Abstract
Intraoperative computed tomography (iCT) has gained increasing impact among modern neurosurgical techniques. Multislice CT with a sliding gantry in the OR provides excellent diagnostic image quality in the visualization of vascular lesions as well as bony structures including skull base and spine. Due to short acquisition times and a high spatial and temporal resolution, various modalities such as iCT-angiography, iCT-cerebral perfusion and the integration of intraoperative navigation with automatic re-registration after scanning can be performed. This allows a variety of applications, e.g. intraoperative angiography, intraoperative cerebral perfusion studies, update of cerebral and spinal navigation, stereotactic procedures as well as resection control in tumour surgery. Its versatility promotes its use in a multidisciplinary setting. Radiation exposure is comparable to standard CT systems outside the OR. For neurosurgical purposes, however, new hardware components (e.g. a radiolucent headholder system) had to be developed. Having a different range of applications compared to intraoperative MRI, it is an attractive modality for intraoperative imaging being comparatively easy to install and cost efficient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The development of neurosurgical techniques and the demand for treating more complex lesions with the aim of continuous reduction of surgery related morbidity has generated an increasing demand for sophisticated intraoperative imaging modalities. Various technologies are available, albeit with different indications. Intraoperative ultrasound has proven to be a rather easy to use, straight forward solution for the intraoperative localisation of deep-seated lesions like cavernomas, metastases or haemorrhages as well as, to a certain extent, for resection control of tumours. Being rather inexpensive with no additional costs of installation, the use of ultrasound is dependent on the personal experience of the user. In addition, despite improved technology, its resolution may be limited depending on the particular type of pathology [1, 2]. For the resection of high grade gliomas, the use of tissue fluorescence after administration of 5-aminolevulinic acid (5-ALA) has been proven to be efficient to delineate tumour margins and residual tumour tissue. The rate of “radicality” in the microsurgical resection of high grade gliomas has been improved significantly hereby. However, this technology is only useful for malignant gliomas, especially glioblastomas; moreover, the drug itself has so far not yet been approved by health care authorities in all parts of the world [3].
Intraoperative imaging using magnetic resonance (MR) scanners, either high field or low field, provide good visualisation of soft tissue abnormalities like gliomas, pituitary adenomas, and other tumours. However, this technology is very expensive and implementation into a pre-existing operating theatre demands a lot of resources. Moreover, the use of intraoperative MRI is limited for cranial applications due to the geometry of the scanner in relation to the table system. The surgical workflow has to be adapted to the work in a magnetic field with special demands for surgical instruments as well as anaesthesiological monitoring systems [4–6].
Recently, computed tomography (CT) technology has made a tremendous progress. Multislice scanners (e.g. 40-slice scanning systems) provide a very high image spatial resolution with very short imaging acquisition times [7]. Software for image processing has generated tools to provide high quality 3-D CT-angiography, which has been shown to provide images at a quality level superior to MR-angiography. Quantitative perfusion parameters like cerebral blood volume, blood flow and time to peak may be displayed as colour coded maps. By virtue of electronic artefact suppression and use of adequate acquisition parameters, imaging of metallic implants in spine surgery can be achieved and image distorsion can be eliminated to a large extent so that implants are precisely depicted and correction can be performed, if necessary.
For intraoperative applications, CT scanners with a wide bore are superior to those with narrow opening enabling better access to the patient. The longitudinal coverage has also been enhanced so that the whole body of the patient (from head to toe) can be scanned without repositioning. This allows intraoperative CT scanners to be used by many surgical disciplines (e.g. ENT surgeons, vascular surgeons, traumatologists, orthopaedic surgeons) in addition to neurosurgery. Thus, such an installation can be used in a multidisciplinary scenario and thereby enhancing cost effectiveness.
After the development of a setting with a scanner with a sliding gantry on rails and integrating a neuronavigation device for cranial and spinal applications, we analysed the usefulness of this setting for spinal and neurovascular surgery. Moreover, the impact on workflow was examined. Experience has been gained according to the visibility in spinal and vascular surgery as well as work flow analysis, which will be presented here [8, 9].
Methods/Technology
A 40-slice-CT scanner (Somatom Sensation Open Sliding Gantry, Siemens Healthcare, Forchheim, Germany) with a sliding gantry and a diameter of 82cm mounted on rails within the floor of the OR was installed in a pre-existing operating room. As operating table a carbon table plate, segmented to allow virtually all neurosurgical positionings, was used (Trumpf, Puchheim, Germany). The system was adjusted to a ceiling mounted navigation system (Vector Vision Sky, BrainLab, Feldkirchen, Germany). Invasive head fixation was performed with a radiolucent head clamp (Mayfield radiolucent skull clamp A-2002, Integra, Plainsborough, New Jersey, USA). For application of contrast media in case of CT angiography, a motor injection pump (Stellant MEDRAD Inc., Indianola, Pennsylvania, USA) was employed.
During image acquisition, the gantry moves over the patient without necessity to adjust the position of ventilation systems or catheters for scanning.
CT imaging was performed with a collimation of 40×0.6mm at 120kV and 140mAs with a rotation time of 1s for CTA and 20×1.2mm at 120kV and 350mAs for cranial CT. The automated dose modulation software was used. Multiplanar reconstructions (MPR) were calculated with a slice thickness of 1–3mm in axial, sagittal and coronal planes. Data could be imported into the frameless infrared–based neuronavigation system (Vector Vision Sky). For re-registration, the gantry of the scanner is equipped with fiducials equivalent to the fiducials at the head holder or the spinal fiducial clamp in case of spinal surgery.
Techniques of Intraoperative Computed Tomography Angiography (iCTA) and Perfusion Computed Tomography (PCT)
For the application of contrast agent, the injector is connected to a central line or at least 10 gauge lumen peripheral venous catheter. Monitoring of the whole procedure is secured by direct visual contact through a lead-glass window and indirectly via monitor camera from a neighboring room to get a visual control of all parts of the OR.
For iCTA a CT scout for planning of the scan range of the head and upper neck is acquired first. Then CTA is acquired in caudo-cranial scan direction from C1 to the vertex. A user modified bolus tracking technique (repeated sequential CT scans every 1s roughly at the level of the carotid bifurcations to monitor the contrast arrival at the cervical arteries, scan is then started manually when contrast enhancement in the arteries is visible) and a weight-adapted contrast agent protocol is used for CTA. This was done to obtain high contrast attenuation values in the cerebral arteries and low contrast enhancement overlay in the veins and sinus.
CTA is then followed by PCT. The scan range is manually selected to avoid beam hardening artifacts starting 1cm superior to the aneurysm clip and with a distance of at least 1cm to head clamps. Five second after injection of 50ml contrast agent (Imeron 300) at 7ml/s followed by a saline flush of 50ml at 7ml/s the PCT starts. Therefore sequential scans with 24mm slice thickness are acquired every second over a period of 40s. A standard, vendor given PCT analysis software is used for perfusion analysis. Color-coded parameter-maps of cerebral blood flow (CBF), cerebral blood volume (CBV) and time to peak (TTP) are calculated.
Results
Work Flow
After final positioning of the patient according to the envisioned procedure, a “safety check” was performed. Hereby the gantry is moved over the patient in order to detect any possible collision during the scanning procedure. A special anti-collision system prevents any conflict in case the movement of the gantry is obstructed. All positionings including complex ones like park bench were feasible; the only exception for intraoperative scanning is the semi sitting position.
In case of spinal instrumentation, pre-operative imaging was performed at that time with the data fed into the navigation system in order to allow intraoperative navigation. Hereby, the real position of the spine during surgery is the basis for navigation. This is especially valuable if luxation of the spine has to be reduced in the OR (e.g. in case of cervical instability) since any navigation on the basis of image sets obtained prior to the positioning of the patient on the OR table may be misleading.
The intraoperative examination time for vascular lesions including 3-D CT-angio and a perfusion-CT was 12min including 3min for additional draping /undraping of the patient for the scanning procedure, 1:30min for image acquisition and 3:00min for reconstruction of the data set. For spinal instrumentation a mean time frame of 9min was needed for scanning and data acquisition/image evaluation until resumption of surgery.
Radiation Exposure
The highest radiation exposure for intraoperative CT-scanning was obtained in CT angiography (including CT perfusion studies). Here, the mean effective dose of CTA and CT perfusion together was 3.69mSv. This is comparable to 3.6mSv, which is the value typically required for a 4-vessel catheter angiogram and which does not include a perfusion study [8].
Evaluation of Imaging
With CT guided spinal navigation, a computed spatial accuracy of 0.8±0.1mm could be achieved. In a first series a total of 414 screws were analysed. Intraoperative CT could detect a minor misplacement (2–4mm) in 16 screws (3.8% of all screws) and a major misplacement (>4mm) in four screws (1.0%). All misplaced screws could be re-positioned during the same surgery. Hereby, the necessity for screw revision surgery could be reduced to zero from previously 4.4% in the pre-iCT era (Fig.1). Especially in cervical spine screw placements and in the cranial cervical junction, no major misplacements of screws occurred. There was no increase of infection rate or other procedure related morbidity compared to our pre-iCT series. Duration of surgery in the cranial cervical junction was 127±21min, thoracic spine stabilisation (eight screws) 170±38min and for lumbar stabilisation (four screws) 100±24min, all including the time for imaging [10].
Vascular Neurosurgery
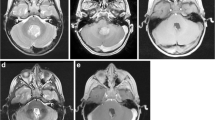
In a pilot series of neurovascular surgery, intraoperative CT angiography and intraoperative CT perfusion were performed. The image quality of CT angiograms was rated excellent by a radiologist (D.M.) in all 13 cases (Fig. 2). CT perfusion imaging was rated excellent or good in 10/11 cases, in one case artefacts resulted in major degradation of the image quality. CT angiography and CT perfusion changed the surgical strategy in two cases, in which clip repositioning, either due to residual portions of the aneurysm or because of an inadvertent occlusion of a vessel, was corrected. The image quality of iCTA and the intraoperative perfusion map were rated by the surgeon to be adequate for intraoperative decision making [8].
Discussion
The results of our studies indicate that intraoperative CT (iCT) is a promising method enhancing precision of neurosurgery and ameliorating the outcome of patients. State of the art CT technology incorporating multislice (multi detector row) systems (MSCT) allow for very short acquisition times and excellent image quality as well as intuitive multiplanar and 3D representations. Moreover, MSCT enables to perform advanced applications, such as 3-D angiography, cerebral perfusion imaging and the visualization of implants in spinal surgery. In contrast to MR-based intraoperative imaging, there is no need for dedicated surgical instruments. The system can easily be handled with no negative impact on the surgical workflow. Radiation exposure in modern MSCT-systems is acceptable as compared to conventional fluoroscopy. The learning curve for the staff including anaesthesiologists and scrub nurses as well as surgeons was very steep. Even if newly developed radiolucent head clamps with pins made of artificial sapphire were used, artefacts due to the pins may be met. Recently polymeric composite pins proved to be best suited in terms of artefact reduction with grip force comparable to standard titanium pins [11].
In comparison to intraoperative MRI, iCT is definitely superior in intraoperative spinal imaging. Moreover, iCT provides better depiction of bony structures and enables to acquire high quality CT angiography and cerebral perfusion studies. Furthermore, CT is especially useful for the control of catheter placement, e.g. in shunt surgery. MR angiography is severely limited in the depiction of vascular structures adjacent to aneurysm clips, which is clearly a major drawback of MR angiography. As a recent development for vascular imaging, intraoperative fluorescence angiography (ICG) has been shown to be extremely helpful [12]. Whether iCTA and CT perfusion might be complementary is subject of a presently ongoing study. Compared to intraoperative MRI, iCT has a reduced sensitivity in the detection and delineation of low grade gliomas and small pituitary adenomas.
Intraoperative CT imaging is a versatile, cost efficient and easy to handle – easy to install technology, with the potential of a multidisciplinary use especially in spine, skull base and vascular surgery.
Conflict of interest statement
We declare that we have no conflict of interest.
References
Rasmussen IA Jr, Lindseth F, Rygh OM, Berntsen EM, Selbekk T, Xu J, Nagelhus Hernes TA, Harg E, Håberg A, Unsgaard G (2007) Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien) 149:365–378
Roth J, Biyani N, Beni-Adani L, Constantini S (2007) Real-time neuronavigation with high-quality 3D ultrasound SonoWand in pediatric neurosurgery. Pediatr Neurosurg 43:185–191
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-Glioma Study Group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Black PM, Moriarty T, Alexander E 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA (1997) Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 41:831–842, discussion 842–845
Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R (2006) Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurol Res 28:482–487
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2005) Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 56:130–137, discussion 138
Hundt W, Rust F, Stäbler A, Wolff H, Suess C, Reiser M (2005) Dose reduction in multislice computed tomography. J Comput Assist Tomogr 29:140–147
Schichor C, Rachinger W, Morhard D, Zausinger S, Heigl TJ, Reiser M, Tonn JC (2009) Intraoperative computed tomography angiography with computed tomography perfusion imaging in vascular neurosurgery: feasibility of a new concept. J Neurosurg 112(4):722–728
Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, Schichor C, Tonn JC (2009) Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery 64:231–239, discussion 239–240
Zausinger S, Scheder B, Uhl E, Heigl T, Morhard D, Tonn JC (2009) Intraoperative computed tomography with integrated navigation system in spinal stabilizations. Spine 34(26):2919–2926
Ardeshiri A, Radina C, Edlauer M, Ardeshiri A, Riepertinger A, Nerlich A, Tonn JC, Winkler PA (2009) Evaluation of new radiolucent polymer headholder pins for use in intraoperative computed tomography. J Neurosurg 111(6):1168–1174
Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF (2005) Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103:982–989
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag/Wien
About this chapter
Cite this chapter
Tonn, J.C. et al. (2011). Intraoperative Computed Tomography. In: Pamir, M., Seifert, V., Kiris, T. (eds) Intraoperative Imaging. Acta Neurochirurgica Supplementum, vol 109. Springer, Vienna. https://doi.org/10.1007/978-3-211-99651-5_25
Download citation
DOI: https://doi.org/10.1007/978-3-211-99651-5_25
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-211-99650-8
Online ISBN: 978-3-211-99651-5
eBook Packages: MedicineMedicine (R0)