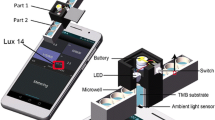
Abstract
3D-printed portable devices have immense and proven potential to transform the field of electrochemiluminescence (ECL) for diverse biochemical applications. 3D printing (3DP) offers unparalleled ability to build tiny devices in a single step with high accuracy and compatibility, and integrability as per the requirement. In this study, for the first time, a six-well 3D-printed closed bipolar electrochemiluminescence (3DP-CBPE-ECL) device has been successfully fabricated and validated by performing single-step detection of various biochemicals such as glucose and choline. Luminol/H2O2-based enzymatic reactions were performed with optimized parameters for selective sensing of glucose and choline. The single-step detection of glucose and choline was accomplished for the linear ranges of 0.1 to 10 mM and 0.1 to 5 mM, with a limit of detections (LODs) of 24 µM and 10 µM, respectively. A smartphone was leveraged to execute multiple activities such as powering the ECL device, capturing ECL images, and calculating the ECL intensity of the obtained ECL signal. The feasibility of a six-well 3DP-CBPE-ECL device was tested by sensing glucose and choline simultaneously in a single device at three different concentrations. Furthermore, the concentration of glucose and choline was calculated in real blood serum using the conventional additive (spiking) method, demonstrating the high practicability of the fabricated ECL device and yielding promising findings. Finally, based on the obtained results and other advantages such as low-cost, fast prototyping and requirement of a minimum sample volume, the fabricated six-well 3DP-CBPE-ECL device has shown potential to be used in the field of biochemical applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three-dimensional printing (3DP) is a layer-by-layer fast prototyping or additive manufacturing technique that has piqued the interest of researchers and is widely used in diverse applications including automotive, defense industries [1, 2], point-of-care testing, biochemical applications, lab-on-chip, organ printing, industrial design, and healthcare [3, 4]. When compared to traditional fabrication methods such as photolithography, 3DP fabrication methodology provides many various crucial benefits such as low-cost, fast prototyping [5], reduced manufacturing time, and complicated designs that may be easily created [6, 7]. Further, numerous biochemical sensing techniques, such as chemiluminescence, electrochemical, and electrochemiluminescence, are compatible with the 3DP technique [8, 9]. However, selecting an optimal and adaptive detection methodology to incorporate 3DP devices for biochemical sensing is critical for such sensing techniques. Amongst them, ECL, whereby electrical energy is converted to the radiative one [10,11,12], is the most promising approach since it provides major advantages such as being very selective and sensitive, having a wide operating range, having minimal background noise, and many more [13,14,15].
ECL devices with bipolar electrodes (BPEs) have been widely employed as an effective analytical tool for sensing numerous biomolecules [16, 17]. BPEs operate as conductors as they are immersed in the channel and have no direct connections to the applied external potential. As ECL-BPE devices are easily fabricated, controlled, and integrated with POCT devices, they have been employed as a powerful tool in electrochemistry [16, 18]. Generally, two types of BPE systems have been reported: open BPE-ECL (OBPE-ECL) and closed BPE-ECL (CBPE-ECL) systems [19, 20]. The open BPE-based ECL approach has two major drawbacks: very low current efficiency and background noise signals produced by driving electrodes, making it impractical to use open BPE-ECL systems in practical applications [21]. On the other hand, the closed BPE-ECL approach can improve current efficiency to 100% ideally and by placing anode and cathode of BPE into two different wells (reporting and supporting wells), the background noise from driving electrodes can easily be eliminated [22,23,24]. Considering the advantages of the CBPE-ECL system, herein, a novel six-well 3D-printed ECL device has been realized and was validated experimentally by detecting various biomolecules like glucose and choline.
Continuous monitoring of glucose and choline is essential to work the human body correctly. Hence, in the presented work, a novel six-well 3D-printed closed bipolar electrode-based ECL device was fabricated and single-step detection of glucose and choline was performed. The primary focus was to develop a portable, rapid diagnosis ECL system to detect various biomolecules accurately. Keeping that in mind, to obtain better selectivity and sensitivity, enzymatic reactions were carried out to detect glucose and choline. All the materials and equipment necessary to fabricate the six-well 3DP-CBPE-ECL system are inexpensive and widely available. Additionally, the proposed device was fabricated using a one-step manufacturing process, which avoids following the lengthy fabrication flow and sophisticated equipment.
Experimental section
Materials and equipment
Luminol, glucose, choline, glucose oxidase (GOx), and choline oxidase (COx) were obtained from Sigma Aldrich, India. As the luminol is soluble in base, in order to make luminol stock solution, the base solution was first prepared using sodium hydroxide (NaOH). The NaOH was procured from Sisco Research Laboratories, India. The same step-by-step procedure was used to make luminol stock solution, as detailed in a previously published article by our group [25]. Stock solution of luminol (10 mM) was prepared in 50 mL (47 mL luminol + 3 mL NaOH having 0.1 M concentration) DI water. Further, different concentrations of luminol (1 to 7 mM) were prepared with standard dilution method in DI water using stock solution of luminol. Similarly, stock solutions of glucose and choline (10 mM each) were prepared in DI water, and different molar concentrations were prepared using standard dilution method. Isopropanol (IPA) and dimethylforamide (DMF) were procured from SRL, India.
To fabricate ECL devices, Creator PRO Flashforge 3D printer was effectively used. Commercially available graphene filament (1.75 mm diameter) was used and procured from Black Magic 3D, USA, to fabricate the closed BPE electrodes for the six-well ECL device. The white PLA filament (1.75 mm diameter) was purchased from Amazon, India. To capture ECL signals, the Samsung Galaxy M11-12 MP smartphone was exactly placed over a 3D-printed black box at an ideal distance. A DC-DC (2.4 to 24 V) buck-boost converter was utilized to power the ECL device, essentially eliminating the need for an external power supply.
Working principle and fabrication of six-well 3DP-CBPE-ECL
Enzymatic reactions based on luminol/H2O2 have been carried out to better comprehend the working principle of the fabricated ECL device. Anode and cathode of BPE electrodes were placed in different channels. When a sufficient external voltage was provided to the ECL device, a strong electric field was formed across the closed bipolar electrode (CBPE), triggering ECL processes such as oxidation and reduction on the anode and cathode of the CBPE, respectively, resulting in the emission of an ECL signal at the anode of the CBPE. The working principle of the six-well 3DP-CBPE-ECL device with anodic, cathodic, and enzymatic reactions is depicted in Fig. 1a.
A dual-extruder FDM 3D printer was efficiently used to fabricate a six-well 3DP-CBPE-ECL. Prior to the fabrication, the ECL device design was created using computer-aided design (CAD) software-fusion 360 and later, design was saved in “.stl” format. Following that, graphene and white PLA filament were used to create electrodes and channels by adjusting the temperature (220 °C). The electrodes and device dimension are shown in Fig. 1b and c. The generated file was then stored in the “.x3g” format, which is compatible with 3D printers. Finally, the completed design was sent to a dual-extruder FDM 3D printer through a graphical user interface (GUI). Image of final fabricated six-well 3DP-CBPE-ECL device with electrodes and channels is shown in Fig. 1d.
After the device fabrication, the six-well 3DP-CBPE-ECL devices were DMF treated to enhance device performance on several fronts, such as the surface of the electrode being more porous, to achieve better detection limit [26]. All six wells of the device were filled with DMF for a 10-min optimum time [27]. The device was then cleaned with 60% diluted IPA. Finally, DMF-treated devices were allowed to dry for 6 to 7 h at room temperature.
Data acquisition and analysis
Herein, for the first time, smartphone was efficiently leveraged to carry out the following functions: (1) to power the ECL device through buck-boost converter which successfully makes the system portable and eliminates the usage of external bulky power supply, (2) to capture the ECL signals, and (3) to calculate the ECL intensity of captured signals which eliminates to use third-party software such as ImageJ and MATLAB. The ECL intensity calculator mobile app was developed in Java built-in Android Studio with read–write storage. The ECL intensity calculator android app in mobile is shown in Fig. 2b. The android app was designed in such way that it can calculate the intensity of real-time captured images or saved images in a gallery. The captured ECL image using an android app is shown in Fig. 2c. After capturing the ECL signal, a high intensity portion of an image was clipped and ECL intensity was calculated by pressing the “MEASURE” button shown on the display displayed in Fig. 2d.
Results and discussion
Characterization
The morphological changes on the surface of graphene electrodes for the fabricated ECL device were investigated using SEM analysis before and after DMF treatment. Figure 3a and b show the SEM images of graphene filaments before and after DMF treatment [28]. With optimized parameters, the performance of six-well 3DP-CBPE-ECL device was validated by doing single-step detection of glucose and choline for six different concentrations.
Analytical performance of six-well 3DP-CBPE-ECL
Before validating the analytical performance of the ECL device, it was mandatory to optimize several parameters over which ECL signal intensity was highly dependent. Primarily, in luminol/H2O2-based chemistry, ECL signal strength is dependent on parameters such as concentration of pH, luminol, and voltage. All of the optimized values in this study were obtained directly from a prior article published by our group. For validation of the six-well 3DP-CBPE-ECL, the optimal values for luminol (4 mM), pH (9), and voltage (7 V) were used. The six-well 3DP-CBPE-ECL device was validated by detecting glucose and choline in a single step. First, before the sensing of glucose and choline, the response of blank was calculated. For this, all the six wells were filled with 4-mM luminol concentration and ECL intensity was calculated. It was observed that when only luminol concentration was used, no ECL signal was obtained. Next, the glucose was detected by keeping six different concentrations in six distinct channels, as illustrated in Fig. 4a. To sense the glucose, glucose oxidase (10 mg/mL) was used and an enzymatic approach as described below was used. Experimentally, it was observed that minimum time of 3 min was required to react glucose with glucose oxidase to produce H2O2. Hence, luminol, glucose, and glucose oxidase were pipetted into the channel for an optimized time period of 3 min. The ECL signal was then achieved by applying an optimized value of external voltage (7 V) to the anode and cathode of the ECL device, and the corresponding ECL signal was successfully captured with android smartphone. ECL signal intensity for different six concentrations is shown in Fig. 4b. The single-step detection of glucose was accomplished for the linear range 0.1 to 10 mM with a limit of detection (LOD) of 24 µM.
a Schematic representation of glucose with different concentrations, b ECL intensity vs glucose, glucose = 0.1, 1, 3, 5, 7, and 10 mM, luminol = 4 mM, GOx = 10 mg/mL, applied voltage = 7 V, c schematic representation of choline with different concentrations, d ECL intensity vs choline, choline = 0.1, 0.5, 0.7, 1, 3, and 5 mM, luminol = 4 mM, COx = 10 mg/mL, applied voltage = 7 V, e simultaneous detection of glucose and choline using six channel CBPE-ECL device, f real-time ECL signal image corresponding to various concentrations of glucose and choline [N = 3]
Similarly, choline was detected by pipetting six different concentrations of choline with luminol and choline oxidase into the six distinct channels shown in Fig. 4c. Choline, luminol, and choline oxidase were pipetted into the channel for 10 min before applying an external voltage to the ECL device. Choline detection was carried out by obtaining linear range 0.1 to 5 mM with LOD 10 µM. For each different concentrations of choline, ECL signal intensity was calculated and a related linear fit graph was plotted which is shown in Fig. 4d. To test the workability of the six-well CBPE-ECL device, simultaneous detection of varied concentrations of glucose and choline was performed, and it was found that the fabricated ECL device effectively sensed both analytes at the same time. The simultaneous detection of glucose and choline was accomplished by pipetting three different concentrations of each (glucose = 0.1, 5, and 10 mM and choline = 0.1, 3, and 3 mM) into separate channels and calculated ECL intensity as indicated in Fig. 4e and f. Table 1 shows the comparative study for different ECL biosensing platforms with presented 3D-printed ECL platforms to sense glucose and choline.
Real sample analysis of glucose and choline
Finally, a real sample analysis was performed to assess the practicability of the fabricated ECL device. The original glucose concentration was estimated by intersecting the extrapolation of the fitting line with the concentration axis [33]. The original values of glucose before the sample being spiked were found to be 3.9 mM. Standard spiking (addition) method was adopted for the real sample analysis of glucose and choline. Following method was adopted to do the real sample analysis of glucose.
An unknown blood sample was collected from the medical center (BITS Pilani Hyderabad Campus, Hyderabad, India) and diluted to ten times to avoid interference. In our case, ten times dilution was performed by adding 100 µL of real sample into 900 µL of DI water. Real sample analysis was performed using the standard spiking method for different concentrations of glucose and choline, and yielded a satisfactory recovery rate.
Following method was adopted to do the real sample analysis. The real sample was diluted to 10 times i.e. In present study, 100 µL of real sample was added to 900 µL DI water. Then, known concentration of glucose (1000 µM) and diluted real sample having equal volume (30 µL each) added into well along with glucose oxidase (10 mg/mL having 20 µL volume) and ECL intensity was calculated. Recovery rate was calculated using the following formula [36].
In a similar way, real sample analysis was carried out for different concentrations of choline. In spite of rigorous literature survey, no proven clinical method to detect choline was found. Hence, herein, no clinical data for choline could be provided. Multiple experiments were carried out with the 3DP-CBPE-ECL device to detect choline in real samples but we could not get convincing results. This could be due to the fact that choline concentrations in real serum are much below the detection limit. As a result, the conventional additive approach was used to detect choline, and the results were satisfactory. The recovery rate with standard deviation for various concentrations of glucose and choline is tabulated in Table 2.
Interference study of glucose and choline with other biomolecules
The interference study of glucose and choline with other biomolecules such as ascorbic acid, uric acid, and creatinine was carried out to prove the selectivity of the device. Following method was adopted for the interference study of glucose with other biomolecules. First, known concentrations of glucose (5 mM in 30 µL), luminol (4 mM in 30 µL), and glucose oxidase (10 mg/mL in 20 µL) were pipetted into well and ECL intensity was calculated. Then, interfering compound such as uric acid (0.5 mM in 30 µL) was added to same well and ECL intensity was calculated. Less than 5% change was observed in ECL intensity which indicated that the fabricated device provided good selectivity. Similarly, an interference study of glucose with other interfering compounds (ascorbic acid, choline, and creatinine) was carried out, and the selectivity of the device was confirmed. Figure 5a and b show the interference study of glucose and choline with other interfering compounds.
Interference study: a interference study of glucose with choline, creatine, uric acid, ascorbic acid: glucose = 5 mM, choline = 1 mM, creatine = 0.5 mM, uric acid = 0.5 mM, ascorbic acid = 0.1 mM, luminol = 4 mM, GOx = 10 mg/mL, and applied voltage = 7 V. b Interference study of choline with glucose, creatine, uric acid, ascorbic acid: choline = 2 mM, glucose = 1 mM, creatine = 0.5 mM, uric acid = 0.5 mM, ascorbic acid = 0.1 mM, luminol = 4 mM, COx = 10 mg/mL, and applied voltage = 7 V, n = 3
Conclusion
In this study, a portable and compact, 3D-printed six-well closed bipolar device was fabricated and validated by performing single-step detection of glucose and choline. After performing several optimizations, the single-step detection of glucose and choline was accomplished for the linear ranges of 0.1 to 10 mM and 0.1 to 5 mM, respectively, with a LODs of 24 µM and 10 µM. Following are the main key advantages of fabricated six-well 3DP-CBPE-ECL device: (1) single-step, low-cost fabrication (3D-printing) approach was used to fabricate ECL devices, (2) most popular luminol/H2O2-based enzymatic chemical reactions were used to detect glucose and choline, and (3) the smartphone was leveraged to its full potential by executing many activities such as capturing ECL images, powering the ECL device, and calculating the ECL intensity of the obtained ECL signal. Hence, based on the results and other key important functions, the fabricated six-well 3DP-CBPE-ECL device has shown potential to employ in a variety of biochemical applications.
References
Dodziuk H (2016) Applications of 3D printing in healthcare. Kardiochirurgia i Torakochirurgia Pol 13:283–293. https://doi.org/10.5114/kitp.2016.62625
Ballard DH, Trace AP, Ali S et al (2018) Clinical applications of 3D printing: primer for radiologists. Acad Radiol 25:52–65. https://doi.org/10.1016/j.acra.2017.08.004
Chen G, Xu Y, Kwok PCL, Kang L (2020) Pharmaceutical applications of 3D printing. Addit Manuf 34:101209. https://doi.org/10.1016/j.addma.2020.101209
He Y, Wu Y, Fu JZ et al (2016) Developments of 3D printing microfluidics and applications in chemistry and biology: a review. Electroanalysis 28:1658–1678. https://doi.org/10.1002/elan.201600043
Pal A, Amreen K, Dubey SK, Goel S (2021) Highly sensitive and interference-free electrochemical nitrite detection in a 3D printed miniaturized device. IEEE Trans Nanobioscience 20:175–182. https://doi.org/10.1109/TNB.2021.3063730
Duong LH, Chen PC (2019) Simple and low-cost production of hybrid 3D-printed microfluidic devices. Biomicrofluidics 13:024108. https://doi.org/10.1063/1.5092529
Lee JY, An J, Chua CK (2017) Fundamentals and applications of 3D printing for novel materials. Appl Mater Today 7:120–133. https://doi.org/10.1016/j.apmt.2017.02.004
Bhaiyya M, Pattnaik PK, Goel S (2021) Electrochemiluminescence sensing of vitaminB 12 using laser-induced graphene based bipolar and single electrodes in a 3D-printed portable system. Microfluid Nanofluid. https://doi.org/10.1007/s10404-021-02442-x
Kumar PS, Bhand S, Das AK, Goel S (2022) Microfluidic paper device with on-site heating to produce reactive peroxide species for enhanced smartphone enabled chemiluminescence signal. Talanta 236:122858. https://doi.org/10.1016/J.TALANTA.2021.122858
Richter MM (2004). Electrochemiluminescence (ECL). https://doi.org/10.1016/B978-044453125-4.50009-7
Li H, Bouffier L, Arbault S et al (2017) Spatially-resolved multicolor bipolar electrochemiluminescence. Electrochem commun 77:10–13. https://doi.org/10.1016/j.elecom.2017.02.006
Bouffier L, Arbault S, Kuhn A, Sojic N (2016) Generation of electrochemiluminescence at bipolar electrodes: concepts and applications. Anal Bioanal Chem 408:7003–7011. https://doi.org/10.1007/s00216-016-9606-9
Chen L, Zhang C, Xing D (2016) Paper-based bipolar electrode-electrochemiluminescence (BPE-ECL) device with battery energy supply and smartphone read-out: a handheld ECL system for biochemical analysis at the point-of-care level. Sensors Actuators B Chem 237:308–317. https://doi.org/10.1016/j.snb.2016.06.105
Liu R, Zhang C, Liu M (2015) Open bipolar electrode-electrochemiluminescence imaging sensing using paper-based microfluidics. Sensors Actuators B Chem 216:255–262. https://doi.org/10.1016/j.snb.2015.04.014
Isildak I, Navaeipour F, Afsharan H et al (2020) Electrochemiluminescence methods using CdS quantum dots in aptamer-based thrombin biosensors: a comparative study. Microchim Acta 187:25. https://doi.org/10.1007/s00604-019-3882-y
Zhang X, Chen C, Li J et al (2013) New insight into a microfluidic-based bipolar system for an electrochemiluminescence sensing platform. Anal Chem 85:5335–5339. https://doi.org/10.1021/ac400805f
Wagh MD, Puneeth SB, Goel S, Sahoo SK (2021) Development of laser-induced graphene-based automated electro microfluidic viscometer for biochemical sensing applications. IEEE Trans Electron Devices 68:5184–5191. https://doi.org/10.1109/TED.2021.3107374
Guo W, Ding H, Gu C et al (2018) Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. J Am Chem Soc 140:15904–15915. https://doi.org/10.1021/jacs.8b09422
Bhaiyya M, Pattnaik PK, Goel S (2021) Simultaneous detection of vitamin B12 and vitamin C from real samples using miniaturized laser-induced graphene based electrochemiluminescence device with closed bipolar electrode. Sensors Actuators A Phys 331:112831. https://doi.org/10.1016/j.sna.2021.112831
Bhaiyya M, Pattnaik PK, Goel S (2021) ScienceDirect electrochemistry: a brief review on miniaturized electrochemiluminescence devices: from fabrication to applications. Curr Opin Electrochem 30:100800. https://doi.org/10.1016/j.coelec.2021.100800
Zhang X, Zhai Q, Xing H et al (2017) Bipolar electrodes with 100% current efficiency for sensors. ACS Sensors 2:320–326. https://doi.org/10.1021/acssensors.7b00031
Zhang R, Liang Y, Su Y et al (2021) Cloth-based closed bipolar electrochemiluminescence DNA sensors (CCBEDSs): a new class of electrochemiluminescence gene sensors. J Lumin 238:118209. https://doi.org/10.1016/j.jlumin.2021.118209
Liu M, Wang D, Liu C et al (2017) Battery-triggered open wireless electrochemiluminescence in a microfluidic cloth-based bipolar device. Sensors Actuators B Chem 246:327–335. https://doi.org/10.1016/j.snb.2017.02.076
Zhang X, Li J, Jia X et al (2014) Full-featured electrochemiluminescence sensing platform based on the multichannel closed bipolar system. Anal Chem 86:5595–5599. https://doi.org/10.1021/ac501246k
Bhaiyya M, Rewatkar P, Salve M et al (2020) Miniaturized electrochemiluminescence platform with laser-induced graphene electrodes for multiple biosensing. IEEE Trans Nanobioscience 1241:. https://doi.org/10.1109/TNB.2020.3036642
Bhaiyya M, Kulkarni MB, Pattnaik PK, Goel S (2021) IoT enabled PMT and smartphone based electrochemiluminescence platform to detect choline and dopamine using 3D‐printed closed bipolar electrodes. Luminescence 0–2. https://doi.org/10.1002/bio.4179
Manzanares Palenzuela CL, Novotný F, Krupička P et al (2018) 3D-printed graphene/polylactic acid electrodes promise high sensitivity in electroanalysis. Anal Chem 90:5753–5757. https://doi.org/10.1021/acs.analchem.8b00083
Rewatkar P, Goel S (2019) Next-generation 3D printed microfluidic membraneless enzymatic biofuel cell: cost-effective and rapid approach. IEEE Trans Electron Devices 66:3628–3635. https://doi.org/10.1109/TED.2019.2922424
Deng S, Lei J, Cheng L et al (2011) Amplified electrochemiluminescence of quantum dots by electrochemically reduced graphene oxide for nanobiosensing of acetylcholine. Biosens Bioelectron 26:4552–4558. https://doi.org/10.1016/J.BIOS.2011.05.023
Marquette CA, Blum LJ (2004) Conducting elastomer surface texturing: a path to electrode spotting: application to the biochip production. Biosens Bioelectron 20:197–203. https://doi.org/10.1016/J.BIOS.2004.01.033
Zhou Z, Xu L, Wu S, Su B (2014) A novel biosensor array with a wheel-like pattern for glucose, lactate and choline based on electrochemiluminescence imaging. Analyst 139:4934–4939. https://doi.org/10.1039/c4an00687a
Wang D, Liu C, Liang Y et al (2018) A simple and sensitive paper-based bipolar electrochemiluminescence biosensor for detection of oxidase-substrate biomarkers in serum. J Electrochem Soc 165:B361–B369. https://doi.org/10.1149/2.0551809jes
Xiao Y, Xu L, Qi LW (2017) Electrochemiluminescence bipolar electrode array for the multiplexed detection of glucose, lactate and choline based on a versatile enzymatic approach. Talanta 165:577–583. https://doi.org/10.1016/j.talanta.2017.01.019
De Poulpiquet A, Diez-Buitrago B, Dumont Milutinovic M et al (2016) Dual enzymatic detection by bulk electrogenerated chemiluminescence. Anal Chem 88:6585–6592. https://doi.org/10.1021/acs.analchem.6b01434
Rafatmah E, Hemmateenejad B (2019) Colorimetric and visual determination of hydrogen peroxide and glucose by applying paper-based closed bipolar electrochemistry. Microchim Acta 186:684. https://doi.org/10.1007/s00604-019-3793-y
Mohan JM, Amreen K, Kulkarni MB et al (2021) Optimized ink jetted paper device for electroanalytical detection of picric acid. Colloids Surf B Biointerfaces 208:112056. https://doi.org/10.1016/j.colsurfb.2021.112056
Acknowledgements
The authors would like to thank the Department of Science and Technology, Science and Engineering Research Board (DST-SERB), Government of India, Grant No. CRG/2019/005468, for funding this research work. The author would also like to acknowledge Central Analytical Laboratory (CAL), BITS Pilani Hyderabad Campus for their assistance in characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhaiyya, M., Pattnaik, P.K. & Goel, S. Multiplexed and simultaneous biosensing in a 3D-printed portable six-well smartphone operated electrochemiluminescence standalone point-of-care platform. Microchim Acta 189, 79 (2022). https://doi.org/10.1007/s00604-022-05200-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05200-0