Abstract
Background
In this pilot study we compared advantages and drawbacks of near-infrared indocyanine green videoangiography (ICGVA) and intraoperative computed tomography (iCT) to investigate if these are complementary or competitive methods to acquire immediate information about blood vessels and potential critical impairment of brain perfusion during vascular neurosurgery.
Methods
A small subset of patients (n = 10) were prospectively enrolled in this feasibility study and received ICGVA immediately after placement of the aneurysm clips. An intraoperative cranial CT angiography (iCTA) was followed by dynamic perfusion CT scan (iCTP) using a 40-slice, sliding-gantry, CT scanner. The vascular patency of major (aneurysm bearing) arteries, visualisation of arising perforating arteries and brain perfusion after clip application were analysed with both techniques.
Results
The ICGVA was able to visualise blood flow and vascular patency of all major vessels and perforating arteries within the visual field of the microscope, but failed to display vessels located within deeper areas of the surgical field. Even small coverage with brain parenchyma impaired detection of vessels. With iCTA high image quality could be obtained in 7/10 cases of clipped aneurysms. Intraoperative CTA was not sufficiently evaluable in one PICA aneurysm and one case of a previously coiled recurrent aneurysm, due to extensive coil artefacts. Small, perforating arteries could not be detected with iCTA. Intraoperative CTP allowed the assessment of global blood flow and brain perfusion in sufficient quality in 5/10 cases, and enabled adequate intraoperative decision making.
Conclusion
A combination of ICGVA and iCT is feasible, with very good diagnostic imaging quality associated with short acquisition time and little interference with the surgical workflow. Both techniques are complementary rather than competing analysing tools and help to assess information about local (ICGVA/iCTA) as well as regional (iCTA/iCTP) blood flow and cerebral perfusion immediately after clipping of intracranial aneurysms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vascular neurosurgery for intracerebral aneurysms has considerably changed with the introduction of endovascular procedures. Improvement of these treatment options has broadened their range of indications in managing ruptured and unruptured aneurysms [10–12]. Due to comparable complication rates and outcome data for surgical and endovascular methods, there has been a growing tendency towards endovascular management [10]. As a consequence, neurosurgical operations are performed less frequently to clip aneurysms and, even more importantly, those aneurysms being selected for microsurgical clipping are more complex. Therefore, a decline in surgical expertise is assumed in the future [16], leading to fewer, but highly specialised neurovascular centres to compensate for this development. Whereas new endovascular treatment devices are permanently developed, technical improvements in clipping devices are rare. Most important developments in the surgical field have focused on intraoperative imaging to provide the neurosurgeon with information about residual filling or occlusion of branching arteries after clip positioning [1–3, 14, 15]. The use of intraoperative digital subtraction angiography (DSA) has been suggested for this purpose and had a significant impact on the neurosurgical procedure in as much as 34 % of cases [1, 2, 4]. Yet, due to limited availability and high technical complexity, its use as a routine procedure is limited. Moreover, it was shown that the detection of small branching and perforating arteries is restricted with intraoperative angiography [15]. Indocyanine green videoangiography (ICGVA) has been demonstrated to successfully visualise blood flow in small perforating vessels during intracranial vascular surgery [5, 7, 14, 15]. Due to its common use and fast application, it also reduced the time interval between positioning of the aneurysm clip and the possible repositioning in case of insufficient exclusion of the aneurysm or imminent ischaemia due to clip stenosis.
Recently, we could demonstrate, that both intraoperative computed tomography angiography (iCTA) and intraoperative perfusion computed tomography (iCTP) are feasible and provide valuable information without disturbing the surgical workflow [13]. Intraoperative CT imaging was shown to provide helpful information with a direct impact on intraoperative decision-making in order to maintain cerebral perfusion, thereby reducing the risk of iatrogenic ischaemia.
Although both indocyanine green videoangiography (ICGVA) and iCTA have demonstrated their value, the combination of both might be too time consuming and even redundant. Moreover, aneurysm treatment is contemplated more and more in the light of cost-effectiveness studies, where surgical clipping has been shown to be superior compared with coiling techniques. Therefore, we carried out this prospective study in a high-volume neurovascular centre in order to evaluate if ICGVA and iCT are redundant or complementary intraoperative imaging methods.
Materials and methods
Patients
During the study period, 72 patients with aneurysms were treated in our department, of whom ten patients (men/women: 3/7) with ruptured or unruptured (3/7) aneurysms were selected for this prospective feasibility study to obtain intraoperative imaging data with all modalities (ICGVA, iCTA, iCTP). A total of ten aneurysms were operated on in the following locations: middle cerebral artery (MCA; n = 2; left/right: 2/0), internal carotid artery (ICA; n = 4; 1/3), anterior cerebral artery (ACA; n = 1; 0/1), anterior communicating artery (ACOM; n = 2; 1/1) and posterior inferior cerebellar artery (PICA; n = 1; 0/1). One patient with a right-sided ICA aneurysm had been treated by endovascular coiling 9 months before, and one patient had previous subarachnoid haemorrhage from another aneurysm. The mean age at the time of surgery was 52 years (range: 35–72). Clinical data from all patients are summarised in Table 1. All patients had signed informed consent for all intraoperative imaging techniques. The legal requirements of the state and the board of physicians regarding storage of data and radiation exposure were met.
Indocyanine green videoangiography (ICGVA)
For ICGVA, the operative microscopes were equipped to acquire, process and store ICG fluorescence images with high resolution and contrast (Pentero; Carl Zeiss Meditec, Jena, Germany). Therefore, after the aneurysm has been clipped, a bolus of fluorescent indocyanine green (absorption peak 805 nm, emission peak 835 nm) was injected intravenously in a dosage of approximately 0.2–0.5 mg/kg b.w., as already described by others [14]. The ICG fluorescence was visualised in real-time and recorded by the integrated unit. Images were then repeatedly displayed for evaluation of the visualisation quality, especially of the minor vessels and to have a closer look on the initial inflow of the fluorescent dye.
Intraoperative computed tomography angiography (iCTA) and perfusion computed tomography (iCTP)
Intraoperative CTA was performed in a specifically equipped operating room (OR), as described previously [17]. In short, a sliding gantry of a 40-slice CT scanner with an 82-cm bore diameter (Somatom Open Sliding Gantry; Siemens Medical Solutions, Erlangen, USA) is installed on rails to move over an adjustable radiolucent carbon operating table (Trumpf, Ditzingen, Germany) where the patient’s head is fixed in a radiolucent headclamp (Mayfield® radiolucent skull clamp A-2002; Integra, Plainsboro, NJ, USA). For iCT scanning, all metal-containing instruments were removed in the close vicinity of the scanned area (field of view) and the operative field was covered with an additional sterile drape. Then, a CT scout of the head and upper neck was followed by caudo-cranial CTA with scan range from C1 to vertex. CT-adjusted injection of iodine contrast medium was carried out, employing a dual-head motor pump (Stellant; Medrad, Volkach Germany) and a bolus-tracking technique to achieve high-contrast attenuation values in the cerebral arteries and low overlay in the veins and sinus. The CTA was then started manually (collimation of 40 × 0.6 mm at 120 kV, 120mAs, pitch 1.1 and a rotation time of 0.5 s, resulting in a normalised effective dose of approximately 0.5 mSv) and an automated contrast agent injection was done by using a dedicated weight-adapted protocol (1.35 ml contrast agent per 1 kg body weight Imeron 300 [Bracco-Altana-Pharma, Konstanz, Germany], respectively 0.4 g iodine per 1 kg bodyweight at an injection rate of 6.0 ml/s, followed by 100 ml saline at 6.0 ml/s). Intraoperative CTP was performed after iCTA with a scan range starting 1 cm cranial to the aneurysm clip with a distance of at least 1 cm to the head clamps. Two 14.4-mm-thick slices were acquired every second for 40s using 80 kV and 200mAs with a total collimation of 24 × 1.2 mm, resulting in a normalised effective dose of approximately 3.2 mSv. Perfusion scanning started 5 s after injection of 50 ml contrast agent (Imeron 300) at 7 ml/s and a saline flush of 50 ml at 7 ml/s. After iCT, the additional drape was removed, the table repositioned and the surgical procedure resumed.
Reconstruction of CT raw data and image analysis
Image analysis was performed immediately after the scanning procedure by the neuroradiologist, in close collaboration with the neurosurgeon. Special attention was paid to the assessment or exclusion of residual aneurysm parts and impaired brain perfusion. Slice thickness for axial reformations was 1.0 mm with an increment of 0.75 mm, which were then loaded into a standard 3D workstation (Syngo MMWP, Siemens Medical Solutions, Erlangen, Germany), allowing cross-sectional viewing of multiplanar reconstructions (MPRs) in the sliding thin-slap technique, as well as reconstructions using volume rendering technique (VRT). Maximum intensity projections (MIPs) were calculated in all three planes with a slice thickness and increment of 2.5 mm. A standard, vendor given PCT analysis software was used for perfusion analysis. Colour-coded parameter-maps of cerebral blood flow (CBF), cerebral blood volume (CBV) and time to peak (TTP) were calculated.
Results
ICGVA
ICGVA was applicable for every location within the skull with high quality. It led to good visualisation of the aneurysm bearing as well as most major or perforating arteries within the field of view in 8/10 patients. On the other hand, ICGVA failed to display any vessel beyond the microscopic field and even minor overlay with brain parenchyma, endovascular coils or aneurysm clips (1/10; Table 1, patient no. 10) impaired detection of ICG within the vessels. ICGVA may also not be able to demonstrate functionally sufficient patency of branching arteries within the visual field, as has been the case in 1/10 patients (Fig. 3, Table 1, no. 4) in our series. In this case, ICGVA displayed good ICG fluorescence in the branching artery after clip application to occlude a right-sided pericallosal aneurysm. Yet, this was not functionally sufficient to avoid ischaemia in the depending area (Fig. 3), even if routine micro-Doppler did also not indicate altered blood flow due to relevant stenosis when applied before and after clip application. Hence, ICGVA may be technically easily applicable in all patients to display most arteries within the visual field but may not indicate haemodynamically relevant stenoses of arteries extending beyond the visual field and might therefore miss critical brain perfusion within distant brain areas.
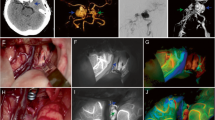
Patient (Table 1, no. 2) with recurrent aneurysm of the ICA/ophthalmic artery. Pre-interventional DSA (a) of an unruptured aneurysm which had been treated endovascularly but developed a recurrence at the entrance (b). After clip placement (c), ICGVA was applied (d) and demonstrated patency of the branching artery (arrow) without signs of stenosis. In contrast, iCTA (e) was not evaluable due to coil artefacts (arrow). Intraoperative CTP (f), which was applied in ICA/MCA segments, did not show artefacts due to clip or coil placement
Preoperative DSA (a) revealed untreated, unruptured aneurysm of the ICA/ophthalmic artery in this patient (Table 1, no. 6). Intraoperatively, the aneurysm (dashed line) was hidden underneath the right optic nerve (b). After application of the aneurysm clip (c) intraoperative micro-Doppler demonstrated patency of the ophtalmic artery, which was confirmed by ICGVA (d). Whereas branching of the ophthalmic artery could not be detected in the sagittal plane of the iCTA (e, black arrow), the intraorbital course of the artery was clearly detectable (f, white arrow). Intraoperative CTP was properly evaluable but did not reveal additional information in this context
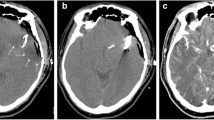
Complex aneurysm of the pericallosal artery as displayed by 3D reconstruction of DSA in this patient (Table 1, no. 4) (a). Patency of the branching artery was analysed by use of intraoperative micro-Doppler after aneurysm clipping (b) and ICGVA (c). Both methods assumed good local blood flow in one small perforating artery during surgery, which was not seen in the iCTA (d). Since intraoperative micro-Doppler and ICGVA did not reveal haemodynamically relevant stenosis, the neurosurgeon did not believe the results of the iCTA, which was considered not sufficient enough for detection of this small vessel, although iCTP (e) demonstrated perfusion deficit. Shortly after surgery infarction occurred in the postoperative CT (f). Luckily, there was no clinical correlation and the patient recovered without symptoms
iCTA
Intraoperative CTA was performed with adequate imaging quality in 7/10 patients. Decision-making was not possible in 1/1 patient with a PICA aneurysm of the posterior fossa (Table 1, patient no. 8), since beam-hardening artefacts originating from the petrosal bone were present. Additionally, 1/9 patients with supratentorially located aneurysms could not be properly evaluated in iCTA due to previous endovascular coiling (Fig. 1, Table 1, no. 2), which led to extensive imaging artefacts. Moreover, iCTA revealed insufficient reliability in the detection of small (perforating) arteries <1 mm diameter (e.g. A. recurrens Heubneri) as demonstrated in one case, in which undetected stenosis by clip application in a small perforation artery resulted in surgery-related ischaemia of one patient (Table 1, no. 5). Of note, intraoperative ICGVA and micro-Doppler analysis also did not reveal this relevant stenosis, leading to small infarction of the caudate nucleus but without neurological deficits.
All other imaging studies in the supratentorial region were of adequate quality for intraoperative decision-making and in 6/9 patients with supratentorial aneurysms the parent and branching vessels were sufficiently displayed after clip placement. Yet, as shown in Fig. 2 (Table 1, patient no. 6), direct visualisation of aneurysm-bearing small arteries at the skull base (e.g. ophthalmic artery) could be impaired after clip application. In this patient, branching of the ophthalmic artery could not be detected in the sagittal plane of the iCTA, but relevant stenosis of the ophthalmic artery could be excluded by use of iCTA due to contrast enhancement of more distal parts of the artery in its intraorbital course. Micro-Doppler and ICGVA also demonstrated patency of the ophthalmic artery after application of the aneurysm clip.
iCTP
In contrast to ICGVA and iCTA, iCTP resulted in good visualisation of the regional brain perfusion in 8/9 patients with supratentorial aneurysms. Impairment of imaging quality by coil or clip artefacts could be avoided in these cases due to the distant region of interest. Yet, the analysed area is limited to a maximum of 28.8-mm-thick brain region in caudo-cranial extension and the region of interest has to be chosen with care in order to display the relevant brain areas. Retrospectively, this was not done properly in one patient with an ACOM aneurysm in our series (Table 1, no. 9). Nevertheless, iCTP detected clipping related perfusion deficits in one patient where this became imminent, as shown in Fig. 3 (Table 1, no. 4). In this patient, ICGVA displayed good ICG fluorescence and intraoperative micro-Doppler assumed good local blood flow in one small perforating artery, which was assumed to be too small to be visualised by iCTA (Fig. 3d). Therefore, since ICGVA and micro-Doppler did not indicate haemodynamically relevant stenosis and iCTA was thought to be technically insufficient to display this small branching vessel, the neurosurgeon did not believe in the results of the iCTP, which was considered not to be of sufficient quality for detection of this small vessel. Shortly after surgery cerebral infarction of the depending area became obvious on a regular postoperative CT but, fortunately, the patient recovered without any symptoms. As already demonstrated for iCTA, iCTP could also not be employed for decision-making in one patient with a PICA aneurysm of the posterior fossa due to beam hardening artefacts.
Discussion
Worldwide, digital subtraction angiography (DSA) is still considered as the “gold standard” for the detection of cerebral aneurysms after subarachnoid haemorrhage. It is also recommended to monitor surgical results after aneurysm clipping either intraoperatively or postoperatively [2, 8, 9, 19]. Yet, intraoperative DSA is not available in all centres and is also not considered a routine method for all cases due to its invasive and time-consuming nature. Therefore, alternative methods to acquire reliable results during surgical intervention are most desirable.
Both techniques investigated in this study (ICGVA and iCTA/iCTP) have been described as fast and reliable with an impact on intraoperative decision-making [7, 14, 15, 17]. However, one might assume that application of both techniques is redundant and too time-consuming during vascular neurosurgery for aneurysm clipping. Since clinical guidelines are missing, high expenses like installation of specifically ICGVA-equipped microscopes or, even more, iCT have to be decided with care since there is no evidence so far to show superiority of one technique over the other. In our study, we investigated for the first time feasibility and usefulness of ICGVA and iCT during aneurysm surgery and we evaluated indications and limitations for each imaging method.
ICGVA has been implemented into aneurysm surgery [13, 14] and has become a routine procedure for most vascular neurosurgeons [14, 15]. It also provided valuable information in 8/10 patients of our series. Yet, even if it has the advantage that it can be easily embedded into the surgical workflow, in line with previous reports on the use of ICGVA during vascular neurosurgery [5, 14, 15], it only helped to identify the local anatomy and vascular patency of those vessels within the visual field of the operative microscope. Moreover, these problems are even more severe, if the aneurysm clip has been applied in the operative field. Therefore, these limitations may sometimes indicate an additional DSA, for example when the aneurysm or its branching vessels are located in the depth of the operative field or when the aneurysm is hidden behind relevant brain structures.
CTA has been demonstrated to be equally sensitive for detection of intracranial aneurysms larger than 3 mm [6] and it allows to display small aneurysm remnants even after clipping with adequate results [20]. Since CTA is also less invasive than DSA, all together these capabilities would render CTA an ideal method for this purpose. However, it has not been used for intraoperative imaging in this context so far [18]. In this study, intraoperative imaging using iCTA was acceptable for decision-making in 7/10 patients but failed in one patient with a previously coiled supratentorial aneurysm and in one case with an infratentorial aneurysm localisation due to beam hardening artefacts by the petrosal bone. Local conditions within the operative field like the assessment of an aneurysm remnant or clip stenosis could well be detected in these cases by iCTA as demonstrated, for example, in Fig. 2. Moreover, patency of small branching arteries >1 mm could also be assessed even near the anterior skull base, but it has to be admitted that ICGVA was of greater use in this context (Fig. 1). For all that, iCTA provided additional information in two patients in our small series of ten representative cases. In one patient, patency of the ophthalmic artery was demonstrated by its intraorbital course, which was not possible to be verified by ICGVA alone (Fig. 2, Table 1, no. 6). In another case, iCTA did correctly indicate insufficient blood flow of a branching artery after clip application, whereas ICGVA did demonstrate good fluorescence simulating sufficient blood supply for the depending brain area (Fig. 3). Remarkably, imaging quality was not reduced due to clip artefacts in the region of interest (ROI) in any patient in our cohort, even if such drawbacks have been reported [13]. Artefacts due to previously coiled aneurysms made decision making via iCTA impossible though (Fig. 1). We would therefore recommend additional iCTA if patency of the aneurysm or branching arteries cannot be definitely confirmed by ICGVA and perforating arteries have a diameter >1 mm.
In addition to the intraoperative application of CTA, which has been demonstrated to be of great use in selected and complex aneurysms, iCTP has not been investigated previously. One major advantage of iCTP is that it is performed distant from the CT plane with clips or coils, so that image quality is not degraded due to clip or coil artefacts (Fig. 3). Therefore, it yields crucial information about distant and regional blood flow in dependent areas of even small branching and perforating arteries. This was demonstrated quite obviously in one of our cases (Fig. 3, Table 1, patient no. 4), where ICGVA and micro-Doppler did not indicate haemodynamically relevant stenosis and iCTA was thought to be technically insufficient to display this small branching vessel. As a matter of fact, the neurosurgeon did not believe in the results of the iCTP, which was considered not to be of sufficient quality for detection of this small vessel by the neurosurgeon and the neuroradiologist. On the other hand, one has to keep in mind that calculation of cerebral blood volume (CBV) and cerebral blood flow (CBF), as well as time-to peak (TTP), may need up to 1 min, and the time required for proper evaluation may exceed that acceptable for decision of clip repositioning. Therefore, scanning protocols—especially for iCTP—have to be prepared during skull opening by the technician or neuroradiologist to assure that contrast medium infusion will be applicable immediately after clipping of the aneurysm. Additionally, data acquisition has to be performed by an experienced neuroradiologist and preliminary calculations or incorrect detection of the depending ROI might mislead the neurosurgeon to unjustified security (Table 1, patient no. 9). In our opinion, iCTP is therefore indicated in patients with complex or previously coiled or clipped aneurysms, especially if these are located near small branching or perforating arteries, which cannot be displayed by ICGVA or iCTA. If reconstruction of a larger aneurysm-bearing artery will be necessary, iCTP might also be helpful in order to obtain information about the regional and global blood flow of the depending brain areas. In these cases, longer acquisition time will be counterbalanced by the benefit to be able to replace the aneurysm clip in due time.
Conclusion
Combination of ICGVA and iCTA/iCTP is feasible with very good diagnostic imaging quality associated with short acquisition time and little interference with surgical workflow. Each method has its indications and limitations which have to be taken into account for intraoperative decision-making. ICGVA represents a modern standard procedure for analysis of local vessel patency, whereas iCTA/iCTP additionally supply information on dependent cerebral perfusion, which might be helpful in selected complex vascular neurosurgical cases in specialised centres.
References
Alexander TD, Macdonald RL, Weir B, Kowalczuk A (1996) Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery 39(1):10–17
Chiang VL, Gailloud P, Murphy KJ, Rigamonti D, Tamargo RJ (2002) Routine intraoperative angiography during aneurysm surgery. J Neurosurg 96(6):988–992
Dashti R, Laakso A, Niemela M, Porras M, Hernesniemi J (2009) Microscope-integrated near-infrared indocyanine green videoangiography during surgery of intracranial aneurysms: the Helsinki experience. Surg Neurol 71(5):543–550
Dehdashti AR, Thines L, Da Costa LB, terBrugge KG, Willinsky RA, Wallace MC, Tymianski M (2009) Intraoperative biplanar rotational angiography during neurovascular surgery. Technical note. J Neurosurg 111(1):188–192
de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A (2008) Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 62(6 Suppl 3):1300–1310
Doenitz C, Schebesch KM, Zoephel R, Brawanski A (2010) A mechanism for the rapid development of intracranial aneurysms: a case study. Neurosurgery 67(5):1213–1221
Faber F, Thon N, Fesl G, Rachinger W, Guckler R, Tonn JC, Schichor C (2011) Enhanced analysis of intracerebral arterioveneous malformations by the intraoperative use of analytical indocyanine green videoangiography: technical note. Acta Neurochir (Wien) 153(11):2181–2187
Friedman JA, Kumar R (2009) Intraoperative angiography should be standard in cerebral aneurysm surgery. BMC Surg 9:7
Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP, Zabramski JM, Porter RW, Albuquerque FC, McDougall CG, Fiorella DJ (2004) Comparison of routine and selective use of intraoperative angiography during aneurysm surgery: a prospective assessment. J Neurosurg 100(2):230–235
Molyneux AJ (2005) Indications for treatment of cerebral aneurysms from an endovascular perspective: the creation of an evidence base for interventional techniques. Neurosurg Clin N Am 16(2):313–316, ix
Molyneux AJ, Kerr RS (2002) The future management of subarachnoid haemorrhage. J Neuroradiol 29(2):74–75
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366(9488):809–817
Pechlivanis I, Konig M, Engelhardt M, Scholz M, Heuser L, Harders A, Schmieder K (2009) Evaluation of clip artefacts in three-dimensional computed tomography. Cen Eur Neurosurg 70(1):9–14
Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52(1):132–139
Raabe A, Beck J, Seifert V (2005) Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near-infrared video technology. Zentralbl Neurochir 66(1):1–6
Raftopoulos C, Vaz G (2011) Surgical indications and techniques for failed coiled aneurysms. Adv Tech Stand Neurosurg 36:199–226
Schichor C, Rachinger W, Morhard D, Zausinger S, Heigl TJ, Reiser M, Tonn JC (2010) Intraoperative computed tomography angiography with computed tomography perfusion imaging in vascular neurosurgery: feasibility of a new concept. J Neurosurg 112(4):722–728
Tonn JC, Schichor C, Schnell O, Zausinger S, Uhl E, Morhard D, Reiser M (2011) Intraoperative computed tomography. Acta Neurochir Suppl 109:163–167
Wachter D, Psychogios M, Knauth M, Rohde V (2010) IvACT after aneurysm clipping as an alternative to digital subtraction angiography—first experiences. Cen Eur Neurosurg 71(3):121–125
Zachenhofer I, Cejna M, Schuster A, Donat M, Roessler K (2010) Image quality and artefact generation post-cerebral aneurysm clipping using a 64-row multislice computer tomography angiography (MSCTA) technology: a retrospective study and review of the literature. Clin Neurol Neurosurg 112(5):386–391
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The comparison of the described techniques is of scientific interest and iCTA may definitely be helpful for the vascular neurosurgeon in some special situations in addition to or instead of the other techniques with their limitations. The problem may be that we do not know without intraoperative—and also somewhat time-consuming—conventional angiography which of the techniques (ICGVA or iCTA) is to be trusted during surgery. With usually limited resources, the purchase of the relatively slow and complex technique of iCTA would further increase the indirect expenses of clipping in addition to already having the expensive microscopes equipped with ICGVA. Naturally, patient safety comes first, but it is doubtful whether iCTA is a necessity in everyday practice, even in a dedicated neurovascular centres.
Mika Niemelä
Juha Hernesniemi
Helsinki, Finland
Rights and permissions
About this article
Cite this article
Schnell, O., Morhard, D., Holtmannspötter, M. et al. Near-infrared indocyanine green videoangiography (ICGVA) and intraoperative computed tomography (iCT): are they complementary or competitive imaging techniques in aneurysm surgery?. Acta Neurochir 154, 1861–1868 (2012). https://doi.org/10.1007/s00701-012-1386-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1386-1