [2, 3].
The advent of high-field intraoperative MRI (IoMRI) integrated platform has changed the way of dealing with brain tumors and especially gliomas. The use of such IoMRI helps to address several surgical issues. First, the update of the neuronavigation data using intraoperative images helps to face the brain shift issue. The IoMRI also assists the surgeon dissection to reach higher EOR highlighting tumor remnants on intraoperative imaging [1]. More recently, diffusion tensor imaging (e.g., intraoperative tractography) emerged as a useful tool to preserve the main neurological functions [21]. The use of the IoMRI was reported as significantly increasing the resection rate of LGGs and high-grade gliomas (HGGs) while preserving the function [13, 19, 23, 25].
We reported our surgical experience using a high-field IoMRI (1.5 T) under general anesthesia without intraoperative stimulation for a wide range of gliomas: de novo or recurrent, with or without contrast enhancement, close or not to eloquent area. Our objective was to report our experience using IoMRI during neuronavigated glioma surgery, in terms of EOR and functional outcomes.
Materials and methods
Patients
We prospectively included all consecutive adult patients operated on for a glioma using high-field IoMRI from July 2014 to April 2017. Our institutional ethic committee approved this data register.
A flow chart depicted our glioma surgical activity during the study period (Fig. 1). The tumor location was reported for each patient in the following categories: frontal, fronto-callosal, fronto-temporo-insular, temporal, parietal, and occipital specifying whether it was the left or right hemisphere. The following brain areas were considered to be “eloquent”: primary sensorimotor areas, supplementary motor areas, visual areas, language areas (fronto-opercular cortex and posterior temporal cortex in dominant hemisphere, the arcuate fasciculus, the inferior fronto-occipital fasciculus [IFOF]), limbic and paralimbic structures (including both insula), and the basal nuclei.
The patients harboring a lesion potentially closed to the speech areas underwent a preoperative functional MRI (activation of language) to check their dominant hemisphere. A lesion in the speech areas in the dominant side was preferentially operated on awake surgery conditions rather than with IoMRI in asleep condition. Complementary diffusion tensor imaging with fiber tracking (DTI-FB) was performed if necessary, to specify the position of main neurological bundles (e.g., corticospinal tract, arcuate fasciculus, IFOF, optical radiations). All patients underwent a recent preoperative MRI neuronavigation sequence, usually the day before surgery. The postoperative MRI was performed less than 48 h after surgery, then at 3 and 6 months.
The functional outcomes were assessed by a board neurosurgeon preoperatively, at discharge; then at 3, 6, and 12 months, using the Karnofsky performance score (KPS). Additional neuropsychological examination was performed in patients harboring cognitive, memory, or behavior complaints. The neuropsychological assessments detailed as follows: overall cognitive efficiency (Montreal Cognitive Assessment, MoCa), the working memory, verbal and episodic hearing, learning, executive tasks, attention, language (speaking and listening), visuoconstructional and gnosic functions, and reasoning. To assess the social cognition, three tasks coming from the BCS, a French computer battery designed to assess emotional and sociocognitive abilities, were used [8]. For each patient, the definitive histologic subtype was reviewed by a senior neuropathologist, based on the 2016 WHO classification.
Surgical procedures
All patients were operated under general anesthesia without intraoperative stimulation in our integrated imaging platform, including a 1.5 Tesla MRI (General Electric®, Boston, MA) located next to the OR. No 5 ALA fluorescence-guided resection was performed. Therefore, patients underwent surgery outside the 5-G line; thus, normal instruments and a surgical microscope were used for surgery. We used a MRI-compatible head holder to position the patient. The imaging sequences used for neuronavigation were 3D T1 after gadolinium injection, or 3D FLAIR, with 1-mm slice thickness. The surgical microscope (OPMI Pentero® Zeiss, Germany) was connected to the imaging network and could be used as neuronavigation.
For enhanced tumors after T1 Gadolinium infusion, the resection goal was to remove all enhancing tumor. For non-enhanced lesions, removal of all detectable tumor tissue on T2/FLAIR sequence was the main objective (both in suspected grades II and III). A new IoMRI was performed when the neuronavigation became insufficiently accurate and/or to assess the presence of residual tumor. The IoMRI images were used to update the navigation data after each control.
Before translating the patient into the MRI, a checklist was methodically filled, to ensure the absence of metallic material in the surgical site that could interfere with the magnetic field. Concerning the IoMRI sequences, a volumetric 3D T1 was systematically performed to readjust the neuronavigation. Additional sequences, such as 3D FLAIR (especially for LGG), diffusion (B1000 and ADC), and T2 with gradient echo, could be performed. An intraoperative tractography was performed in case of gliomas close to functional areas (e.g., corticospinal tract). The neuronavigation data update procedure was performed using the automatic coregistration provided by Brainlab® Munich, Germany. The quality and accuracy of the coregistration were double-checked by an imaging engineer and the board neurosurgeon.
Volumetric analyses
Two independent observers (including a board radiologist and a senior neurosurgeon) measured all preoperative, intraoperative, and postoperative glioma volumes, using the software Intellispace Portal (Philips®, Amsterdam, Netherlands), module “Tumor tracking.” The lesions without contrast enhancement or slightly enhanced after gadolinium infusion were contoured using the T2/FLAIR hypersignal. Highly contrast-taking tumors were segmented using T1 hypersignal after gadolinium infusion (T1G+). After segmentation, volumes were calculated in cubic centimeters. If a discrepancy > 10% was reported between the 2 observers, we compared both segmentations to achieve a consensus volume. We also reported the EOR in percentages.
Statistical analyses
The institutional biostatistics department performed the statistical analyses. Qualitative variables are expressed as numbers (percentage). Quantitative variables are expressed as the median (interquartile range (IQR)). Normality of distributions was assessed using histograms and Shapiro-Wilk test. Comparisons between two groups of patients (T2/FLAIR vs. T1G+ groups) were performed using the U test of Mann-Whitney for quantitative variables and using chi-square test for qualitative variables. To study the impact of the number of IoMRI controls on the postoperative tumor volume, by adjusting on the parameters defined a priori (e.g., preoperative volume, tumor de novo or recurrence, and eloquent area), a nonparametric analysis of covariance was performed. The functional score (KPS) measured at different postoperative times was compared with the preoperative evaluation using a Wilcoxon signed-rank test. Statistical testing was done at the two-tailed α level of 0.05. Data were analyzed using the SAS software package, release 9.3 (SAS Institute, Cary, NC).
Results
Population data
One hundred patients were included, 62 men and 38 women (sex ratio M/F = 1.6). The median age at diagnosis was 44 years (IQR, 34–51 years). Eighty-six patients were right-handed, 12 were left-handed, and 2 were ambidextrous. Ninety-three patients had a KPS ≥ 80 at diagnosis. The symptoms at diagnosis were as follows: epilepsy 55%, headaches 18%, focal deficit 17% with phasic disorders in 7 cases.
The anatomical distribution of operated tumors was as follows: frontal, 39 (20 right, 19 left); frontal and corpus callosum, 5 (4 right, 1 left); insula, 20 (17 right, 3 left); temporal, 21 (14 right, 7 left); and parieto-occipital, 15 (11 right, 4 left). Forty-eight patients had a lesion close to an eloquent area (e.g., speech areas, primary motor areas).
The repartition of the histologic grades WHO 2016 according to the FLAIR and T1G+ groups are reported in Table 1. Thirty-four patients harbored recurrent gliomas as follows: 12 WHO grade II, 12 WHO grade III, and 10 WHO grade IV.
According to the preoperative MRI, 63 tumors were contoured on T2/FLAIR and 37 on T1G+ sequences. In the FLAIR group, patients were younger (p < .0001), with a higher KPS (p = 0.004). The FLAIR tumors also involved more frequently eloquent areas (p = 0.017) and were larger (p = 0.006) than in the T1G+ group.
The mean hospital stay was of 7.8 days (4 to 23).
Operative data
All patients underwent at least one IoMRI. No technical incident has been reported. The median duration of the first IoMRI control (including patient transfer) was 34 min (IQR, 25–42 min). Thirty-one patients underwent a second IoMRI, with a median duration of 21 min (IQR, 17–28 min). No patient underwent more than two IoMRI. The median total operative time was 4:25 (IQR, 4:02 to 5:37).
The median preoperative tumor volume was 28.4 cm3 (IQR, 11–58). The intraoperative volume after the first IoMRI was 3 cm3 (IQR, 0.2–9.6), after the second IoMRI was 4.2 cm3 (IQR, 0.2–7). The final median tumor volume was 0 cm3 (IQR, 0–2.5). The final median extent of resection was 100% (IQR, 93.6–100).
Additional resection was performed in 90% of cases after the first IoMRI among FLAIR tumors and in 60% of cases among T1G+ tumors. After the second IoMRI, 71% of FLAIR lesions benefited from additional resection and 29% of T1G+ lesions. Additional resection was performed 6 times more frequently in the FLAIR group than in the T1G+ group (odds ratio = 5.7, CI (1.9–17)). Other parameters such as a tumor close to an eloquent area or a recurrent tumor did not influence the rate of additional resection (respectively p = 0.07 and p = 0.87).
At the first IoMRI, the median tumor residue was significantly higher in the FLAIR group (p = 0.0001) whereas at the second IoMRI, no more difference was reported between the FLAIR and T1G+ groups (p = 0.56) (Fig. 2).
Evolution of the median tumor volumes (cm3) in each group. The initial median volume of FLAIR tumors was larger than T1G+ lesions (p = 0.006). At first IoMRI, the median tumor residue was significantly larger in the FLAIR group (p = 0.0001). No significant difference was reported between the 2 groups on the postoperative MRI (p = 0.56)
Regarding the tumor location, larger tumor residues were reported in the insula area than in the frontal or temporal lobes, on the IoMRI and on the postoperative MRI (p = 0.004). The tumors close to an eloquent area harbored higher residual volumes on the first IoMRI than other tumors (p = 0.001). On postoperative MRI regarding the tumor remnant, no more significant difference was reported between tumors located close to eloquent area and other location (p = 0.29).
Functional outcomes
In our series, the median KPS at discharge was of 90 (IQR, 80–100, mean 84.9), at 3 months was of 90 (IQR, 80–100), at 6 months was of 90 (IQR, 80–100), and at 1 year was of 90 (IQR, 90–100) (Fig. 3). The respective percentage of patients harboring a KPS < 90 at each examination for the HGG and LGG groups were as follows: preoperatively 18% and 0%, at discharge 39% and 37% at 3 months 35% and 19%, at 6 months 36% and 15%, at 1 year 21% and 17%. Thirty-six patients harbored a KPS decrease at discharge in comparison with preoperative KPS. At 3 months after surgery, 25 patients still harbored a KPS decrease. On the other side, 22 patients improved their KPS at 3 months in comparison with their preop status. Fifty-five patients were free of symptoms at discharge. Postoperative morbidities were as follows: 10 cases of transient motor deficit (of which 7 were supplementary motor area syndrome), 11 cases of transient phasic disorder, and 11 cases of permanent visual field impairment (homonymous hemianopia). Eleven patients harbored cognitive impairments during early postoperative course (6 frontal syndromes (5 apragmatism, 1 disinhibition), 3 confusions (in relation with phasic disorders), 2 attention disorders with visuocontructive troubles). After a neuropsychologic examination at 3 months, the frontal disorders and confusion were no more reported. The attention disorders were less important but still present. The WHO grades of operated tumors were as follows: 7 grade I, 36 grade II, 28 grade III, and 29 grade IV. Thirty-five patients underwent MRI follow-up, 21 from chemotherapy alone, 7 from radiotherapy alone, and 37 from radio chemotherapy. In our series, regarding the KPS evolution until 12 months after surgery, no statistical difference was found between low-grade (I + II) and high-grade (III + IV) gliomas (p = 0.35) (Fig. 3).
Illustrative cases
Representative cases are shown in Figs. 4 and 5.
This is the case of a 46-year-old man, right handed, harboring a recurrence of a left frontal astrocytoma grade II. The FLAIR hypersignal was increasing at the boarder of the previous cavity. A second surgery was decided after collegiate discussion. The EOR was 100%. During the first days after surgery, the patient presented with a SMA syndrome which regressed after 1 week. (A) Preoperative FLAIR MRI reporting a superior and medium frontal glioma. (B) Speech activation functional MRI: an activated spot (corresponding to the Broca area) was located at the posterior and lateral part of the lesion. (C) Preoperative DTI-FT of the inferior fronto-occipital fasciculus (IFOF) located under the lesion. (D) Intraoperative DTI-FT of the cortico-spinal tract, posterior to the surgical cavity. (E) 1st IoMRI: tumor residue at the posterior part of the cavity. Additional resection decided. (F) 2nd IoMRI: no residue funded. Closure was performed
This is the case of a 40-year-old woman, right handed, with no medical antecedent, KPS 90. She presented with insular seizures (somatosensitive and digestive symptoms). The preoperative MRI reported a probable right fronto-temporo-insular low-grade glioma (no contrast enhancement). She underwent a preoperative functional MRI with speech area activation showing only left side activation. The preoperative volume was 42.8 cm3. The surgery was performed with two IoMRI followed by additional resection. The EOR was 91% with a tumor residue < 4 cm3. The histological result reported an astrocytoma WHO grade II. No postoperative deficit was noted
Discussion
The concept of strategic tumor residue
In our experience, the use of IoMRI modified our way of dissecting glioma [15]. During the inclusion period (2014–2017), the way of using IoMRI has changed in our team. The first months, we were reaching maximal EOR and then we performed an IoMRI. Later, we moved to a step by step dissection to enhance the safety of the procedure. Our surgeries tended to be longer, with two to three intraoperative controls. Then, we achieved a compromise between very close IoMRI controls and shorter surgical procedures. For instance, we stopped performing a final control after closure under general anesthesia. We did not aim at maximal tumor resection straightaway. Especially for low-grade gliomas, a step-by-step dissection was performed, interrupted by on one or two IoMRI. We performed 6 times more frequently additional resection after IoMRI for low-grade than for high-grade gliomas. At the end of surgery, the initial volume discrepancy between FLAIR and T1G+ tumors was not reported anymore. The presence of nodular FLAIR hypersignal on the edges of the surgical cavity represented an argument to pursue the resection in case of LGGs. The use of high-field IoMRI was particularly helpful for low-grade gliomas harboring blurred borders. It helped us to discriminate true FLAIR tumor remnants from intraoperative peri cavity remodeling due to edema or peripheral ischemia. In these cases, a dedicated neuroradiologist could provide valuable assistance. Our results were in line with Mohammadi et al. who reported in their volumetric analysis that intraoperative residual tumor was higher in non-enhancing tumors than in enhancing tumors and that IoMRI improved the resection rate of non-and minimally enhancing tumors more than those with enhancing tumors [19]. In our series, the FLAIR group mostly corresponded to LGGs. However, as reported in Table 1, 23 lesions belonging to the FLAIR group were grade WHO III and IV. On the other side, 3 grade WHO I belonged to the T1G+ group (pilocytic astrocytoma and ganglioglioma). These results illustrated the change the new WHO 2016 introduced in our practice. Because of biomolecular data, some lesions looking like LGGs on MRI were finally classified as HGGs.
Gliomas next to eloquent area
The IoMRI was useful to manage tumor resection close to an eloquent area [23]. In this series, all surgeries were performed under general anesthesia without electro cortico-subcortical stimulation. Preoperative diffusion tensor imaging-fiber tracking (DTI-FT) was routinely performed to assess the most at risk neurological bundles (e.g., corticospinal tract, optic radiations). During the surgery, DTI-FT was reassessed during IoMRI and uploaded into the navigation dataset. In our experience, the surgeon did not stop prematurely his resection due to intraoperative DTI changes after updating navigation registration. Although the tumor residual at the first IoMRI was higher than tumors far from eloquent area, at the end of surgery, the EOR was similar to non-eloquent area lesions. This staged volume approach using IoMRI and intraoperative DTI-FT helped to reach a similar rate of resection in comparison with other gliomas (median EOR = 100%). The permanent postoperative morbidity among the 48 lesions close to an eloquent area was 2.8% [23]. Thus, IoMRI with DTI-FT updating of navigation data helped to overcome the pitfalls of an exclusive preoperative and inaccurate navigation plan [5, 16].
In our series, 20 gliomas involved the insula area of the non-dominant hemisphere (right side for all cases). This tumor location was challenging with an important functional and vascular risk. Previously, such insular gliomas underwent biopsies and then radiotherapy or chemotherapy. According to our experience, the IoMRI-guided surgery provided high resection rates for insular glioma located in the non-dominant hemisphere. In our institution, we usually performed for left insular glioma awake surgery with cortico-subcortical stimulation. Consecutive IoMRI controls enhanced the tumor resection by 25% between the first and the final IoMRI. The final median tumor residual in this subgroup was < 3.7 cm3 (highest median tumor residue in our series). The final EOR was of 93%, which was higher than Berger et al. who reported an EOR of 85%, or Ius et al. with an EOR of 80% [11, 12]. Even in this subgroup, the median KPS was 90 (IQR, 80–90) at discharge and at 3 months after surgery.
Recurrent gliomas
Revision surgery for gliomas is demanding because of previous treatments that modified the brain aspect and blurred the tumor borders. According to our series, the use of IoMRI allowed reaching the same EOR as for de novo gliomas (34 recurrent tumors, median EOR = 100%). The tumor borders thanks to the neuronavigation update were clearly identified. In our series, for high-grade gliomas, a complete resection of contrast enhancing tumor was performed in 100% of cases. Oppenlander et al. reported an 80% threshold of resection to improve the overall survival of patients harboring glioblastoma recurrence [20]. According to Quick et al., only complete removal of contrast enhancing tumor was significantly correlated with survival after re-resection according to multivariate analysis [22]. From this point of view, recurrent gliomas represented relevant candidates for IoMRI-guided surgery.
Functional outcomes
In our series, 55% of patients were free of symptoms at discharge. Nonetheless, 45% harbored new or worsened clinical deficits during the early postoperative course. Thirty-six patients harbored a KPS decrease at discharge. Most of these deficits were transient (e.g., SMA syndromes). In most cases, persistent deficits were visual field defects, such as homonymous hemianopia. The patients were previously informed of this frequent visual impairment in case of parieto-occipital gliomas. From our point of view, oncologic benefits outweighed the campimetric preservation, especially for high-grade gliomas. For LGG, the functional impact of visual field defect has to be discussed with the patient, taking into account the patient profession, the patient need to drive, etc. Regarding the neuro cognitive outcomes, permanent impairments related to surgery were uncommon, in line with previous publication [10]. As the other additional surgical techniques aiming at improving the EOR (e.g., fluorescence-guided resection [1, 6]), the use of IoMRI heightened the postoperative transient neurological deficit without hampering long-term functional outcomes. The use of awake craniotomy with cortical and subcortical stimulation was also related to higher transient postoperative deficit [6, 9]. Maldaun et al. and Coburger et al. combined in their series awake surgery and the use of IoMRI. They reported a lower range of postoperative transient deficit (26% and 35%) [4, 17]. We recently began to perform such resection using awake surgery and IoMRI and we will report our results.
In any case, the median KPS at discharge was 90 (capable of normal activity, with few symptoms or signs of disease), enabling the vast majority of patients to return home. This high level of autonomy was preserved at 3, 6 and 12 months postoperatively. Surprisingly, the KPS evolution during the 12 months after surgery was not statistically different between the patients harboring LGG and HGG. This finding supported that GTR for HGG allowed better PFS and improved tolerance to adjuvant therapies such as radiotherapy and chemotherapy [2, 3].
Limitations
The functional outcomes were assessed by one investigator (HAL). It would have been of greater value to perform an external multi observer KPS evaluation. Moreover, we used “Observer reported outcomes” (ORO) to evaluate the KPS. To better assess complaints and patient quality of life, we could have used “Patient reported outcomes” (PRO).
In this study, we did not use fluorescence-guided surgery (FGR), such as 5-ALA, in combination with IoMRI-guided surgery. During the inclusion period, we were participating in the RESECT clinical trial, a France-wide study evaluating the medico-economic aspect of FGR applied to glioblastoma. We could not use IoMRI with FGR at this time to avoid biasing the oncological outcomes. The synergy of FGR- and IoMRI-guided surgery for glioma has been highlighted [26]. Our objective will be to evaluate prospectively in a randomized manner the benefits of the IoMRI-guided surgery and FGR on OS for patients harboring HGGs.
Conclusion
High-field IoMRI neuronavigated surgery significantly enhanced the EOR of gliomas, especially for non- or minimal enhancing tumors (mainly LGG). The use of IoMRI introduced the concept of “staged volume” surgery, including the idea of strategic tumor residue. This way of resecting tumor was safe and effective, with low permanent morbidity. The next step will be to evaluate combined adjuvant surgical techniques such as FGR or awake conditions and IoMRI [7, 17].
References
Barone DG, Lawrie TA, Hart MG (2014) Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev 1:CD009685. https://doi.org/10.1002/14651858.CD009685.pub2
Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, Boeve BF, Arusell RM, Clark MM, Buckner JC (2005) A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery 57:495
Brzozowska A, Toruń A, Mazurkiewicz M (2015) The impact of surgery on the efficacy of adjuvant therapy in glioblastoma multiforme. Adv Clin Exp Med 24:279–287. https://doi.org/10.17219/acem/40456
Coburger J, Merkel A, Scherer M, Schwartz F, Gessler F, Roder C, Pala A, Konig R, Bullinger L, Nagel G, Jungk C, Bisdas S, Nabavi A, Ganslandt O, Seifert V, Tatagiba M, Senft C, Mehdorn M, Unterberg AW, Rossler K, Wirtz CR (2016) Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery 78:775–786. https://doi.org/10.1227/NEU.0000000000001081
D'Andrea G, Familiari P, Di Lauro A, Angelini A, Sessa G (2016) Safe resection of gliomas of the dominant angular gyrus availing of preoperative FMRI and intraoperative DTI: preliminary series and surgical technique. World Neurosurg 87:627–639. https://doi.org/10.1016/j.wneu.2015.10.076
Della Puppa A, De Pellegrin S, d'Avella E, Gioffre G, Rossetto M, Gerardi A, Lombardi G, Manara R, Munari M, Saladini M, Scienza R (2013) 5-aminolevulinic acid (5-ALA) fluorescence guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir 155:965–972; discussion 972. https://doi.org/10.1007/s00701-013-1660-x
Dupont C, Vermandel M, Leroy HA, Quidet M, Lecomte F, Delhem N, Mordon S, Reyns N (2018) INtraoperative photoDYnamic therapy for GliOblastomas: study protocol for a phase I clinical trial. Neurosurgery. https://doi.org/10.1093/neuros/nyy324
Ehrle N, Henry A, Pesa A, Bakchine S (2011) Assessment of sociocognitive functions in neurological patients presentation of a French adaptation of two tools and implementation in frontal dementia. Geriatr Psychol Neuropsychiatr Vieil 9:117–128. https://doi.org/10.1684/pnv.2010.0252
Eseonu CI, Rincon-Torroella J, ReFaey K, Lee YM, Nangiana J, Vivas-Buitrago T, Quinones-Hinojosa A (2017) Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: evaluating perioperative complications and extent of resection. Neurosurgery 81:481–489. https://doi.org/10.1093/neuros/nyx023
Habets EJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJ (2014) Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir 156:1451–1459. https://doi.org/10.1007/s00701-014-2115-8
Hervey-Jumper SL, Li J, Osorio JA, Lau D, Molinaro AM, Benet A, Berger MS (2016) Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J Neurosurg 124:482–488. https://doi.org/10.3171/2015.4.JNS1521
Ius T, Isola M, Budai R, Pauletto G, Tomasino B, Fadiga L, Skrap M (2012) Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg 117:1039–1052. https://doi.org/10.3171/2012.8.JNS12393
Kuhnt D, Ganslandt O, Schlaffer SM, Buchfelder M, Nimsky C (2011) Quantification of glioma removal by intraoperative high-field magnetic resonance imaging: an update. Neurosurgery 69:852–862; discussion 862-853. https://doi.org/10.1227/NEU.0b013e318225ea6b
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Leroy HA, Delmaire C, Le Rhun E, Drumez E, Lejeune JP, Reyns N (2018) High-field intraoperative MRI in glioma surgery: a prospective study with volumetric analysis of extent of resection and functional outcome. Neurochirurgie 64:155–160. https://doi.org/10.1016/j.neuchi.2018.02.003
Maesawa S, Fujii M, Nakahara N, Watanabe T, Wakabayashi T, Yoshida J (2010) Intraoperative tractography and motor evoked potential (MEP) monitoring in surgery for gliomas around the corticospinal tract. World Neurosurg 74:153–161. https://doi.org/10.1016/j.wneu.2010.03.022
Maldaun MV, Khawja SN, Levine NB, Rao G, Lang FF, Weinberg JS, Tummala S, Cowles CE, Ferson D, Nguyen AT, Sawaya R, Suki D, Prabhu SS (2014) Awake craniotomy for gliomas in a high-field intraoperative magnetic resonance imaging suite: analysis of 42 cases. J Neurosurg 121:810–817. https://doi.org/10.3171/2014.6.JNS132285
McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, AR Q√±o-H (2009) Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110:156–162. https://doi.org/10.3171/2008.4.17536
Mohammadi AM, Sullivan TB, Barnett GH, Recinos V, Angelov L, Kamian K, Vogelbaum MA (2014) Use of high-field intraoperative magnetic resonance imaging to enhance the extent of resection of enhancing and nonenhancing gliomas. Neurosurgery 74:339. https://doi.org/10.1227/NEU.0000000000000278
Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, Ashby LS, Brachman D, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N (2014) An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 120:846–853. https://doi.org/10.3171/2013.12.JNS13184
Ottenhausen M, Krieg SM, Meyer B, Ringel F (2015) Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus 38:E3. https://doi.org/10.3171/2014.10.FOCUS14611
Quick J, Gessler F, Dutzmann S, Hattingen E, Harter PN, Weise LM, Franz K, Seifert V, Senft C (2014) Benefit of tumor resection for recurrent glioblastoma. J Neuro-Oncol 117:365–372. https://doi.org/10.1007/s11060-014-1397-2
Reyns N, Leroy HA, Delmaire C, Derre B, Le-Rhun E, Lejeune JP (2017) Intraoperative MRI for the management of brain lesions adjacent to eloquent areas. Neurochirurgie 63:181–188. https://doi.org/10.1016/j.neuchi.2016.12.006
Sanai N, Polley M-YY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003. https://doi.org/10.1016/s1470-2045(11)70196-6
Suero Molina E, Schipmann S, Stummer W (2017) Maximizing safe resections: the roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg Rev. https://doi.org/10.1007/s10143-017-0907-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Comité d’éthique institutionnel du CHU de Lille) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Leroy, HA., Delmaire, C., Le Rhun, E. et al. High-field intraoperative MRI and glioma surgery: results after the first 100 consecutive patients. Acta Neurochir 161, 1467–1474 (2019). https://doi.org/10.1007/s00701-019-03920-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03920-6