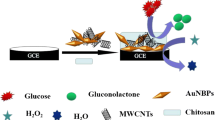
Abstract
An innovative supramolecular architecture is reported for bienzymatic glucose biosensing based on the use of a nanohybrid made of multi-walled carbon nanotubes (MWCNTs) non-covalently functionalized with a Schiff base modified with two phenylboronic acid residues (SB-dBA) as platform for the site-specific immobilization of the glycoproteins glucose oxidase (GOx) and horseradish peroxidase (HRP). The analytical signal was obtained from amperometric experiments at − 0.050 V in the presence of 5.0 × 10−4 M hydroquinone as redox mediator. The concentration of GOx and HRP and the interaction time between the enzymes and the nanohybrid MWCNT–SB-dBA deposited at glassy carbon electrodes (GCEs) were optimized through a central composite design (CCD)/response surface methodology (RSM). The optimal concentrations of GOx and HRP were 3.0 mg mL−1 and 1.50 mg mL−1, respectively, while the optimum interaction time was 3.0 min. The bienzymatic biosensor presented a sensitivity of (24 ± 2) × 102 µA dL mg−1 ((44 ± 4) × 102 µA M−1), a linear range between 0.06 mg dL−1 and 21.6 mg dL−1 (3.1 µM–1.2 mM) (R2 = 0.9991), and detection and quantification limits of 0.02 mg dL−1 (1.0 µM) and 0.06 mg dL−1 (3.1 µM), respectively. The reproducibility for five sensors prepared with the same MWCNT–SB-dBA nanohybrid was 6.3%, while the reproducibility for sensors prepared with five different nanohybrids and five electrodes each was 7.9%. The GCE/MWCNT–SB-dBA/GOx-HRP was successfully used for the quantification of glucose in artificial human urine and commercial human serum samples.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon-based nanomaterials have been largely used in biosensing applications due to their extraordinary properties. In particular, electrochemical biosensors modified with carbon nanotubes (CNTs) have demonstrated a largely improved performance [1,2,3]. However, it is important to remark that the key challenge to make possible the fully exploitation of the exceptional characteristics of carbon nanotubes is to minimize their aggregation. Consequently, different surface modifications have been implemented to modify the surface chemistry of these 1D carbon materials [4,5,6]. Among them, the non-covalent functionalization with biomolecules has proved to be a very advantageous alternative since it not only allows the exfoliation of the nanostructures, but also gives them specific properties [7,8,9,10]. Recently, we have reported the non-covalent functionalization of multi-walled carbon nanotubes (MWCNTs) with a rationally designed Schiff base modified with phenylboronic acid residues (SB-dBA) to obtain nanohybrids with properties that mimic the specific interaction of lectins with glycocompounds [11].
In this work, we present a novel strategy for the preparation of a bienzymatic glucose biosensor through the use of glassy carbon electrodes (GCEs) modified with a nanohybrid of MWCNTs non-covalently functionalized with SB-dBA as platform for the specific anchoring of the glycoproteins glucose oxidase (GOx) and horseradish peroxidase (HRP). This new approach represents an interesting alternative for the highly sensitive and selective amperometric glucose biosensing using a cheap and easy-to-prepare platform without the need of expensive biomolecules like lectins or avidin or covalent attachment to anchor the biorecognition element.
The combination of peroxidases or pseudoperoxidases and GOx has demonstrated to be an excellent alternative for the development of bi- or pseudobi-enzymatic glucose biosensors. In the last years, several electrochemical bienzymatic biosensors have been proposed for glucose determination using GOx/HRP, GOx/peroxidases other than HRP or GOx/nanozymes. Gallay et al. [12] presented a bienzymatic glucose biosensor based on the use of a GCE modified with the nanohybrid obtained by non-covalent functionalization of MWCNTs with avidin and GOx, and final anchoring of HRP. Rama et al. [13] used mass-produced stainless-steel pins modified with carbon ink to develop simple and portable amperometric glucose biosensors using GOx/HRP and ferrocyanide as electron-transfer mediator. Ortiz et al. [14] reported an amperometric glucose biosensor using MWCNTs non-covalently functionalized with concanavalin A as platform to allow the immobilization of GOx and HRP. Márquez et al. [15] described the use of an electrodeposited calcium alginate hydrogel as a biocompatible matrix for HRP and GOx immobilization using 3,3′,5,5′-tetramethylbenzidine as mediator. Amor-Gutierrez et al. [16] presented a simple and low-cost multiplexed paper-based electrochemical device for the quantification of glucose in the presence of GOx and HRP using ferrocyanide as electron-transfer mediator. Liu et al. [17] reported the sensitive glucose biosensing through the simultaneous entrapment of GOx and HRP during the HRP-catalyzed polymerization of noradrenaline in the presence of hydrogen peroxide. Wang et al. [18] proposed the use of reduced graphene oxide as platform for the immobilization of GOx, HRP, and the redox mediator poly(toluidine blue). The modification of GCE with a nanohybrid of MWCNTs functionalized with cytochrome c and GOx, using hydroquinone as redox mediator, was reported by Eguílaz et al. [19]. Izadyar et al. [20] proposed the use of GOx and a recombinant Mn peroxidase from corn for the successful bienzymatic biosensing of glucose. Gao et al. [21] presented a platform made of Au@Fe3O4 nanoparticles modified with GOx and chloroperoxidase by layer-by-layer assembly. Gallay et al. [22] reported a pseudobienzymatic glucose biosensor based on the use of GCEs modified with a nanohybrid of avidin-modified MWCNTs, biotinylated GOx, and in situ electrogenerated Ru nanoparticles to mimic peroxidase.
In the following sections, we discuss the optimization of the experimental conditions to prepare the bienzymatic amperometric biosensor using a response surface methodology (RSM) coupled with a CCRD (central composite rotatable design) matrix, the analytical performance, and the practical application of the resulting biosensor in samples of clinical relevance.
Experimental
Reagents
Glucose oxidase (GOx) (type X-S, Aspergillus niger, 50,000 units/g solid, catalog number G7141), horseradish peroxidase (HRP) (type I, ≥ 50 units/mg solid, catalog number P8125), human immunoglobulin G (IgG), human serum albumin (HSA), ascorbic acid (AA), uric acid (UA), hydroquinone (HQ), and multi-walled carbon nanotubes powder (MWCNTs, diameter (30 ± 15) nm, length 1–5 μm) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were reagent grade and used without further purification. The supporting electrolyte was 0.050 M phosphate buffer solution pH 7.40. All the aqueous solutions were prepared with ultrapure water (ρ = 18 MΩ cm) from a Millipore-MilliQ system (Molsheim, France).
Apparatus and procedure
Electrochemical experiments were performed with a TEQ_4 potentiostat. Electrochemical impedance spectroscopy (EIS) measurements were carried out with a PGSTAT30 potentiostat (Methrom, Herisau, Switzerland). The working electrode was a GCE modified with the MWCNT–SB-dBA nanohybrid, GOx, and HRP (GCE/MWCNT–SB-dBA/GOx-HRP). The auxiliary and reference electrodes were a platinum wire and a Ag/AgCl (3 M NaCl), respectively. All potentials are referred to the latter.
A TB04TA ultrasonic cleaner (Testlab, Bernal, Argentina) of 40-kHz frequency and 160 W of ultrasonic power was used for performing the sonication treatments.
Optimization of the experimental conditions using CCD-RSM design
A CCRD combined with RSM was used for the simultaneous optimization of statistically significant variables (factors) on the analytical performance of the bienzymatic amperometric glucose biosensor. In this work, a set of 17 experiments, including three replicates at the central point, were designed using CCRD with three factors (GOx concentration, HRP concentration, and interaction time) at three levels using DesignExpert version 12.0 (Stat-Ease Inc., Minneapolis, USA). The amperometric response obtained in the presence of 5.0 × 10−4 M hydroquinone and 3.6 mg dL−1 glucose was chosen as the dependent variable (response).
Preparation of glassy carbon electrode modified with MWCNT–SB-dBA nanohybrid and the glycoenzymes (GCE/MWCNT–SB-dBA/GOx-HRP)
MWCNT–SB-dBA nanohybrid was prepared according to the conditions reported by Tamborelli et al. [11]. Briefly, 2.0 mg of MWCNTs was mixed with 0.50 mg SB-dBA dissolved in 1.0 mL of N,N-dimethylformamide (DMF) followed by sonication with an ultrasonic bath for 30.0 min (Fig. 1).
Before modification, GCEs (3.0 mm diameter) were polished with slurries of different alumina size (1.0, 0.3, and 0.05 μm), followed by an exhaustive rinsing with MilliQ water, sonication for 5 s in water, and modification by deposition of 10 µL of MWCNT–SB-dBA nanohybrid. Once the solvent was evaporated at room temperature, the bienzymatic glucose biosensor was obtained by dropping 10 µL of a solution containing 3.00 mg mL−1 GOx + 1.50 mg mL−1 HRP (prepared in 0.050 M phosphate buffer pH 7.40) at the resulting GCE/MWCNT–SB-dBA, allowing to interact for 3.0 min (Fig. 1). After washing with 0.050 M phosphate buffer solution pH 7.40, the bienzymatic biosensor was ready to use.
Procedure
All electrochemical experiments were performed at room temperature in a 0.050 M phosphate buffer solution pH 7.40. Amperometric experiments were performed under mechanical stirring at − 0.050 V in the presence of 5.0 × 10−4 M hydroquinone as redox mediator.
EIS parameters were the following: amplitude: 0.010 V, frequency range: 1.0 × 10−2 to 1.0 × 105 Hz, and working potential: 0.050 V. Hydroquinone/benzoquinone (1.0 × 10−3 M, prepared in the phosphate buffer solution) was used as a redox probe. The EIS spectra were analyzed and fitted using the Z-view program.
Analytical applications for glucose quantification in samples of clinical interest
We evaluated the usefulness of the proposed bienzymatic biosensor for glucose quantification in commercial human serum samples (Standatrol S-E, Wienner lab., Rosario, Santa Fe, Argentina) and artificial human urine samples prepared according to the procedure described previously by Sarigul et al. [25].
A deproteinization step was necessary to eliminate the bilirubin interference for the amperometric determination of glucose in these serum samples [23]. The reconstituted commercial human serum samples were diluted four times with a cold mix of methanol:ethanol (1:1) and vortex-mixed for 1.0 min. Then, the samples were kept on ice bath for 20.0 min and centrifuged at 8000 rpm for 20.0 min [24]. After that, the supernatant was transferred into a vial and stored at 4 °C until further analysis.
Enriched samples of artificial human urine were obtained by adding glucose concentrations equivalent to the glucose levels detected with the reagent strips used in the clinical laboratory for testing glucose in urine samples.
Results and discussion
Specificity of the interaction between GOx and HRP with GCE/MWCNT–SB-dBA
SB-dBA have demonstrated the advantages to present a dual role, both as an exfoliation agent for MWCNTs and as a specific site for anchoring glycobiomolecules [11]. In this work, the two glycoproteins GOx and HRP were anchored at the SB-dBA that supports the MWCNTs to obtain a bienzymatic glucose biosensor.
Figure 2A displays the amperometric response obtained at GCE/MWCNT–SB-dBA and GCE/MWCNT (without the ligand) modified by interaction with 3.00 mg mL−1 GOx and 1.50 mg mL−1 HRP for 3.0 min. There is a well-defined response for successive additions of glucose at the GCE modified with MWCNT–SB-dBA nanohybrid and the enzymes (red line), while no response was obtained at GCE modified with MWCNTs (blue line) since GOx and HRP cannot be immobilized at the electrode due to the absence of the phenylboronic acid residues of the Schiff base. These results demonstrate the importance of SB-dBA as anchoring point for the specific immobilization of the two glycoenzymes used to develop the bienzymatic biosensor. Figure 2B displays a bars plot for the charge transfer resistances (RCT) obtained from the Nyquist diagrams shown as inset for GCE/MWCNT–SB-dBA and GCE/MWCNT before and after the interaction with 3.00 mg mL−1 GOx and 1.50 mg mL−1 HRP for 3.0 min using 1.0 × 10−3 M hydroquinone/benzoquinone as redox probe. The corresponding equivalent circuit used for fitting the spectra (Randles circuit) is also indicated in the inset, where Rs is the resistance of the solution, RCT corresponds to the charge transfer resistance, Cdl is the double-layer capacitance, and W the Warburg impedance. The RCT increases from (0.9 ± 0.1) × 102 Ω at GCE/MWCNT–SB-dBA (black bar, a) to (1.7 ± 0.1) × 102 Ω at GCE/MWCNT–SB-dBA/GOx-HRP (red bar, b) due to the blocking of the surface as a consequence of the non-conductive nature of the proteins, indicating that the glycoenzymes are immobilized at GCE/MWCNT–SB-dBA. In the case of GCE/MWCNT, almost no change in RCT was obtained after the interaction with 3.00 mg mL−1 GOx and 1.50 mg mL−1 HRP for 3.0 min ((0.83 ± 0.03) × 102 and (0.93 ± 0.08) × 102 Ω for GCE/MWCNT (blue bar, c) and GCE/MWCNT/GOx-HRP (green bar, d), respectively), confirming the high specificity of the glycoproteins interaction at GCE/MWCNT–SB-dBA. RCT for GCE (pink bar, e) is also shown for comparative purposes.
A Amperometric response obtained for successive additions of 3.6 mg dL−1 glucose at GCE/MWCNT–SB-dBA/GOx-HRP (red line) and GCE/MWCNT/GOx-HRP (blue line). GOx concentration: 3.00 mg mL−1. HRP concentration: 1.50 mg mL−1. MWCNT–SB-dBA···glycoenzymes interaction time: 3.0 min. Redox mediator: 5.0 × 10−4 M hydroquinone. Working potential: − 0.050 V. B Bars plot for the charge transfer resistance (RCT) obtained at GCE/MWCNT–SB-dBA (black bar, a), GCE/MWCNT–SB-dBA/GOx-HRP (red bar, b), GCE/MWCNT (blue bar, c), GCE/MWCNT/GOx-HRP (green bar, d), and GCE (pink bar, e). GOx concentration: 3.00 mg mL−1; HRP concentration: 1.50 mg mL−1; MWCNT–SB-dBA···glycoenzymes interaction time: 3.0 min. Redox probe: 1.0 × 10−3 M hydroquinone/benzoquinone. Amplitude: 0.010 V. Frequency range: 1.0 × 10−2 to 1.0 × 10.5 Hz. Working potential: 0.050 V. Inset: Nyquist plots obtained at the corresponding electrodes and the equivalent circuit used to fit the experimental results. Supporting electrolyte: 0.050 M phosphate buffer solution pH 7.40
Optimization of GCE/MWCNT–SB-dBA/GOx-HRP preparation using central composite design/response surface methodology
GOx and HRP concentrations and the interaction time of the enzymes with GCE/MWCNT–SB-dBA play a critical role on the performance of the GCE/MWCNT–SB-dBA/GOx-HRP biosensor. The statistical optimization of these independent variables was performed using a central composite rotatable design (CCRD) combined with response surface methodology (RSM) to maximize the amperometric response of 3.6 mg dL−1 glucose at − 0.050 V in the presence of 5.0 × 10−4 M hydroquinone as redox mediator. Table 1 shows the experimental design values of the three factors used in the matrix of the experiments, as well as the responses obtained (reduction currents of the redox mediator after the addition of glucose).
The relationship between the experimental response and the optimization variables can be given by the following second-order polynomial equations in terms of coded factors (Eq. 1) or actual factors (Eq. 2):
where I represents the reduction current of the redox mediator, while A, B, and C correspond to the HRP concentration, the GOx concentration, and the interaction time, respectively. Analysis of variance (ANOVA) was used to evaluate the quality of this quadratic regression model, and the results are shown in Table 2. The F value (48.17) and the remarkably low p value (< 0.0001) indicate that the proposed model is statistically significant. In addition, Fig. 3 shows that there is a very good fitting between the actual results and the currents predicted by the model.
According to the coded model (Eq. 1), HRP concentration (A) and the interaction time (C) have a negative effect since an increase in of any of them produces a decrease in the biosensor response. On the contrary, an increment in the concentration of GOx (B) produces a higher response, indicating that this variable has a positive effect. Figure 4 displays 3D surface plots for the reduction current of the redox mediator after glucose addition based on the quadratic regression model for different concentrations of HRP and GOx at three time levels (3.0, 10.5, and 18.0 min). When the GOx concentration increases from 1.50 to 3.00 mg mL−1 and HRP concentration decreases from 3.00 to 1.50 mg mL−1, the current shows a drastic enhancement, demonstrating the importance of the ratio between GOx and HRP concentrations on the amperometric response of the bienzymatic glucose biosensor. In fact, a considerably higher GOx concentration (compared to HRP) is required to obtain the most efficient cascade response, indicating that the determining step is the production of hydrogen peroxide as a byproduct of the GOx-catalyzed glucose oxidation. Regarding the interaction time, for longer times (from 3.0 to 18.0 min), the current decreases, probably due to an increase of the amount of non-conductive proteins on the electrode surface, with the consequent negative influence on the electron transfer process, and/or a competition between the glycoenzymes for the available sites at the platform due to the different sizes and density of glycosidic residues. From CCRD/RSM, the optimal level of the variables predicted to obtained the maximum amperometric current with GCE/MWCNT–SB-dBA/GOx-HRP was GOx concentration = 3.00 mg mL−1, HRP concentration = 1.50 mg mL−1, and interaction time = 3.0 min. The results presented here demonstrate that RSM is a valuable tool to obtain the most efficient biosensor response through the adequate optimization of the amount of enzymes and time to build the bienzymatic biosensor.
Amperometric experiments performed with GCE/MWCNT–SB-dBA/HRP (Figure S1,a) did not show any response after successive additions of 3.6 mg dL−1 glucose, as expected, due to the absence of GOx, the enzyme responsible to generate the hydrogen peroxide. Similar experiments performed with GCE/MWCNT–SB-dBA/GOx presented a well-defined response after the additions of glucose due to the reduction of the oxidized redox mediator (Figure S1,b). The presence of GOx and HRP at the surface of GCE/MWCNT–SB-dBA produced a response that is the double of the one obtained in the presence of GOx, demonstrating the importance of the cascade enzymatic reaction to amplify the response (Figure S1,c). The inset shows bar plot for the currents obtained from the amperometric recordings after three additions of 3.6 mg dL−1 glucose.
Analytical performance of the biosensor
Figure 5A depicts the amperometric response for successive additions of glucose obtained at GCE/MWCNT–SB-dBA/GOx-HRP at − 0.050 V in the presence of 5.0 × 10−4 M hydroquinone. A well-defined signal, due to the reduction of the quinone produced during HRP regeneration, was obtained even for 0.06 mg dL−1 glucose. Figure 5B displays the corresponding calibration plot obtained from the amperogram shown in Fig. 5A. There is a linear relationship between 0.06 mg dL−1 and 21.6 mg dL−1 (3.1 µM–1.2 mM) (R2 = 0.9991), with a sensitivity of (24 ± 2) × 102 µA dL mg−1 ((44 ± 4) × 102 µA M−1), a detection limit of 0.02 mg dL−1 (1.0 µM) (obtained as 3 × standard deviation (SD) of the blank signal/sensitivity), and a quantification limit of 0.06 mg dL−1 (3.10 µM) (obtained as 10 × standard deviation (SD) of the blank signal/sensitivity).
A Amperometric response of GCE/MWCNT–SB-dBA/HRP-GOx to successive additions of glucose. Inset: amperometric recordings obtained for additions of 0.06, 0.12, and 0.24 mg dL−1 glucose. B Calibration plot obtained from the amperometric recordings shown in (A). GOx concentration: 3.00 mg mL−1. HRP concentration: 1.50 mg mL−1. Interaction time: 3.0 min. Redox mediator: 5.0 × 10−.4 M hydroquinone. Working potential: − 0.050 V. Supporting electrolyte: 0.050 M phosphate buffer solution pH 7.40
Table 3 summarizes the analytical characteristics of the most relevant electrochemical bienzymatic glucose biosensors reported in the last years. GCE/MWCNT–SB-dBA/GOx-HRP presented a detection limit lower than most of these biosensors [13, 15, 16, 18,19,20, 22], similar to the one proposed by Gallay et al. [12], and higher than those reported by Ortiz et al. [14] and Liu et al. [17]. However, it is important to mention that [14] requires a more expensive platform based on the use of concanavalin A and [17] needs a polymerization step for the preparation of each biosensor.
The reproducibility for five biosensors prepared with the same MWCNT–SB-dBA nanohybrid (reproducibility intra-dispersion) was 6.3%, while the reproducibility for five different nanohybrids (reproducibility inter-dispersion using five electrodes for each dispersion) was 7.9%. Sensitivities obtained from amperometric experiments at − 0.050 V remained in a (90 ± 1)% of the one obtained the first day after 3 days for biosensors stored at 4 °C. The selectivity of the biosensor was evaluated in the presence of possible interferents usually present in human blood serum. Figure S2 displays the amperometric response obtained after the addition of glucose and possible interferents present in human serum at physiological level. With the exception of bilirubin, no interference was observed in the presence of the other compounds.
The biosensor was challenged with artificial human urine and human blood serum. In the case of urine, since glucose was not present in the sample, it was spiked with glucose at concentrations similar to those detected through the different colors of the commonly used laboratory reagent strips for the detection of glucose in urine: 75, 225, 450, 900, and 1800 mg dL−1. Figure 6 depicts bar plots for the recovery percentages obtained for the different glucose concentrations added to the urine sample as well as the associated error. An excellent correlation was obtained, with percentages of 94.0, 90.0, 104.3, 105.7, and 101.8, respectively, for the glucose concentrations previously indicated and errors lower than 10%. Taking into account the bilirubin interference shown in Figure S2, the reconstituted human serum samples were pretreated as indicated in the Experimental section. The glucose concentration obtained after three determinations was (88 ± 5) mg dL−1, demonstrating an excellent agreement with the value reported by Wiener laboratory (84 mg dL−1).
Bar plot for the recovery percentages and associate errors obtained from the amperometric response of GCE/MWCNT–SB-dBA/GOx-HRP in artificial urine samples enriched with the glucose concentrations detected with the urinalysis reagent strips used in the clinical laboratory. Redox mediator: 5.0 × 10−4 M hydroquinone. Working potential: − 0.050 V. Supporting electrolyte: 0.050 M phosphate buffer solution pH 7.40. Number of biosensors used for each concentration = 3
Conclusions
We proposed an innovative strategy to build an amperometric bienzymatic glucose biosensor based on the use of non-covalently functionalized MWCNTs with a new Schiff base modified with phenylboronic acid residues as a platform for the anchoring of GOx and HRP and response surface methodology to optimize the experimental conditions for the electrochemical quantification of glucose. The lectin-mimic properties of the rationally designed Schiff base offer a simple and cheap way to immobilize GOx and HRP without using expensive biomolecules like avidin or lectins. The efficient cascade transport of electrons through the enzymes and the fast charge transfer of the redox mediator at the MWCNT-modified GCE made possible the development of a highly competitive bienzymatic biosensor with successful application for the quantification of glucose in biological fluids. The effectiveness of the proposed biosensor demonstrates once more the advantages of a careful selection of the carbon nanotube functionalization agent to tune their properties and obtain a custom-made biosensing platform.
Data availability
The data will be available upon request to the corresponding author.
References
Srivastava A, Azad UP (2023) Nanobioengineered surface comprising carbon-based materials for advanced biosensing and biomedical application. Int J Biol Macromol 253:126802
Maduraiveeran G, Jin W (2021) Carbon nanomaterials: synthesis, properties and applications in electrochemical sensors and energy conversion systems. Mater Sci Eng B 272:115341
Speranza G (2021) Carbon nanomaterials: synthesis, functionalization and sensing applications. Nanomater 11(4):967
Chen R, Chen H, Peng H et al (2023) Multi-walled carbon nanotube array modified electrode with 3D sensing interface as electrochemical DNA biosensor for multidrug-resistant gene detection. Biosensors 13(8):764
Flores-Lasluisa JX, Navlani-García M, Berenguer-Murcia A et al (2024) 10 years of frontiers in carbon-based materials: carbon, the “newest and oldest” material. Story Far Front Mater 11:1381363
Eguílaz M, Dalmasso P, Rubianes MD et al (2019) Recent advances in the development of electrochemical hydrogen peroxide carbon nanotubes-based (bio)sensors. Curr Opin Electrochem 14:157–165
Primo EN, Gutierrez FA, Rubianes MD, Rivas GA (2015) Bamboo-like multiwalled carbon nanotubes dispersed in double stranded calf-thymus DNA as a new analytical platform for building layer-by-layer based biosensors. Electrochim Acta 182:391–397
Gutierrez FA, Rubianes MD, Rivas GA (2019) New bioanalytical platform based on the use of avidin for the successful exfoliation of multi-walled carbon nanotubes and the robust anchoring of biomolecules. Application for hydrogen peroxide biosensing. Anal Chim Acta 1065:12–20
Eguílaz M, Gutiérrez A, Rivas G (2016) Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: enhanced direct electron transfer and analytical applications. Sens Actuators B Chem 225:74–80
Mujica ML, Tamborelli A, Vaschetti VM et al (2022) Two birds with one stone: integrating exfoliation and immunoaffinity properties in multi-walled carbon nanotubes by non-covalent functionalization with human immunoglobulin G. Mikrochim Acta 190:73
Tamborelli A, Mujica ML, Sánchez-Velasco O et al (2024) A new strategy to build electrochemical enzymatic biosensors using a nanohybrid material based on carbon nanotubes and a rationally designed Schiff base containing boronic acid. Talanata 270:125520
Gallay P, Rubianes MD, Gutierrez FA (2019) Avidin and glucose oxidase-non-covalently functionalized multi-walled carbon nanotubes: a new analytical tool for building a bienzymatic glucose biosensor. Electroanalysis 31:1888–1894
Rama EC, Costa-García A, Fernández-Abedul MT (2017) Pin-based electrochemical glucose sensor with multiplexing possibilities. Biosens Bioelectron 8:34–40
Ortiz E, Gallay P, Galicia L et al (2019) Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with concanavalin a: a new building-block with supramolecular recognition properties for the development of electrochemical biosensors. Sens Actuators B Chem 292:254–262
Márquez A, Jiménez-Jorquera C, Domínguez C, Muñoz-Berbel X (2017) Electrodepositable alginate membranes for enzymatic sensors: an amperometric glucose biosensor for whole blood análisis. Biosens Bioelectron 97:136–142
Amor-Gutiérrez O, Costa-Rama E, Fernández-Abedul MT (2019) Sampling and multiplexing in lab-on-paper bioelectroanalytical devices for glucose determination. Biosens Bioelectron 135:64–70
Liu L, Chen C, Chen C et al (2019) Poly(noradrenalin) based bi-enzyme biosensor for ultrasensitive multi-analyte determination. Talanta 194:343–349
Wang F, Gong W, Wang L, Chen Z (2015) Enhanced amperometric response of a glucose oxidase and horseradish peroxidase based bienzyme glucose biosensor modified with a film of polymerized toluidine blue containing reduced graphene oxide. Mikrochim Acta 182:1949–1956
Eguílaz M, Venegas CJ, Gutiérrez A et al (2016) Carbon nanotubes non-covalently functionalized with cytochrome c: a new bioanalytical platform for building bienzymatic biosensors. Microchem J 128:161–165
Izadyar A, Van MN, Rodriguez KA et al (2021) A bienzymatic amperometric glucose biosensor based on using a novel recombinant Mn peroxidase from corn and glucose oxidase with a Nafion membrane. J Electroanal Chem 895:115387
Gao F, Jiang Y, Hu M et al (2016) Bienzymatic nanoreactors composed of chloroperoxidase–glucose oxidase on Au@Fe3O4 nanoparticles: dependence of catalytic performance on the bioarchitecture. Mater Des 111:414–420
Gallay P, Eguílaz M, Rivas G (2020) Designing electrochemical interfaces based on nanohybrids of avidin functionalized-carbon nanotubes and ruthenium nanoparticles as peroxidase-like nanozyme with supramolecular recognition properties for site-specific anchoring of biotinylated residues. Biosens Bioelectron 148:111764
Lolekha PH, Jaruthunyaluck S, Srisawasdi P (2001) Deproteinization of serum: another best approach to eliminate all forms of bilirubin interference on serum creatinine by the kinetic Jaffe reaction. J Clin Lab Anal 15:116–121
Obeso D, Contreras N, Dolores-Hernández M et al (2022) Development of a novel targeted metabolomic LC-QqQ-MS method in allergic inflammation. Metabolites 12:592
Sarigul N, Korkmaz F, Kurultak İ (2019) A new artificial urine protocol to better imitate human urine. Sci Rep 9:20159
Acknowledgements
The authors wish to acknowledge the financial and institutional support of CONICET (PIP 2022-11220210100957CO), ANPCyT-FONCyT (PICT 2018-03862, PICT 2019-1114), Universidad Nacional de Córdoba (PIDTA 2023), and Universidad Tecnológica Nacional (PID MSECCO0010216TC and PID MAPPBCO0008422) from Argentina; and Financiamiento Basal Program AFB220001, FONDECYT Iniciacion 11190540 and ANID-Fondecyt Grant N° 1250720 from Chile. AT, BV, and MLM acknowledge their fellowships to CONICET.
Funding
Argentina: CONICET (PIP 2022-11220210100957CO), ANPCyT-FONCyT (PICT 2018–03862, PICT 2019–1114), Universidad Nacional de Córdoba (PIDTA 2023), and Universidad Tecnológica Nacional (PID MSECCO0010216TC and PID MAPPBCO0008422).
Chile: Financiamiento Basal Program AFB220001, FONDECYT Iniciación 11190540, and ANID-Fondecyt Grant N° 1250720 from Chile.
Author information
Authors and Affiliations
Contributions
A.T.: Methodology, Formal analysis, Investigation, Visualization. V.V.: Conceptualization, Validation, Writing – review & editing, Supervision, Funding acquisition. B.V.: Methodology, Formal analysis. M.L.M.: Methodology, Formal analysis, Investigation, Visualization. S.B.:Writing – review & editing. D.V.-Y.: Writing – review & editing. P.H.-I.: Conceptualization, Resources, Writing – original draft, Writing – review & editing, Funding acquisition, Supervision. G.R.: Conceptualization, Validation, Resources, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition. P.D.: Conceptualization, Validation, Writing – review & editing, Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This research did not involve animal samples. Human serum and urine samples used were commercial and artificial, respectively.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamborelli, A., Vaschetti, V., Viada, B. et al. Multi-walled carbon nanotubes functionalized with a new Schiff base containing phenylboronic acid residues: application to the development of a bienzymatic glucose biosensor using a response surface methodology approach. Microchim Acta 191, 558 (2024). https://doi.org/10.1007/s00604-024-06608-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-024-06608-6