Abstract
Amino-functionalized polyhedral oligomeric silsesquioxanes (POSS-8NH2) were covalently bound to the surface of polydopamine-coated magnetized graphene oxide. It was then reacted with 4-formylphenylboronic acid to prepare a “cubic boronic acid”-bonded magnetic graphene oxide adsorbent. The new adsorbent exhibits better selectivity and much higher adsorption capacity for ortho-phenols over adsorbents where small boronic ligands are directly bound to the surface of the material. It is shown to enable selective and faster enrichment of the catecholamines epinephrine (EP), dopamine (DA) and isoprenaline (IP) with high selectivity over many potential interferents that can occur in urine. The analytes were then quantified by HPLC with fluorometric detection. Under optimal conditions, response is linear (R2 ≥ 0.9907), limits of detection are low (0.54–2.3 ng·mL−1), and reproducibility is acceptable (inter- and intra-day assay RSDs of≤10.9%). The method was successfully applied to the determination of endogenous EP and DA and exogenous IP in urine samples.

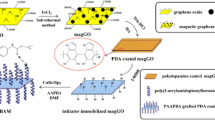
Schematic of boronic acid (BA)-modified polyhedral oligomeric silsesquioxanes (POSS) on polydopamine-coated magnetized graphene oxide (magGO). The material (magGO@POSS-BA) has good selectivity and higher adsorption capacity to ortho-phenols and can be applied to enrich the catecholamines in urine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A large number of cis-diol-containing compounds (cis-diols) and ortho-phenols play significant physiological functions and pharmacological characteristics in the body, such as glycoproteins, carbohydrates, catecholamines and nucleosides. As such, the separation and analysis of cis-diols and ortho-phenols are of great significance [1]. At present, boronate affinity chromatography is viewed as one of the effective technologies to separate and enrich cis-diols and ortho-phenols. In this technology, boronic acids on the surface of adsorbent can form five- or six-member cyclic esters with cis-diol groups in alkaline solution and these cyclic esters dissociate at acidic pH [1]. Based on this principle, boronate affinity materials (BAMs) can specifically adsorb cis-diols and ortho-phenols from the complex biological samples. Besides, boronate affinity technique is easy-to-manipulate capture/release, low cost and good compatibility with mass spectrometry. For these reasons, the boronate affinity technique has drawn increasing attention in the separation and enrichment of cis-diols and ortho-phenols.
As a key in boronate affinity technology, BAMs should possess the two features of good adsorption selectivity and high adsorption capacity. Adsorption selectivity mainly comes from the structure of boronate, whereas adsorption capacity depends on the binding amount of boronate [2]. So far, the prevailing method for preparation of BAMs is to activate the matrix with silane coupling agents (act as spacers) and then to immobilize various boronic ligands on various matrix [3]. By this method, the BAMs usually have low adsorption capacity. To increase the binding amount of boronate, the new matrix with high specific surface area tends to be used, such as porous organic and inorganic materials [2], nanoparticles [4], magnetized graphene [5] and metal-organic frameworks [6]. Besides the high specific surface area, magnetized graphene can endow the BAMs with the advantage of magnetic separation. Anyway, the adsorption capacities of this type of BAMs are usually in the range from 0.46 to 97 μmol·g−1 towards adenosine [7,8,9,10,11] and remain to be improved.
Catecholamines epinephrine (EP), dopamine (DA) and isoprenaline (IP) are three receptor agonists acting on adrenergic receptors. The former two belong to endogenous neurotransmitters released from neurons, and their levels are related with human health, and thus can serve as biomarkers for the diagnosis, therapy and prognosis of several neurological and cardiovascular disorders [12]. Whereas IP is often employed to treat bronchial asthma, cardiac arrest and atrioventricular block [13]. For these reasons, the measurement of EP, DA and IP in biological samples is of great importance. However, they are usually present in very low abundance in biological systems and co-exist with high-abundance interferents, so the effective sample pretreatment procedure is necessary before the instrument analysis. As one of the most popular sample preparation methods, solid-phase extraction has been widely used. Alumina [14], cation exchanger [15], BAMs [16], Cu(II)-immobilized [17] and reversed-phase [18] adsorbent are the present types of solid-phase extraction materials. Among various adsorbents, BAMs exhibit special selectivity towards ortho-phenols IP, EP and DA. However, the reported BAMs had either lower adsorption capacity [7,8,9,10,11], or showed slow mass transfer for the analytes (30~90 min) [16, 19], limiting the extraction performance.
This work aims to use amino-functionalized polyhedral oligomeric silsesquioxanes (POSS-8NH2) as a spacer to prepare a selective and high-capacity boronate affinity magnetized graphene oxide (magGO@POSS-BA). POSS is a family of inorganic-organic hybrid molecules which consist of a cage-like inorganic core and eight vertex groups. Each POSS molecular can provide eight reaction sites, and thus POSS reagents are ideal building blocks for functional composites. So far, various POSS reagents are usually employed as the cross-linker/monomers to prepare varieties of porous inorganic-organic hybrid polymers [16, 20, 21]. In contrary, POSS-8NH2 was employed as an amplifier. Finally, the magGO@POSS-BA was employed to extract IP, EP and DA from human urine to show the extraction efficiency of the high-capacity adsorbent.
Experimental
Materials and chemicals
Amino-functionalized polyhedral oligomeric silsesquioxanes (POSS-8NH2; 96%) was obtained from American Hybrid Plastics Ltd., USA (http://www.hybridplastics.com). Dopamine (DA, 98%), 4-formylphenylboronic acid (FPBA, 97%), catechol(99.0%), 5-hydroxytryptamine and adenosine (99%) were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China, http://www.aladdin-e.com). Quinol (99.0%), protocatechualdehyde (98%) and p-hydroxybenzaldehyde (98%) were from Sinopharm Chemical Regent Co. Ltd. (Shanghai, China, http://www.sinopharmholding.com). Ethyl theophylline and dihydroxypropyltheophylline (98%) were from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China, https://www.cnbg.com.cn). Epinephrine hydrochloride (EP, 98%) and isoprenaline hydrochloride (IP, 98%) were purchased from Xiya Reagent Ltd. (Linyi, China, http://www.xiyashiji.com). Acetic acid (HAc, 99.0%), NaBH3CN (98%) and HPLC grade methanol were supplied by Yongda Chemical Reagent Co. Ltd. (Tianjin, China, http://www.tjydhxsj.com). All other reagents were of analytical grade.
Instrumentation
The morphology and size of materials were observed by transmission electron microscopy (TEM, H-600, Hitachi, Japan, http://www.hitachi.com) and scanning electron microscope (SEM, SV8010, Hitachi, Japan, http://www.hitachi.com). The chemical composition was analyzed by Fourier-transform infrared spectrometer (FT-IR, TENSOR27, Bruker, Germany, https://www.labx.com) and X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Fisher Scientific, USA, https://www.thermofisher.com). The magnetic property was determined by a vibrating sample magnetometer (VSM, MPMS-XL-7, Quantum Design, USA, https://www.qdusa.com).
The chromatographic separation was performed on a Shimadzu HPLC system consisting of two LC-20AT pumps, a SPD-20A UV-Vis and a RF-10A fluorescence detector (Kyoto, Japan, https://www.shimadzu.com). The column (250 mm × 4.6 mm I.D., TSK-GEL ODS-100 V, 5 μm) was obtained from Tosoh Co. (Kyoto, Japan, https://www.tosoh.com). The column was maintained at 25 °C and the flow-rate of mobile phase was 1.0 mL·min−1. The injection volume was 20 μL.
Preparation of boronic acid-modified polyhedral oligomeric silsesquioxanes on polydopamine-coated magnetized graphene oxide (magGO@POSS-BA)
The preparation procedure of magGO@POSS-BA is shown in Fig. 1. Of the four steps, graphene oxide (GO), magnetized GO (magGO) and polydopamine-coated magGO (magGO@PDA) were prepared according to the reference [22].
0.2 g magGO@PDA and 0.4 g POSS-8NH2 were dispersed in 30.0 mL distilled water and the mixture was adjusted to pH 8.5 with 1.0 mol·L−1 NaOH aqueous solution. Then, the mixture was mechanically stirred for 24 h at room temperature. The magGO@POSS-NH2 was separated, washed with distilled water and ethanol under the aid of a magnet and finally dried in a vacuum.
0.2 g magGO@POSS-NH2, 0.4 g FPBA and 0.5 g NaBH3CN were dispersed in 30 mL of methanol under ultrasonication. After the mixture was stirred at room temperature for 24 h, the magGO@POSS-BA was separated via magnetic separation operation. It was washed with methanol, 5% NaHCO3 and distilled water, respectively, and finally was dried in a vacuum. The magGO@POSS-BA was prepared three times in parallel to investigate the batch-reproducibility.
Preparation of the control magGO@BA
The adsorbent without amino-functionalized polyhedral oligomeric silsesquioxane as a spacer, namely magGO@BA, was prepared as a control. Briefly, 0.2 g magGO@PDA, 0.4 g FPBA and 0.5 g NaBH3CN were dispersed in 30 mL of methanol under ultrasonication. After the mixture was stirred at room temperature for 24 h, the magGO@BA was separated and washed with methanol, 5% NaHCO3 and distilled water, respectively, and finally was dried in a vacuum.
Adsorption selectivity of the adsorbent
Three pairs and two groups of compounds were chosen to assess the selectivity of the adsorbent. Three pairs were quinol and catechol; DA and 5-hydroxytryptamine; protocatechualdehyde and p-hydroxybenzaldehyde, in which two compounds had similar molecular structure. The first group consisted of quinol, resorcinol, catechol and phenol, and the second was of DA, IP, 5-hydroxytryptamine, ethyl theophylline and dihydroxypropyltheophylline. Each pair and group of compounds were dissolved into 20 mM NH3-NH4Cl buffer (pH 8.5) to make 10.0 μg·mL−1 of each compound. Five solutions were subjected to magnet-assisted miniaturized dispersive solid-phase extraction (M-d-μSPE) procedure. Briefly, 10.0 mg of the adsorbent was dispersed in 3.0 mL of each solution. After the solution was shaken at 25 °C for 1.0 min, the adsorbent was separated and washed twice with 1 mL of NH3-NH4Cl buffer under the aid of a magnet. Next, the adsorbent was immediately immersed in 1.0 mL of 5% HAc to release the analytes. Then, the supernatant was collected under a magnetic field, and filtered with 0.45 μm membrane before HPLC analysis.
For enrichment factors (EFs) of compounds, 10.0 mg of the adsorbent and 5 mL of 0.3 μmol·mL−1 catechol, IP and adenosine were respectively subjected to the same M-d-μSPEM procedure as the above. EFs are calculated according to the following equation:
Where C0 and Ci are the concentration of the analyte in the initial solution and final supernatant, respectively.
Adsorption capacity of the adsorbent
The adsorption capacity of the adsorbent was tested by catechol, IP and adenosine. Briefly, 10.0 mg of the adsorbent was dispersed in a series of 5.0 mL standard solutions of each analyte at different concentrations in 20 mM NH3-NH4Cl buffer. After the adsorption equilibrium was reached, the supernatant was collected under a magnetic field. The supernatant was analyzed by HPLC-UV, and the adsorption capacity (Qe, μmol·g−1) is calculated according to the following equation:
Where C0 (μg·mL−1) and Ce (μg·mL−1) are the concentrations of analyte in the initial and supernatant, respectively. V (mL) is the volume of the initial solution, m (mg) is the amount of the adsorbent, and M (g·mol−1) is the molecular weight of the analyte. Adsorption isotherms were obtained by plotting of Qe against Ce.
Extraction of catecholamines from urine
The human urine samples from healthy volunteers in our group and patients from Shaanxi University of Chinese Medicine (Xi’an, China) were collected under the guidelines of the Ethics Committee of the Institute. The urine samples were stored at −20 °C before further treatment. To obtain the blank urine, the urine from a healthy volunteer was kept at 37°Cfor 48 h to degrade the endogenous catecholamines oxidatively below detectable levels. 4.9 mL of blank urine was spiked with 0.1 mL of a series of standard solutions of DA, IP and EP at different concentrations in 20 mM NH3-NH4Cl buffer to make the concentration range from 10 to 500 ng·mL−1. 0.1 mL of acetonitrile was added to the spiked solution; the solution was shaken vigorously on a rotary mixer and centrifugated at 12,863 g for 10 min to complete the deproteinization process. The supernatant was separated and adjusted to pH 8.5 with ammonium hydroxide. Then, 10.0 mg of magGO@POSS-BA was dispersed in the solution. The mixture was shaken for 1 min; the adsorbent was separated and washed twice with 1 mL of NH3-NH4Cl buffer (pH 8.5) with the aid of a magnet. Next, the adsorbent was immediately immersed in 1.0 mL of 5% HAc for 1 min. The supernatant was collected under a magnetic field and was dried under a nitrogen stream. The residue was redissolved in 0.2 mL of mobile phase and filtered with 0.45 μm membrane before HPLC with a fluorescence detector (FLD). The FLD excitation and emission wavelength were set at 280 nm and 330 nm, respectively. The calibration plots were plotted by the peak areas (y) versus the concentrations of analytes (x).
The limits of detection (LODs) and limits of quantification (LOQs) of the method are calculated using Eqs. 3 and 4, respectively:
Where σ is the standard deviation of the blank and S is the slope of calibration plot.
The extractions of three spiked urine samples with different concentration levels (20, 100 and 500 ng·mL−1) were performed under optimal conditions. The recoveries of the analytes are calculated according to the following equation:
where Ci (ng·mL−1) and Vi (mL) are the concentration and volume of the treated solution, respectively; C0 (ng·mL−1) is the spiked concentration, and V0 (mL) is the added volume.
Results and discussion
Preparation and characterization of the adsorbent
Figure 1 shows the preparation steps and the structure of magGO@POSS-BA. GO, magGO and magGO@PDA can be easily prepared according to the reference [22]. Because the coated poly(dopamine) can easily react with amines [23], POSS-8NH2 is bonded onto the magGO@PDA to produce magGO@POSS-NH2. There are two kinds of -NH2, one coming from POSS-8NH2 and the other from poly(dopamine). The -NH2 groups from the octahedral POSS-8NH2 can react with FPBA to form a “cubic boronic acid” around POSS core. Therefore, POSS amplifies boronic acids on the surface of the adsorbent. Since POSS-8NH2 is of nanoparticles, the introduction of POSS also roughens the surface of the adsorbent, alleviating the steric hindrance as the target approaches the boronate sites. As for the -NH2 groups from poly(dopamine), they can also react with FPBA. To distinguish the contribution from the two kinds of -NH2, a control magGO@BA was prepared by reacting magGO@PDA with FPBA.
In the Electronic Supplementary Material (ESM), Figs. S1 and S2 show the SEM and TEM of the adsorbents, respectively. It can be seen that GO has a layered structure with wrinkles and folds (Fig. S1a and S2a), and Fe3O4 nanoparticles with average diameters of approximately 250 nm are well dispersed on a GO sheet (Fig. S1b and S2b). After being successively modified with PDA, POSS-8NH2 and FPBA, Fe3O4 nanoparticles on the magGO@POSS-BA show a distinct core-shell structure with a shell thickness of about 20 ± 3 nm (Fig. S2c). The magnetic hysteresis loops of materials indicate that the saturation magnetization values of magGO and magGO@POSS-BA are 32.7 emu·g−1 and 26.3 emu·g−1, respectively (Fig. S3). The saturation magnetization of magGO@POSS-BA is reduced to 6.4 emu·g−1. However, the nanocomposites can still be separated from the mixture within 1 min with the aid of a magnet.
The chemical composition of materials was characterized by FT-IR and XPS (Fig. S4), and the results are discussed in ESM. From the B content of materials measured by XPS, the binding content of boronate on magGO@POSS-BA is calculated to be 5.6 mmol·g−1, being 2.5 times as much as the control magGO@BA (Table S1). All results confirm the successful preparation of magGO@POSS-BA.
Selective adsorption of ortho-phenols under a competitive environment
The adsorption selectivity of magGO@POSS-BA and magGO@BA are first evaluated with three pairs of compounds. The ortho-phenols are catechol, DA and protocatechualdehyde, and their corresponding analogs are quinol, 5-hydroxytryptamine and p-hydroxybenzaldehyde, respectively. Fig. S5 shows both adsorbents can extract the ortho-phenols efficiently while minimizing the adsorption of their analogs. Considering that the ortho-phenols often coexist with many interferents in real samples, magGO@POSS-BA was tried to capture ortho-phenols from a multicomponent mixed solution to evaluate the selectivity of the adsorbent under competitive adsorption environment. Figure 2 indicates that magGO@POSS-BA can also enrich the ortho-phenols while removing other phenols almost completely, showing a good selectivity towards ortho-phenols in a complex solution.
Chromatograms of the untreated solution (i) and the eluate treated with magGO@POSS-BA (ii). Condition: a, 50 mM HAc-methanol (75:25); b, Mobile phase is 10% methanol+90% 10 mM KH2PO4 (pH 3.0) at the first 10 min, and then was changed to 25% methanol in 5 min. UV wavelength: 280 nm. Peaks: 1, quinol; 2, resorcinol; 3, catechol; 4, phenol; 5, DA; 6, IP; 7, 5-hydroxytryptamine; 8, ethyl theophylline; 9, dihydroxypropyltheophylline
Effect of polyhedral oligomeric silsesquioxanes (POSS) on enhancing the adsorption capacity
The adsorption capacity is another crucial factor for evaluating an adsorbent. The adsorption capacities of BAMs are often determined via the adsorption isotherm method. In the structure of magGO@POSS-BA, POSS acts as the core of a “cubic boronic acid”. To highlight the effect of POSS on enhancing the adsorption capacity of the adsorbent, magGO@BA is employed as a control. Regarding that the adsorption capacities of BAMs are often characterized by catechol and adenosine, the two compounds as well as IP were selected as model analytes. From the adsorption isotherms (Fig. S6), the saturation adsorption capacities of magGO@POSS-BA are 197.3 μmol·g−1 for catechol, 137.0 μmol·g−1 for adenosine and 212.5 μmol·g−1 for IP, respectively, which are remarkably higher than those of magGO@BA (70.9 μmol·g−1 for catechol; 48.6 μmol·g−1 for adenosine; 74.7 μmol·g−1 for IP). Such high capacities are contributed to the grafting of POSS-8NH2, which greatly amplifies boronic acids on the surface of magGO@POSS-BA. As a result, the enrichment ability towards the analytes is improved. Figure 3 shows that magGO@POSS-BA can give much higher enrichment factors (EFs) towards catechol, adenosine and IP than the control magGO@BA. In Fig. 3, the used amounts of both adsorbents are the same, whereas the amounts of analytes in solution slightly exceed the amounts that magGO@POSS-BA can adsorb according to its maximum adsorption capacity. In this case, high-capacity magGO@POSS-BA can adsorb much more targets than low-capacity magGO@BA, achieving higher EF. The result suggests that it had better use the high-capacity magGO@POSS-BA in real sample because it can extract the targets more completely than the same amount of low-capacity adsorbent.
Comparison of adsorption capacity with other adsorbents
To date, there are many BAMs for the enrichment of cis-diols and ortho-phenols. According to the surface structure, the preparation methods of BAMs are generally categorized into two categories. The first kind is to bind small boronic ligands directly on various matrix, i.e. Fe3O4@SiO2, magGO, attapulgite, titania [7,8,9,10,11]. This method is popularly used, and this type of BAMs generally give the adsorption capacity in the range from 0.46 to 97.2 μmol·g−1 towards adenosine [7,8,9,10,11] and from 50 to 96 μmol·g−1 towards catechol [24, 25], respectively. In comparison with the type of BAMs, magGO@POSS-BA shows the highest adsorption capacity. The second kind is to modify boronic-functionalized polymer on the surface of the matrix. The second type of BAMs usually contain more binding sites due to the use of polymer [2, 5, 26,27,28,29], and thus some of them show higher adsorption capacities than magGO@POSS-BA [2, 5, 26]. For instance, polymer brush-modified silica [2] and magGO [5] via ATRP had adsorption capacities of 513.6 and 1111 μmol·g−1 to catechol, respectively; polymer-modified monolith via two-step ATRP had adsorption capacity up to 303 μmol·g−1 towards catechol [26]. However, the three polymer-modified BAMs were rather difficult to make as ATRP reaction needs an anaerobic operation in the preparation procedure, and polymer-modified BAMs usually provide low mass-transfer [2, 16]. For polymer brush-modified silica, it took 60 min to reach the adsorption equilibrium [2]. Feng’ group also prepared a BAM via co-polymerization of octavinyl POSS and 3-acrylamidophenylboronic acid on the surface of Fe3O4 nanoparticles [16]. In their work, octavinyl POSS acted as the cross-linker, and the enrichment of analytes needed extraction time of 60 min and desorption time of 30 min. However, our adsorbent with POSS-8NH2 as amplification scaffold is structurally of the first type and has fast mass-transfer, so it can finish the extraction process within 1.0 min, being more faster over the polymer-modified adsorbents. Overall, magGO@POSS-BA shows lower adsorption capacity than some of the second type of BAMs, but it is easily made and can extract the analytes rather fast. Based on the boronic affinity principle, BAMs can specifically adsorb ortho-phenols and cis-diols, i.e., catecholamines and cis-diol-containing biomolecules, glycoproteins, saccharides and nucleosides [1]. Predictably, magGO@POSS-BA can also extract these substances quickly. In the following, magGO@POSS-BA is employed to extract catecholamines from the urine samples to show its potential in the pretreatment of complex biological sample.
Reusability and inter-batch reproducibility of boronic acid-modified polyhedral oligomeric silsesquioxanes on polydopamine-coated magnetized graphene oxide (magGO@POSS-BA)
It was reported that POSS-8NH2 is unstable in water due to the hydrolysis but the rate is rather slow. Adding longer aliphatic chains in POSS-8NH2 can protect the siloxane cages and can better prevent the degradation of POSS cage [30]. Consequently, the stability of magGO@POSS-BA in water is improved significantly due to the introduction of phenylboronic in POSS-8NH2. To prove the stability and assess the recycle of magGO@POSS-BA, it was subjected to magnet-assisted miniaturized dispersive solid-phase extraction (M-d-μSPE) for eight cycles. The recoveries of EP are shown in Fig. S7. The recovery of EP only drops by 9.1% after eight cycles of M-d-μSPE. To evaluate the batch-reproducibility, magGO@POSS-BA was prepared three times in parallel, and the adsorption capacities of three batches of adsorbents were determined (Table S2). The RSDs for the adsorption capacities are 8.8% for catechol, 4.8% for IP and 11.8% for adenosine, respectively. Since there are four steps in the preparation of adsorbents, such RSDs are acceptable. These results indicate the adsorbent is reproducible and recyclable for use as an eligible adsorbent.
Extraction conditions of catecholamines
Since EP, DA and IP are ortho-phenols, they can be enriched by magGO@POSS-BA. Firstly, the following parameters affecting enrichment performance were optimized: (a) pH value of the sample solution; (b) type of eluent; (c) adsorption time; (d) desorption time; (e) adsorbent dosage. Respective data and Figures are given in Fig. S8, and the detail discussion is in ESM. Finally, the following experimental conditions are found to give best results: pH of sample solution is 8.5; 5% HAc aqueous solution is selected as eluent; adsorption and desorption time are 1 min, and the adsorbent dosage is 10 mg for 3.0 mL of the sample solution, respectively.
Validation of magnet-assisted miniaturized dispersive solid-phase extraction (M-d-μSPE) coupled with HPLC with fluorometric detection
To achieve reliable quantification, it is desired to establish calibration plots by using a matched matrix that spiked with standard analytes. According to the reported literatures [31], there is a normal range for endogenous DA and EP in the human urine. Therefore, endogenous DA and EP must be removed in the matched matrix, otherwise their presence can result in a false positive value. Zhang et al. reported that the blank plasma was prepared by degrading oxidatively the endogenous catecholamines in plasma [14]. This method is feasible because endogenous DA and EP are of ortho-phenols and can be easily oxidized in the solution. Similarly, this method was employed to prepare the blank urine samples to well-match the real urine matrix. Then, the calibration plots were established using the spiked samples for quantification.
Analytical performance characteristics of the method, including linear range, LODs, LOQs, recovery and precision, were performed to validate the analytical method (Table S3 -S5). The linear range is between 10 and 500 ng·mL−1 for three targets with a correlation coefficient greater than 0.9907. LODs are calculated to be 0.54 ng·mL−1 for EP, 2.28 ng·mL−1 for DA and 0.73 ng·mL−1 for IP, respectively. The recoveries of the method were measured by analyzing the spiked human urine at three different concentrations (20, 100 and 500 ng·mL−1). Table S4 and S5 show that the intra- and inter-day assay recoveries are in the range of 85.7–101.7% with the RSDs less than 10.9%. The values of precision and accuracy meet the requirement that RSDs should not exceed 15% for bioanalytical test methods according to the international recommendation [32].
To evaluate the application of the M-d-μSPE-HPLC system in the analysis of biological samples, the urine samples from three healthy volunteers and one patient were treated by the M-d-μSPE and analyzed by HPLC-FLD. Figure 4 is the typical chromatograms of the untreated urine, extracted urine and standard solution. For the untreated urine, very sensitive FLD was employed, but there are still so many interfere peaks that the quantitative determinations of EP, DA and IP are disturbed. After extraction with magGO@POSS-BA, the peaks of the targets are strengthened and most interfering peaks are eliminated, indicating the contribution of enrichment protocol in the removal of interferents from the complicated biological samples. Table 1 is the concentrations of three analytes in the urine of the volunteers. It was reported that DA is usually from ten to one hundred ng·mL−1, and EP is from a few to dozens ng·mL−1 in the urine of health people [14, 31]. The mean values of EP and DA for three healthy volunteers are in good agreement with the normal levels, and are also similar to those obtained previously by other researchers [14, 16]. For healthy people, there is no IP in urine, whereas IP in the urine of the patient is found to be 4.3 ng·mL−1 at about 2 h after 10 mg of IP was taken via sublingual administration. These results indicate our method is potential in the analysis of endogenous EP and DA and exogenous IP in urine.
Chromatograms of (i) urine sample spiked with 100 ng·mL−1, (ii) eluate of the spiked urine sample after extraction by magGO@POSS-BA and (iii) standard solution of EP, DA and IP. Mobile phase: methanol (solvent A) and 10 mM NaH2PO4 (solvent B), gradient: 2% A in 0–5 min, then 2% A-15% A within 10 min. The FLD excitation and emission wavelength were set at 280 nm and 330 nm, respectively. Peaks: 1. EP; 2. DA; 3. IP
Table 2 lists a comparison between the method and other methods in the literature. In comparison, the practicability and superiority of the method are considered. The precision and accuracy of the method are comparable to most references. The linear range of the method is also wider and covers the normal range, especially the abnormal higher concentration range. When it comes to LODs, the proposed method is comparable or superior to most of the methods except that using more sensitive mass spectrometer detector [14]. Such a low LOD completely meets the analysis of EP, DA and IP in urine. In particular, the costed time (only 2 min) of the method is greatly shorter than the previously reported methods, favoring the rapid analysis of sample in clinical diagnosis. Therefore, our method has potential in the analysis of complicated biological samples.
Conclusions
A high-capacity boronate affinity adsorbent was constructed by taking advantage of GO and the POSS-8NH2. The special structure of POSS-8NH2 plays an important role in the improvement of adsorption capacity as well as the mass transfer of analytes. This method using POSS as an amplifier can be extended to the preparation of other affinity and ion-exchange adsorbents since these two types of adsorbents have special requirements for high capacity. As an application of the new adsorbent, the magnet-assisted miniaturized dispersive solid-phase extraction (M-d-μSPE-HPLC) is a fast, selective and sensitive method in the determination of catecholamines in human urine. Because of the same boronic affinity principle, magGO@POSS-BA has also potential in the extraction of glycoproteins, saccharides, and nucleosides from the real biological sample.
References
Li DJ, Chen Y, Liu Z (2015) Boronate affinity materials for separation and molecular recognition: structure, properties and applications. Chem Soc Rev 44:8097–8123. https://doi.org/10.1039/C5CS00013K
Wang W, He MF, Wang CZ, Wei YM (2015) Enhanced binding capacity of boronate affinity adsorbent via surface modification of silica by combination of atom transfer radical polymerization and chain-end functionalization for high-efficiency enrichment of cis-diol molecules. Anal Chim Acta 886:66–74. https://doi.org/10.1016/j.aca.2015.06.015
Chen Y, Huang AL, Zhang YN, Bie ZJ (2019) Recent advances of boronate affinity materials in sample preparation. Anal Chim Acta 1076:1–17. https://doi.org/10.1016/j.aca.2019.04.050
Jiang LW, Chen YB, Luo YM, Tan YM, Ma M, Chen B, Xie QJ, Luo XB (2015) Determination of catecholamines in urine using aminophenylboronic acid functionalized magnetic nanoparticles extraction followed by high-performance liquid chromatography and electrochemical detection. J Sep Sci 38:460–467. https://doi.org/10.1002/jssc.201400920
Deng YN, Gao Q, Ma J, Wang CZ, Wei YM (2018) Preparation of a boronate affinity material with ultrahigh binding capacity for cis-diols by grafting polymer brush from polydopamine-coated magnetized graphene oxide. Microchim Acta 185:189–196. https://doi.org/10.1007/s00604-018-2732-7
Wu YL, Liu QJ, Xie YQ, Deng CH (2018) Core-shell structured magnetic metal-organic framework composites for highly selective enrichment of endogenous N-linked glycopeptides and phosphopeptides. Talanta 190:298–312. https://doi.org/10.1016/j.talanta.2018.08.010
He HB, Sun YR, Li B, Yu QW, Wang TL, Feng YQ (2013) Boronate affinity solid-phase extraction based on functionalized magnesia-zirconia composite for enrichment of nucleosides in human urine. Anal Methods 5:1435–1441. https://doi.org/10.1039/C2AY26420J
Wang ST, Chen D, Ding J, Yuan BF, Feng YQ (2013) Borated titania, a new option for the selective enrichment of cis-diol biomolecules. Chem Eur J 19:606–612. https://doi.org/10.1002/chem.201203109
Li HH, Zhu SQ, Cheng T, Wang SX, Zhu B, Liu XY, Zhang HX (2016) Binary boronic acid-functionalized attapulgite with high adsorption capacity for selective capture of nucleosides at acidic pH values. Microchim Acta 183:1779–1786. https://doi.org/10.1007/s00604-016-1808-5
Du J, He MF, Wang XM, Fan H, Wei YM (2015) Facile preparation of boronic acid-functionalized magnetic nanoparticles with a high capacity and their use in the enrichment of cis-diol-containing compounds from plasma. Biomed Chromatogr 29:312–320. https://doi.org/10.1002/bmc.3277
Pan YN, Gao XM, Li SS, Liu XY, Zhang HX (2018) A boronate-decorated porous carbon material derived from a zinc-based metal-organic framework for enrichment of cis-diol-containing nucleosides. New J Chem 42:2288–2294. https://doi.org/10.1039/C7NJ04575A
Hyland K (1999) Presentation, diagnosis, and treatment of the disorders of monoamine neurotransmitter metabolism. Semin Perinatol 23:194–203. https://doi.org/10.1016/S0146-0005(99)80051-2
Elesber A, Nishimura RA, Rihal CS, Ommen SR, Schaff HV, Holmes DR (2017) Utility of isoproterenol to provoke outflow tract gradients in patients with hypertrophic cardiomyopathy. Am J Cardiol 120:338. https://doi.org/10.1016/j.amjcard.2016.09.005
Zhang GD, Zhang YZ, Ji CJ, McDonald T, Walton J, Groeber EA, Steenwyk RC, Lin ZS (2012) Ultra sensitive measurement of endogenous epinephrine and norepinephrine in human plasma by semi-automated SPE–LC–MS/MS. J Chromatogr B 895–896:186–190. https://doi.org/10.1016/j.jchromb.2012.03.026
Raggi MA, Sabbioni C, Nicoletta G, Mandrioli R, Gerra G (2003) Analysis of plasma catecholamines by liquid chromatography with amperometric detection using a novel SPE ion-exchange procedure. J Sep Sci 26:1141–1146. https://doi.org/10.1002/jssc.200301486
He HB, Zhou ZQ, Dong C, Wang X, Yu QW, Lei YY, Luo LQ, Feng YQ (2016) Facile synthesis of a boronate affinity sorbent from mesoporous nanomagnetic polyhedral oligomeric silsesquioxanes composite and its application for enrichment of catecholamines in human urine. Anal Chim Acta 944:1–13. https://doi.org/10.1016/j.aca.2016.09.012
He MF, Wang CZ, Wei YM (2016) Selective enrichment and determination of monoamine neurotransmitters by cu (II) immobilized magnetic solid phase extraction coupled with high-performance liquid chromatography-fluorescence detection. Talanta 147:437–444. https://doi.org/10.1016/j.talanta.2015.10.017
Talwar D, Williamson C, McLaughlin A, Gill A, O’Reilly DSJ (2002) Extraction and separation of urinary catecholamines as their diphenyl boronate complexes using C18 solid-phase extraction sorbent and high-performance liquid chromatography. J Chromatogr B 769:341–349. https://doi.org/10.1016/S1570-0232(02)00022-3
Xu HH, Wang CZ, Wei YM (2018) A boronate affinity restricted-access material with external hydrophilic bottlebrush polymers for pretreatment of cis-diols in biological matrices. Chin Chem Lett 29:521–523. https://doi.org/10.1016/j.cclet.2017.08.056
Alves F, Scholder P, Nischang I (2013) Conceptual design of large surface area porous polymeric hybrid media based on polyhedral oligomeric silsesquioxane precursors: preparation, tailoring of porous properties, and internal surface functionalization. ACS Appl Mater Interfaces 5:2517–2526. https://doi.org/10.1021/am303048y
Zhang Y, Zhuang YT, Shen HY, Chen XW, Wang JH (2017) A super hydrophilic silsesquioxane-based composite for highly selective adsorption of glycoproteins. Microchim Acta 184(4):1037–1044. https://doi.org/10.1007/s00604-017-2100-z
Wang XY, Song GX, Deng CH (2015) Development of magnetic grapheme @hydrophilic polydopamine for the enrichment and analysis of phthalates in environmental water samples. Talanta 132:753–759. https://doi.org/10.1016/j.talanta.2014.10.014
LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11:1214–1221. https://doi.org/10.1038/nm1314
Li QJ, Lü CC, Li HY, Liu YC, Wang HY, Wang X, Liu Z (2012) Preparation of organic-silica hybrid boronate affinity monolithic column for the specific capture and separation of cis-diol containing compounds. J Chromatogr A 1256:114–120. https://doi.org/10.1016/j.chroma.2012.07.063
Fan H, Wang CZ, Wei YM (2015) Synthesis and application of boronic acid-functionalized magnetic adsorbent for sensitive analysis of salbutamol residues in pig tissues. Biomed Chromatogr 29:1834–1841. https://doi.org/10.1002/bmc.3504
Dong Q, Chi SS, Deng XY, Lan YH, Peng C, Dong LY, Wang XH (2018) Boronate affinity monolith via two-step atom transfer radical polymerization for specific capture of cis-diol-containing compounds. Eur Polym J 100:270–277. https://doi.org/10.1016/j.eurpolymj.2018.02.007
Li DJ, Li Y, Li XL, Bie ZJ, Pan XH, Zhang Q, Liu Z (2015) A high boronate avidity monolithic capillary for the selective enrichment of trace glycoproteins. J Chromatogr A 1384:88–96. https://doi.org/10.1016/j.chroma.2015.01.050
Cheng T, Zhu SQ, Zhu B, Liu XY, Zhang HX (2016) Highly selective capture of nucleosides with boronic acid functionalized polymer brushes prepared by atom transfer radical polymerization. J Sep Sci 39:1347–1356. https://doi.org/10.1002/jssc.201500968
Wang CZ, Xu HH, Wei YM (2016) The preparation of high-capacity boronate affinity adsorbents by surface initiated reversible addition fragmentation chain transfer polymerization for the enrichment of ribonucleosides in serum. Anal Chim Acta 902:115–122. https://doi.org/10.1016/j.aca.2015.11.013
Neyertz S, Brown D, Pilz M, Rival N, Arstad B, Männle F, Simon C (2015) Stability of amino-functionalized polyhedral oligomeric silsesquioxanes in water. J Phys Chem B 119:6433–6447. https://doi.org/10.1021/acs.jpcb.5b01955
Westerlnk BH, Bosker FJ, O’Hanlon JF (1982) Use of alumina, sephadex G 10, and ion-exchange columns to purify samples for determination of epinephrine, norepinephrine, dopamine, homovanillic acid, and 5-hydroxyindoleacetic acid in urine. Clin Chem 28:1745–1748
González O, Blanco ME, Iriarte G, Bartolomé L, Maguregui MI, Alonso RM (2014) Bioanalytical chromatographic method validation according to current regulations, with a special focus on the non-well defined parameters limit of quantification, robustness and matrix effect. J Chromatogr A 1353:10–27. https://doi.org/10.1016/j.chroma.2014.03.077
Ling X, Chen ZL (2018) Boronate affinity solid-phase extraction of cis-diol compounds by a one-step electrochemically synthesized selective polymer sorbent. Anal Bioanal Chem 410:501–508. https://doi.org/10.1007/s00216-017-0740-9
Espina-Benitez MB, Randon J, Demesmay C, Dugas V (2017) Development and application of a new in-line coupling of a miniaturized boronate affinity monolithic column with capillary zone electrophoresis for the selective enrichment and analysis of cis-diol-containing compounds. J Chromatogr A 1494:65–76. https://doi.org/10.1016/j.chroma.2017.03.014
Yang XT, Hu YF, Li GK (2014) Online micro-solid-phase extraction based on boronate affinity monolithic column coupled with high-performance liquid chromatography for the determination of monoamine neurotransmitters in human urine. J Chromatogr A 1342:37–43. https://doi.org/10.1016/j.chroma.2014.03.041
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (Nos. 21775121 and 21974106), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20126101120023) and Discipline Innovation Team Program of Shaanxi University of Chinese Medicine (No. 2019-YL10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 4438 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Tang, Y., Chen, Y. et al. Boronic acid-modified polyhedral oligomeric silsesquioxanes on polydopamine-coated magnetized graphene oxide for selective and high-capacity extraction of the catecholamines epinephrine, dopamine and isoprenaline. Microchim Acta 187, 77 (2020). https://doi.org/10.1007/s00604-019-4036-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4036-y