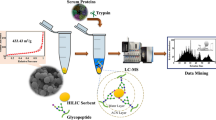
Abstract
A hydrophilic terpolymer MOF composite is designed with high surface area and porosity to enrich mono- and multi-glycosylated peptides facilitating a bottom-up approach. Terpolymer@ZIF-8 is synthesized using free radical polymerization followed by layer by layer ZIF-8 fabrication. Subsequent surface modification was made by aminophenylboronic acid (AMBA). The enrichment ability of terpolymer@ZIF-8@BA is evaluated by using tryptic digest of IgG and HRP to exemplify mono- and multi-glycosylated protein samples. Improved selectivity of 1:200 for spiked HRP in BSA digest and sensitivity down to 1 fmol μL−1 is achieved. Batch to batch reproducibility is better 1% RSD which favors the adoption of the developed method for routine N-linked glycopeptide/protein determination. Cost-effective nature of given approach is given by regeneration of the material up to four cycles. Total 318 N-linked glycopeptides have been identified from 1 μL human serum digest after subjecting the enriched and PNGase-treated deglycosylated peptides to LC-MS. Thus, terpolymer@ZIF-8@BA holds the potential both for mono- and multi-glycosylated peptides from complex biological sample.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycosylation is one of the most common post-translational modifications that occur in more than 50% eukaryotic proteins and modulate in various cellular processes [1]. Above all, it has been found that abnormal glycosylation is closely associated with numerous diseases containing cancer [2], and it has been reported that glycopeptides and glycoproteins may act as biomarkers of particular types of diseases [3, 4]. Consequently, for profound investigation of the connection among diseases and glycosylation, it is critical to know the protein glycosylation procedure, data of glycosylation sites, and important sequence of peptide in medicinal finding [5]. Although high throughput, resolution, and speed of mass spectrometry facilitate the glycosylation, the glycopeptide poor ionization and very low abundance make it a challenging task [6]. So, developing fast and high efficiency enrichment strategies to enrich selectively the N-linked glycopeptides from bio-samples prior to MS would make contributions to glycoproteomics research [7]. Different enrichment strategies which have been developed to enrich the N-linked glycopeptides include hydrazide chemistry [8], lectin affinity chromatography [9], BA (boronic acid chemistry), and hydrophilic interaction chromatography (HILIC) [10, 11]. Among all, this hydrophilic interaction chromatography (HILIC) is widely accepted due to mild enrichment conditions, unbiasness toward glycans composition, high enrichment efficacy, and compatibility to MS. Many substrates containing the hydrophilic functional groups (-COOH, -NH2, -SO3H, -OH, etc.) on their surface for N-linked glycopeptides enrichment attained great attention [12].
New nanocomposite-based HILIC sorbent for the selective enrichment of glycopeptides is in demand. Different enrichment efficacy was observed in various substrates with hydrophilic groups like mesoporous silica [13], polymeric monolithic tip [14], fibrous cellulose [15], magnetic nanoparticles [16], sepharose [17], and MOFs (metal-organic frameworks) [18, 19]. Among those, metal-organic frameworks stand out due to their ultrahigh surface specific area [20], tailorable chemistry, and high acid resistant stability and tunable nanostructure cavities [21]. Therefore, they have been applied as stationary phase in separation science. Moreover, metal-organic frameworks have been widely applied in heterogeneous catalysis [22], surface enhanced Raman spectroscopy (SERS) analysis [23], hydrogen storage [24], and so on.
So, development of high surface area and porosity polymer-MOF composite is achieved in the present study. In this context, we developed zeolitic imidazolate framework (ZIF-8) on the surface of poly(styrene-co-divinylbenzene-co-methacrylic acid) polymer. Terpolymer@ZIF-8 is further functionalized with boronic acid using 3-isocyanatopropyltriethoxysilane as precursor via N-(3 aminopropyl)imidazole, merging the qualities of the polymer with the high surface to volume area of ZIF-8 and extending the selective nature by using BA. The hybrid terpolymer@ZIF-8@BA was applied to tryptic digest of IgG as mono-glycosylated and HRP as multi-glycosylated peptide sample to determine separation capacity, reproducibility, reusability, and selectivity toward glycoproteins.
Experimental
Chemicals and reagents
The chemicals and reagents to carryout research are given in supporting information.
Fabrication of terpolymer@ZIF-8@BA
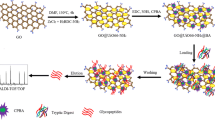
The fabrication route adopted for polymer/MOF composite involves (a) synthesis of terpolymer, (b) terpolymer/MOF composite, and (c) functionalization with boronic acid.
Synthesis of terpolymer
Detailed procedure for the terpolymer synthesis is provided in supporting information.
Terpolymer/MOF composite
Terpolymer was first incubated with 1.0 mol/L N-(3-aminopropyl)-imidazole methanol solution at 60 °C for 3 h, followed by rinsing the terpolymer with methanol until the pH value is neutral. In the next step, material was incubated with Zn (NO3)2∙6H2O at 60 °C for 3 h. After that, material was treated with 2-methylimidazole methanol solution using the same reaction condition for 1 h. Step 2 and step 3 were repeated for 1 h in 5 cycles for layer by layer synthesis. Methanol was used to rinse the column between each step. The terpolymer@MOF composite was collected.
Terpolymer@ZIF-8 was functionalized with aminophenylboronic acid to perform selective enrichment of N-linked glycopeptides. At first, 160 mg of 3-aminophenylboronic acid monohydrate was dissolved in THF. In the above solution, 3-isocyanatopropyltriethoxysilane (240 mL) was added under constant stirring for 24 h at room temperature. The obtained phenylboronic acid functionalized triethoxysilane reagent (APBA) was acidified using acetate buffer (0.1 mol L−1, pH 5.2, 40 mL) and 40 mg of terpolymer@ZIF-8 nanoparticles were dispersed homogeneously. The reaction was allowed to proceed at room temperature for 12 h. The final product (terpolymer@ZIF-8@BA) was collected and dried at 50 °C overnight.
Digestion of standard proteins
The protocol for tryptic digestion of IgG, HRP, and BSA is given in the supporting information.
Enrichment of N-linked glycopeptides
Enrichment of N-linked glycopeptides from tryptic digest of HRP and IgG is carried out using SPE batch extraction method. Terpolymer@ZIF-8@BA (3 mg) was equilibrated with 100 mL of loading buffer (50 mM NH4HCO3). The conditioned affinity material was incubated with 100 μL of digested protein sample at 37 °C for 45 min. The affinity material was collected and washed with the loading buffer to remove non-specifically bound peptides. The captured N-linked glycopeptides were eluted by 10 μL of acidic buffer (50%ACN/49%H2O/1%TFA) at 37 °C for 30 min. The eluted peptides were kept for MS analysis. In HILIC, normally during the enrichment process, loading and washing buffer composition is 50 mM ABC (ammonium bicarbonate) which is mild as compared to hydrazine and boronic acid affinities, but the elution buffer contains 1% TFA along with the 50% ACN that is also compatible with MS.
Regeneration of MOF material
To determine the regeneration ability of terpolymer@ZIF-8@BA, the composite was washed with 250 μL of 30% ACN in 0.1%TFA three times followed by de-ionized water. This removes the memory affect from previous enrichment. Prior to using regenerated material for second cycle of enrichment, it was equilibrated with loading buffer. This regenerates the surface chemistry of material. This cycle of regeneration was repeated up to 4 times.
Human serum digestion
In solution digestion of serum sample, 1.0 μL of serum was diluted with 17 μL of 25 mM ABC (ammonium carbonate) (pH = 7.8–8.0). At centrifugation at 12000g for 2 min, supernatant of diluted serum was collected. Then reduction was done by 10 m M dithiothreitol at 37 °C for 30 min, followed by alkylation using 20 mM of iodoacetamide at 37 °C for 60 min. Finally, for digestion, trypsin was added 1:30 (trypsin:protein) for 16 h at 37 °C. Trypsin was deactivated by adding 1 μL of 1% TFA and stored kept at − 20 °C.
MALDI-TOF/TOF MS analysis
One microliter of eluted fraction containing enriched N-linked glycopeptides was deposited onto 600 μm Anchor Chip Target with 1 μL of matrix solution. The matrix solution was prepared by dissolving 20 mg mL−1 of DHB in TA-30 solution (30% ACN in 0.1% TFA). The eluted peptides were directly analyzed MS as no desalting is required for this strategy. For external calibration, peptide Calibrant II Mono was also spotted. MALDI-TOF/TOF analysis was carried out on Ultraflex Extreme mass spectrometer (Bruker Daltons, Bremen, Germany). All the spectra were acquired in positive reflection mode, 1000 laser shots per spectrum. Resolution was kept 15,000–20,000. All the spectra with mass range 1000–5500 were processed using Flex Analysis Version 3.4 supplied by Bruker Daltonics.
Results and discussion
Fabrication of terpolymer@ZIF-8@BA
ZIF-8 is a member of a subfamily of MOFs and shows good mechanical stability, high porosity, and outer-surface properties [25]. These special properties make it a valuable candidate in separation and enrichment. Terpolymer offers support material to generate desired MOF on surface. The polymeric nature of terpolymer holds strong mechanical properties which endure the resilence during chemical modification. The inert nature reduces the non-specificity which is indirectly significant for selective enrichment. It further reduces agglomeration which is a common limitation in carbon-based or magnetic support materials. These properties of terploymer contribute to its selection for fabrication of terpolymer-ZIF8 composite. In this procedure, ZIF-8 is accumulated on the surface of terpolymer making its surface more available and uniting the benefits of polymer and ZIF-8. The 3-aminophenylboronic acid and triethoxysilane reagent were combined to achieve the reagent that generated a recognition site on the surface of composite. The boronic acid ligand is used to selectively recognize N-linked glycoproteins and N-linked glycopeptides. The preparation of terpolymer@ZIF-8@BA is demonstrated in Fig. 1.
Batch extraction method is adopted for enrichment as the incubation step provides enough time for selective binding of glycosylated peptides with boronic acid. Among reported affinities, boronic acid is chosen due to the reversible nature of binding [26]. The regeneration of material is easy and to consider the cost-effectiveness of research method, boronic acid is the best choice.
Characterization of terpolymer@ZIF-8@BA
Scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray (EDX), and Fourier transform infrared spectra (FTIR) are measurements of terpolymer@ZIF-8@BA measured and are shown in Fig. 2. SEM images (Fig. 2a) of the particles indicate that functionalized MOF particles are crystalline in nature with regular hexagonal morphology. TEM indicates that the single phase of ZIF-8 is obtained high crystallinity. Calculated particle size of the particles is around 55 nm. Usually the ZIF particles are properly dispersed and every crystal is separated from the other, but in this case, particles are closely attached to each other due to the presence of polymeric network to which ZIF crystals are adhered. The agglomerate sizes are approximately 1–2 times the size of particle. The loss and dispersible nature of agglomerates is also observed by TEM (Fig. 2b). Energy-dispersive X-ray spectroscopy analysis reveals that all the representative elements of terpolymer@ZIF-8@BA are present with carbon being the highest percentage (38%). This may be due to the presence of polymeric network attached to ZIF-8. Nitrogen, oxygen, and zinc are also present in the EDS spectrum (Fig. 2c). FTIR analysis shows representative peaks of OH at 3500 cm−1, CH stretching at 3050 cm−1, carbonyl groups at 1710 cm−1, and B-O at 1230 and 950 cm−1. These bands indicate the successful formation of terpolymer@ZIF-8@BA (Fig. 2d).
Porosity and surface area play significant role in enrichment as steric hindrance is reduced among bulky molecules which help to avoid saturation of affinity sites. Thus, material selective nature at low concentration is not compromised due to non-specific background. Layer by layer synthesis of MOF indicates its improved surface area during the fabrication. This is confirmed by using nitrogen adsorption porosimetry to calculate the surface area and pore size of terpolymer@ZIF-8@BA synthesized after first cycle and fifth cycle. Fig. S2A (ESM) shows the adsorption-desorption isotherm of terpolymer@ZIF-8@BA prepared by single cycle. Calculated surface of the material is 184.76 (m2 g−1), and BJH adsorption-desorption pore volume is 2.71 nm. After running 5 cycles, surface area of terpolymer@ZIF-8@BA is increased to 412 m2 g−1 with pore volume 0.075328 cm3 g−1. This sufficient increase in surface area will improve the enrichment ability of material. XRD analysis of terpolymer@ZIF-8@BA shows the peaks at 4.5°, 11°, 13°, 16.5°, 20°, 24°, 28°, 30°, 38.7°, 42°, 44.3°, 46°, and 55.4° which corresponds to carbon and zinc present in the structure Fig. S2B (Electronic supplementary material, ESM).
Enrichment of immunoglobulin G-mono-glycosylation
Human IgG analysis is important as the glycosylation is influenced by genetic makeup and health state. Variation in IgG glycosylation leads to development and progression of many diseases [27]. IgG is mono-glycosylated with conserved N-glycosylation site located at Asn180. The heterogeneity of IgG corresponds to complex type di-antennary N-linked glycans carrying 0–2 galactoses with a major fucose core and a minor having GlcNAc and 1–2 sialic acids. Therefore, the enrichment of N-linked glycopeptides generated as a result of IgG digestion will be helpful to study the variation in glycans attached to the peptide. A simple MS spectrum recorded for digested IgG shows high intensity non-specific background (Fig. 3a). The digest is applied to terpolymer@ZIF-8@BA and after elution, non-specific background is almost negligible with high intensity peaks of characteristics IgG N-linked glycopeptides (Fig. 3b). Using the peptide mass fingerprinting and BioTools, the mass peaks are identified with the attached glycans. Using terpolymer@ZIF-8@BA, 20 N-linked glycopeptides are detected with similar peptide sequence of EEQFN#STFR. The mass shift is due to the attached glycans. This indicates that the surface modification of terpolymer@ZIF-8 via boronic acid facilitate the selective enrichment of N-linked glycopeptides. The improvement in performance of designed composite relies on the enhanced hydrophilicity. The detailed information of the detected N-linked glycopeptides from IgG digest by MALDI-TOF MS analysis is listed in Table S1 (ESM).
Enrichment of HRP multi-glycosylation
Horseradish peroxidase (HRP) has broad applications in biomedical research as diagnostic enzyme. It is used as a model glycoprotein with xylosylated core and fucosylated N-glycans. The attached glycans respond to environmental factors and thus act as antigenic plant allergens. HRP contains eight glycosylation sites at Asn 43, 87, 188, 216, 228, 244, 285, and 298. Therefore, tryptic digest of HRP mimic a complex sample suitable to evaluate the enrichment efficiency of fabricated material. The digest is subjected to MS analysis and it is clear that no significant detection is observed above 3000 Da (Fig. 4a). Only four N-linked glycopeptides are detected which is insufficient to relate HRP glycosylation with respective change. Terpolymer@ZIF-8@BA is used to reduce the non-specific ground and after enrichment 17 N-linked glycopeptides can be identified above S/N ratio (Fig. 4b). The information regarding the peptide m/z, its sequence, and associated glycan is given in Table S2. It is important for the designed affinity to enrich the N-linked glycopeptides with specific glycosylation site reported for the protein under study. This is helpful to determine any modification in normal and affected protein structure and thus can be traced to malfunctioning of said protein. Sequence coverage analysis is performed using the SwissProt database and identified N-linked glycopeptides are sequenced to check the percentage coverage. It is interesting that the data obtained provides 31.42% sequence coverage with complete glycosylation site detection. All the eight glycosylation sites are recovered as shown in the sequence coverage table (Table S5). Thus, terpolymer@ZIF-8@BA is the potential affinity material that can be used to assist the HRP-based proteomic analysis.
Determination of selectivity
Spiked sample of target species in the non-specific background is used to measure the selectivity of designed material. Generally, biological fluid comprises of target molecule in low concentration with complex background. The selective nature of affinity material can be compromised in the presence of species which do have the tendency to bind non-specifically. Therefore, selectivity of terpolymer@ZIF-8@BA for N-linked glycopeptides is measured using tryptic digest of HRP and BSA digest in mass ratio of 1:1, 1:50, 1:100, 1:150, and 1:200. The enrichment protocol is adopted and MALDI-MS analysis is performed for each sample. In 1:1, all characteristic N-linked glycopeptides are observed showing negligible interference from BSA-derived non-glycopeptides (Fig. S3a, ESM). As the ratio of BSA is increased to 1:50 and 1:100, loss of one N-linked glycopeptide is observed at m/z 2361 (Fig. S3b) and 2613 (Fig. S4c). The possible loss is due to low abundance of these N-linked glycopeptides which is further lowered in the presence of BSA peptides. Increasing BSA concentration to 1:150, still HRP N-linked glycopeptides are detected above S/N ratio with loss of N-linked glycopeptides above 4500 Da (Fig. S4d). The possible reasons can be the bulky nature of N-linked glycopeptides with enhanced steric hindrance due by BSA peptides. At 1:200, the number of N-linked glycopeptides is similar to 1:150 but non-glycosylated peptides become visible below 2300 Da (Fig. S5, ESM). Thus, the selectivity experiment reflects that terpolymer@ZIF-8@BA possesses good selective recognition ability toward N-linked glycopeptides in the presence of complex surroundings. This supports the fact that MOF-based composite is more appropriate media to cultivate boronic acid affinity due to improved hydrophilic interactions.
Sensitivity experiment
The common hurdle in targeted proteome analysis is the sensitivity which limits the application of affinity material or developed method for particular biological molecule. High selectivity of terpolymer@ZIF-8@BA will be more favorable if improved sensitivity is achieved at femto molar concentrations of sample. To perform the task, four solutions of different concentrations as 1 pmol μL−1, 100 fmol μL−1, 10 fmol μL−1, and 1 fmol μL−1 are prepared using HRP digest in de-ionized water. After enrichment with terpolymer@ZIF-8@BA, HRP-derived N-linked glycopeptides are detected in MS spectra obtained. At 1 picomolar solution, there is no loss of N-linked glycopeptides Fig. S6a (Electronic supplementary material, ESM), whereas the signal intensity decreased to half when the concentration is lowered to 100 fmol μL−1 (Fig. S6b). At lower concentration down to 10 fmol μL−1, loss of two N-linked glycopeptides is observed (Fig. S7c). It must be noted that mass signals of these N-linked glycopeptides were already of low intensity and when dilution is increased, these relatively less abundant N-linked glycopeptides are not observed above S/N ratio. At 1 fmol μL−1, abundant N-linked glycopeptide peaks are detected easily (Fig. S7d). In short, the sensitivity experiment depicts that terpolymer@ZIF-8@BA has detection limit down to 1 fmol μL−1 of solution. Better sensitivity can be attributed to the hydrophilic porous structure and high surface area of the terpolymer@ZIF-8@BA as compared to reported MOF-based materials in glycoproteomics (Table 1).
Reproducibility
Batch to batch reproducibility is necessary element to determine the feasibility of designed material. Three batches of terpolymer@ZIF-8@BA are used to calculate reproducibility. The MS analysis is performed after enrichment and m/z values of seven N-linked glycopeptides derived from HRP are selected. The data is subjected to statistical analysis and relative standard deviation (RSD) is calculated (Table 2). Similar peak patterns at same intensity highlight the reproducible results obtained from MS analysis (Fig. S8a-c).
Small RSD values show data clustering around mean and thus confirm data precision as shown in Table 2.
Reusability
Cost-effective nature increases the worth of given affinity material as quality analysis requires routine enrichment experiments of target molecules. The reusability of terplymer@ZIF-8@BA is tested by applying HRP digest. After first enrichment, composite is washed with TA-30 to remove any bound peptide and nullify the memory affect. It is followed by washing with de-ionized water to neutralize the applied acidic environment. The regenerated material is equilibrated with loading buffer and subjected to second enrichment cycle. The MS analysis is performed for eluted fraction at the end of each cycle. MS spectrum of forth enrichment cycle shows that terpolymer@ZIF-8@BA enrich N-linked glycopeptides (Fig. S9) without any loss. Thus, the regeneration ability of terpolymer@ZIF-8@BA strengthen its potential application in proteomics.
N-linked glycopeptides enrichment from serum
For the glycoproteome analysis of tryptic digest of real biological sample like serum, high abundance of non-glycosylated peptide complicate the very low-abundance glycosylated peptide during the enrichment process. Based on the high sensitivity and selectivity of terpolymer@ZIF-8@BA HILIC sorbent, we employed this novel HILIC sorbent to capture the glycopeptides from tiny volume (1 μL) of digested serum. Enriched glycopeptides were deglycosylated by PNGase-F before going to nano-LC-MS analysis. A total number of 318 glycopeptides from serum glycoproteins as serotransferrin, apolipoprotein A-I, apolipoprotein B-100, protein AMBP, complement C3, haptoglobin, lactotransferrin, and trypsin-1 are identified using bottom-up approach (Table S3). Role of these enriches glycoproteins in various cancer and their glycosylation has been reported as potential highlight of cancer related to these glycoproteins (Table S4). In hepatocellular carcinomas, serotransferrin glycosylation pattern indicates the increase in highly branched fucosylated glycans [34]. Similarly, Apo-B 100 and compliment C13 has been reported for breast cancer. Elevated levels of Apo-B 100 are a risk factor for intraocular metastasis in breast cancer patients [35]. Lactotransferin, iron binding human milk glycoprotein, is reported to exhibit key role in the protection of breast-fed infants against gastrointestinal tract infections. The importance of these glycoproteins and their potential role in cancer emphasize the need to selective enrichment platform. Therefore, the terplymer@ZIF-8@BA demonstrated remarkable enrichment capability for glycopeptides present in a complex biological matrix. The fabricated terpolymer ZIF8 holds no significant disadvantage that can affect its performance as selective affinity materials toward glycopeptides.
Conclusion
Terpolymer@ZIF-8 provides hydrophilicity which is suitable for desired surface modification. The present study provides synthetic route for hydrophilic terpolymer@ZIF-8 MOF-based composite with flexible surface chemistry. High BET surface area further supports the idea of modification of MOF composite. To exemplify its hydrophilicity, the MOF composite is functionalized with boronic acid applied to enrich N-linked glycopeptides from different standard protein samples. Mono-glycosylated IgG and multi-glycosylated HRP are successfully enriched under optimized enrichment conditions. Terpolymer@ZIF-8@BA exhibits enhanced enrichment performance for glycoproteins. Its batch to batch reproducibility and regenerative nature further complies its worth for desired target less abundant molecules particularly in proteomics.
References
Xu C, Ng DT (2015) Glycosylation-directed quality control of protein folding. Nat Rev Mol Cell Biol 16(12):742–752
Chen M, Shi X, Duke RM, Ruse CI, Dai N, Taron CH, Samuelson JC (2017) An engineered high affinity Fbs1 carbohydrate binding protein for selective capture of N-glycans and N-glycopeptides. Nat Commun 8:15487
Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S (2011) Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469(7331):564–567
Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH (2014) Cell surface protein glycosylation in cancer. Proteomics 14(4–5):525–546
Liu Q, Deng CH, Sun N (2018) Hydrophilic tripeptide-functionalized magnetic metal–organic frameworks for the highly efficient enrichment of N-linked glycopeptides. Nanoscale 10(25):12149–12155
Toghi Eshghi S, Shah P, Yang W, Li X, Zhang H (2015) GPQuest: a spectral library matching algorithm for site-specific assignment of tandem mass spectra to intact N-glycopeptides. Anal Chem 87(10):5181–5188
Yao J, Sun N, Deng C (2018) Recent advances in mesoporous materials for sample preparation in proteomics research. Trends Anal Chem 99:88–100
Sajid MS, Jabeen F, Hussain D, Ashiq MN, Najam-ul-Haq M (2017) Hydrazide-functionalized affinity on conventional support materials for glycopeptide enrichment. Anal Bioanal Chem 409(12):3135–3143
Liu Y, Fu D, Yu L, Xiao Y, Peng X, Liang X (2016) Oxidized dextran facilitated synthesis of a silica-based concanavalin a material for lectin affinity enrichment of glycoproteins/glycopeptides. J Chromatogr A 1455:147–155
Yao J, Wang J, Sun N, Deng C (2017) One-step functionalization of magnetic nanoparticles with 4-mercaptophenylboronic acid for a highly efficient analysis of N-glycopeptides. Nanoscale 9(41):16024–16029
Zhang S, Tang Y, Chen Y, Zhang J, Wei Y (2020) Boronic acid-modified polyhedral oligomeric silsesquioxanes on polydopamine-coated magnetized graphene oxide for selective and high-capacity extraction of the catecholamines epinephrine, dopamine and isoprenaline. Microchim Acta 187(1):77
Saleem S, Sajid M S, Hussain D, Jabeen F, Najam-ul-Haq M, & Saeed A (2020. Boronic acid functionalized MOFs as HILIC material for N-linked glycopeptide enrichment. Anal Bioanal Chem 1-12
Liu L, Zhang Y, Zhang L, Yan G, Yao J, Yang P, Lu H (2012) Highly specific revelation of rat serum glycopeptidome by boronic acid-functionalized mesoporous silica. Anal Chim Acta 753:64–72
Sajid MS, Jovcevski B, Pukala TL, Jabeen F, Najam-ul-Haq M (2019) Fabrication of piperazine functionalized polymeric monolithic tip for rapid enrichment of glycopeptides/glycans. Anal Chem
Sajid MS, Jabeen F, Hussain D, Gardner QTAA, Ashiq MN, Najam-ul-Haq M (2020) Boronic acid functionalized fibrous cellulose for the selective enrichment of glycopeptides. J Sep Sci 43:1348–1355
Li H, Xie T, Ye L, Wang Y, Xie (2017) Core-shell magnetic molecularly imprinted polymer nanoparticles for the extraction of triazophos residues from vegetables. Microchim Acta 183:2677–2695
Yu L, Li X, Guo Z, Zhang X, Liang X (2009) Hydrophilic interaction chromatography based enrichment of glycopeptides by using click maltose: a matrix with high selectivity and glycosylation heterogeneity coverage. Chem Eur J 15(46):12618–12626
Ma W, Xu L, Li Z, Sun Y, Bai Y, Liu H (2016) Post-synthetic modification of an amino-functionalized metal–organic framework for highly efficient enrichment of N-linked glycopeptides. Nanoscale 8(21):10908–10912
Saeed A, Hussain D, Saleem S, Mehdi S, Javeed R, Jabeen F, Najam-ul-Haq M (2019) Metal–organic framework-based affinity materials in proteomics. Anal Bioanal Chem 411(9):1745–1759
Furukawa H, Ko N, Go YB, Aratani N, Choi SB, Choi E, Yaghi OM (2010) Ultrahigh porosity in metal-organic frameworks. Science 329(5990):424–428
Mohyuddin A, Hussain D, Fatima B, Athar M, Ashiq MN, Najam-ul-Haq M (2019) Gallic acid functionalized UiO-66 for the recovery of ribosylated metabolites from human urine samples. Talanta 201:23–32
Gu X, Lu ZH, Jiang HL, Akita T, Xu Q (2011) Synergistic catalysis of metal–organic framework-immobilized Au–Pd nanoparticles in dehydrogenation of formic acid for chemical hydrogen storage. J Am Chem Soc 133(31):11822–11825
Hu YL, Liao J, Wang DM, Li GK (2014) Fabrication of gold nanoparticle-embedded metal-organic framework for highly sensitive surface-enhanced raman scattering detection. Anal Chem 86:3955–3963
Zlotea C, Campesi R, Cuevas F, Leroy E, Dibandjo P, Volkringer C, Latroche M (2010) Pd nanoparticles embedded into a metal-organic framework: synthesis, structural characteristics, and hydrogen sorption properties. J Am Chem Soc 132(9):2991–2997
Bux H, Liang F, Li Y, Cravillon J, Wiebcke M, Caro J (2009) Zeolitic imidazolate framework membrane with molecular sieving properties by microwave-assisted solvothermal synthesis. J Am Chem Soc 131(44):16000–16001
Mohyuddin A, Hussain D, Najam-ul-Haq M (2017) Polydopamine assisted functionalization of boronic acid on magnetic nanoparticles for the selective extraction of ribosylated metabolites from urine. RSC Adv 7(16):9476–9483
Gudelj I, Lauc G, Pezer M (2018) Immunoglobulin G glycosylation in aging and diseases. Cell Immunol 333:65–79
Zhang YW, Li Z, Zhao Q, Zhou YL, Liu HW, Zhang XX (2014) A facilely synthesized amino-functionalized metal–organic framework for highly specific and efficient enrichment of glycopeptides. ChemComm 50(78):11504–11506
Lin H, Shao X, Lu Y, Deng C (2018) Preparation of iminodiacetic acid functionalized silica capillary trap column for on-column selective enrichment of N-linked glycopeptides. Talanta 188:499–506
Li S, Li D, Sun L, Yao Y, Yao C (2018) A designable aminophenylboronic acid functionalized magnetic Fe3O4/ZIF-8/APBA for specific recognition of glycoproteins and glycopeptides. RSC Adv 8(13):6887–6892
Sun N, Wang J, Yao J, Chen H, Deng C (2019) Magnetite nanoparticles coated with mercaptosuccinic acid-modified mesoporous titania as a hydrophilic sorbent for glycopeptides and phosphopeptides prior to their quantitation by LC-MS/MS. Microchim Acta 186(3):159
Shen YF, Yuan FF, Liu XY, Huang YP, Liu ZS (2019) Synergistic effect of organic-inorganic hybrid monomer and polyhedral oligomeric silsesquioxanes in a boronate affinity monolithic capillary/chip for enrichment of glycoproteins. Microchim Acta 186(12):812
Sun N, Wu H, Shen X (2020) Magnetic titanium dioxide nanomaterial modified with hydrophilic dicarboxylic ligand for effective enrichment and separation of phosphopeptides and glycopeptides. Microchim Acta 187(3):1–8
Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA, Rudd PM (2007) Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology 17(12):1344–1356. https://doi.org/10.1093/glycob/cwm100
Liu JX, Yuan Q, Min YL, He Y, Xu QH, Li B, Shi WQ, Lin Q, LiQ H, ZhuP W, Shao Y (2019) Apolipoprotein A1 and B as risk factors for development of intraocular metastasis in patients with breast cancer. Cancer Manag Res 11:2881–2888
Funding
This work was supported by Higher Education Commission (HEC) of Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
Serum samples were obtained from anonymous healthy males from the local hospital in accordance with government regulations and approved by the Ethical Advisory Board, Pakistan.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.98 mb)
Rights and permissions
About this article
Cite this article
Saleem, S., Sajid, M.S., Hussain, D. et al. Highly porous terpolymer-ZIF8@BA MOF composite for identification of mono- and multi-glycosylated peptides/proteins using MS-based bottom-up approach. Microchim Acta 187, 555 (2020). https://doi.org/10.1007/s00604-020-04532-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04532-z