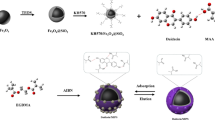
Abstract
D-Glucose was used as a dummy template in a strategy to fabricate boronate-based hollow molecularly imprinted polymers (h-MIPs) for the recognition of glycosides. 3-Aminophenylboronic acid (APBA) was selected as the functional monomer to impart glycoside binding capability. A polystyrene core was synthesized via an emulsifier-free emulsion polymerization. It was then be corroded to form a hollow structure where the binding sites on the inner surface can be fully utilized. Adsorption studies indicate that the resulting h-MIPs can specifically recognize glycosides. The h-MIPs were applied as a sorbent for solid phase extraction of the glycosides (daidzin, glycitin, rutin, and genistin) in soy products. Following desorption with 1% TFA in acetonitrile-water (3:7, v/v), the glycosides were quantified by HPLC with UV detection. The detection limits for the glycosides range from 3.5 to 10.8 ng·mL−1.

Boronate-affinity hollow molecularly imprinted polymers using D-glucose as dummy template were prepared as solid phase extraction adsorbents for selective enrichment of glycosides in soy products prior to quantitation by HPLC-UV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soy products are derived from soybeans, and are major food source in many Asian countries. The soybean contains abundant of glycosides (daidzin, glycitin, rutin, and genistin), which have recently been suggested as a prime source for medicinal chemistry and molecular pharmacology [1, 2]. It is obvious that quantitative analysis of glycosides from soy products is considerably important. Using appropriate analytical methods, the glycoside concentrations can be evaluated and monitored, facilitating fundamental research, as well as the quality control of food and nutraceutical products [3]. Chromatographic methods, especially for HPLC, are considered as the powerful analytical techniques for complex system [4]. However, analytes are always present in real samples at low levels and the sample matrix is very complex. Therefore, sample pretreatment and enrichment processes have become the crucial steps prior to HPLC determination [5].
SPE is the most widely used preconcentration method by which the analytes can be preferably isolated from complex matrices [6]. Among the numerous sorbents of SPE, molecularly imprinted polymers (MIPs) have aroused extensive attention due to their higher sample load capacity, physical robustness, as well as low cost and ease of preparation [7]. But the use of MIPs still meet big challenges such as slow mass transfer rate, template leakage, small binding capacity and poor site accessibility [8]. To overcome these problems, many novel strategies have been generated such as hollow molecular imprinting technique [9] and porous hollow molecular imprinting technique [10]. Compared to traditional MIPs, these novel MIPs exhibited bigger specific surface area, higher binding capacity and faster adsorption rate owing to the hollow structure in the shell.
Dummy template (a structural analogue of template or partial structure of template) can solve the problem of expensive and rare template, it can also lessen the leakage of template [11, 12]. In addition, dummy template MIPs have a higher density of recognition sites than traditional MIPs, which results in higher adsorption capacity [13, 14]. D-glucose, as the partial structure of glycosides, can be selected as the dummy template to prepare the h-MIPs for selective enrichment of glycosides from soy products.
Boronic acids can covalently bind cis-diol-containing compounds such as nucleosides [15], glycoproteins [16], and glycopeptides [17] at relatively high pH values, while the complexes dissociate reversibly when the environmental pH becomes acidic [18]. To date, several types of material modified with boronic acid groups have been widely prepared and applied in the enrichment of cis-diol-containing molecules [10, 19,20,21]. Results show that boronic acid-modified materials, especially h-MIPs, which have higher binding capacity and selective recognition properties, will be a powerful tool for glycosides research.
A boronate-affinity h-MIPs material using D-glucose as dummy template has been synthesized based on PS seed particles. The novel h-MIPs have been applied to the selective extraction of four glycosides from soy products, and then the contents of them have been directly determined by HPLC. This material has the following attractive features: first, APBA, as the boronic acid functional monomer, is introduced owing to several advantages, including easy-to-control binding/release, high specificity, high affinity, and superb tolerance for interference. Moreover, D-glucose as dummy template eliminates the effect of template leakage on quantitative analysis, and improves the sensitivity on qualitative analysis. What is more, the hollow structure of h-MIPs exhibits higher binding capacities and faster adsorption rates, which due to the existence of a large number of the binding sites at the internal and external surfaces in polymer shell. The h-MIPs have been characterized in detail. The adsorption performance and selectivity of h-MIPs have been systematically investigated. The h-MIPs sorbent has high selectivity and sufficient capacity for the separation and enrichment of the glycosides. All the features mentioned above mean that this boronate-affinity dummy template h-MIPs material holds great potential for the rapid separation and selective enrichment of glycosides.
Experimental
Materials and chemicals
D-Glucose, daidzin, glycitin, rutin, genistin, cytidine, daidzein, acrylic acid, APBA, ethylene glycol dimethacrylate (EGDMA), and N,N′-dicyclohexylcarbodiimide (DCC) were purchased from Aladdin Reagent (Shanghai, China, http://www.aladdin-e.com/). Styrene, sodium chloride (NaCl), ammonium persulfate (APS), and tetrahydrofuran (THF) were purchased from Energy Chemical (Shanghai, China, http://www.energy-chemical.com/). Analytical grade ethanol (EtOH) was provided by Beijing Chemical Works (Beijing, China, http://www.beijingchemworks.com/). Azobisisobutyronitrile (AIBN) was supplied by Tianjin Chemical Plant (Tianjin, China, http://tjhgc.atobo.com.cn/), and was purified by recrystallization from EtOH and dried under vacuum at room temperature prior to use. Formic acid (FA), acetic acid (AA), hydrochloric acid (HCl), and trifluoroacetic acid (TFA) were purchased from Shanghai Chemical Reagent (Shanghai, China, http://www.shhxsj.com/). HPLC grade methanol (MeOH) and acetonitrile (ACN) were supplied by Fisher Scientific (New Jersey, USA, http://www.fishersci.ca/ca/en/home.html). All other reagents were bought from various commercial sources and were of analytical or HPLC grade.
Stock solutions of 100 μg·mL−1 glycosides (daidzin, glycitin, rutin, and genistin) in MeOH were stored at 4°C. The standard working solutions were prepared in MeOH from the stock solutions to obtain the required concentrations by diluting with water prior to use. All the soy products purchased from local markets were stored at 4°C. Prior to the SPE-HPLC procedure, the sample solutions (1.0 g) were diluted 100 times and filtered through a 0.45 μm millipore filter to remove impurities.
Instrumentation
HPLC analysis was equipped with a LC-20A HPLC system (Shimadzu, Japan), which consisted of a liquid delivery pump (LC-20AT), an autosample injector (SIL-20A), a column oven (CTO-20A), an ultraviolet detector (SPD-20A), and the labsolutions workstation (Shimadzu, Japan) for the acquisition and analysis of the chromatographic data. A syncronis C18 column (4.6 × 250 mm, 5 μm, Thermoscientific, USA) was employed for the chromatographic separation. Gradient mobile phase consisted of A (0.1% TFA in water) and B (ACN) with a flow rate of 0.4 mL·min−1 was programmed as follows: 0–3 min, 20% B; 3–18 min, 20–35% B; 18–20 min, 35–40% B; 20–22 min, 40–30% B; 22–25 min, 30–20% B; 25–30 min, 20% B. The column temperature was controlled at 35°C, while determine wavelength was acquired at 254 nm. Before use, mobile phases were filtered through a 0.45 μm millipore filter, and then degassed for 15 min with a Model DOA-P504-BN pump (IDEX, USA). A PHSF-3F pH meter (Shanghai Precision and Scientific Instrument, Shanghai, China) was used for pH measurements. A Millipore Milli-Q water purification system (Millipore, Bedford, MA, USA) was used to purify deionized water (DDW), and the DDW produced at 18.2 MΩ cm was prepared for mobile phases and sample solutions. A 79–1 magnetic stirrer (Changzhou Guohua Instruments Co. Ltd., China) was applied. A ZK-82BB electric vacuum drying oven (Shanghai Experimental Instrument Co. Ltd., China) was utilized. A scanning electron microscope (SEM, JSM 6700-F) and a transmission electron microscope (TEM, JSM 2000-F, JEOL Company, Japan) were used to observe the surface morphology. The Fourier transform infrared spectra (FT-IR) were recorded with a Thermo Nicolet 670 FT-IR instrument (Thermo, USA). A Q500 thermal gravimetric (TG) analysis system (TA Company, USA) was employed to investigate the thermal behavior of the materials. An ASE-24 SPE apparatus (Tianjin Automatic Science Instrument, China) was used for separation and enrichment of glycosides.
Preparation of carboxylated PS seed particles
Nanometer-sized, monodisperse carboxylated PS seed particles were prepared by emulsifier-free emulsion polymerization, which was similar to the reported method [22] with minor modification. The detailed synthesis process of the carboxylated PS is illustrated in Electronic Supporting Material.
Preparation of boronate-affinity D-glucose dummy template h-MIPs
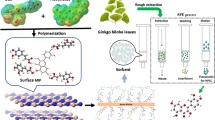
The boronate-affinity D-glucose dummy template h-MIPs were prepared by surface imprinting polymerization of PS seed particles as core based on the steps below. The schematic route is illustrated in Fig. 1.
In the first stage, dummy template (D-glucose, 0.25 mmol) and functional monomer (APBA, 1.00 mmol) were dissolved in 80 mL ACN, and stirred at room temperature for 10 h under a nitrogen atmosphere to prepare the preassembly solution. Then, 0.20 g of carboxylated PS seed particles were suspended in preassembly solution and ultrasonicated for 30 min. Next, 0.05 g DCC was added to the above solution, the reaction system was magnetically stirred at 700 rpm for 2 h. Afterward, 5 mmol EGDMA and 0.08 g AIBN were added to above reaction system and stirred for 12 h at 43°C, and then heated and maintained at 60°C for 24 h and 85°C for 6 h. After polymerization, the surface molecular imprinted polymers were collected by filtration, and rinsed with EtOH until the supernatant was clear. Hollow microspheres were further fabricated by simply dissolving the PS seed particles using THF for 3 h. The imprinted cavities were formed by removing templates using MeOH/AA (8:2, v/v). Finally, the boronate-affinity D-glucose dummy template h-MIPs were dried under vacuum at 60°C. For comparison, the corresponding hollow non-imprinted polymers (h-NIPs) were also prepared in the same way, except that the dummy template (D-glucose) was not added.
Adsorption experiment
The recognition and adsorption capacities of the boronate-affinity h-MIPs/h-NIPs to glycosides were investigated by conducting adsorption equilibrium experiments. 1 mL of 2 μg·mL−1 sample solution (daidzin, glycitin, rutin, and genistin) was pumped through the 0.05 g h-MIPs/h-NIPs materials prepared SPE column with a flow rate of 1.0 mL·min−1. After adsorption, the effluent was collected for HPLC determination after filtering with a 0.45 μm millipore filter.
Typically, the adsorption amounts, Q e (μg·g−1), were calculated by Eq. (1),
where C 0 and C e are the initial concentration before adsorption and equilibrium concentration after adsorption by h-MIPs (or h-NIPs) SPE column, V represents the volume of solution (mL), and m is the mass of h-MIPs (or h-NIPs) (g).
The recognition abilities of h-MIPs and h-NIPs for glycosides were also evaluated by imprinting factor (IF) which is defined as follows:
where Q h-MIPs and Q h-NIPs are the equilibrium adsorption amounts of glycosides for h-MIPs and h-NIPs, respectively.
Selectivity study
To evaluate the selectivity of the h-MIPs on glycosides, four structural analogues containing D-glucose structure (daidzin, glycitin, rutin, and genistin) and two reference compounds (cytidine and daidzein) (Supplementary Fig. S1) were selected to detect the selectivity adsorption of h-MIPs/h-NIPs. The experiment was carried out by introducing 1 mL of 2 μg·mL−1 sample solution (daidzin, glycitin, rutin, genistin, cytidine, and daidzein) into h-MIPs and h-NIPs SPE columns, respectively. After adsorption, the effluent was collected and analyzed by HPLC.
Results and discussion
Choice of materials
The boronate-affinity h-MIPs was synthesized by using D-glucose as dummy template, APBA as functional monomer, and PS as core. This material has the following attractive features for the purpose of the specific and efficient enrichment of glycosides: D-glucose as dummy template eliminates the effect of template leakage. The use of APBA is due to its capability both to react with carboxylated PS seed particles by condensation, and APBA is highly specific to glycosides because the boronic acid groups and the glycosides with cis-diols form a heterocyclic diester in an alkaline solution (see Fig. 1). Moreover, the h-MIPs exhibit higher binding capacities and faster adsorption rates owing to their hollow structure in the shell. Taking into account these considerations, boronate-affinity h-MIPs using D-glucose as dummy template were employed as the material for the enrichment of glycosides.
Characterization of h-MIPs
The resulting h-MIPs were characterized by SEM, TEM, FT-IR, and TG analysis. The SEM and TEM images of the PS seed particles and h-MIPs are shown in Fig. 2. The results indicate that almost all of these PS seed particles exhibit sphere-like morphology and show smooth and good dispersion (Fig. 2a and c). Figure 2b and d represent SEM and TEM images of the h-MIPs respectively, which illustrate that h-MIPs are rigid and keep a perfect spherical shape without breakage and deformation, and the hollow structure of h-MIPs is created by removing the PS seed particles with THF. The hollow structure of h-MIPs survived, which ensured that the analytes enter the imprinting cavities from both sides of the polymer microspheres, suggesting more additional binding sites were produced.
The structures of PS, MIPs, and h-MIPs were separately analyzed by FT-IR. Based on the detailed information in Fig. S2a, the fabrication procedure was successfully performed. The TG determination displayed in Fig. S2b indicated that the h-MIP particles are stable up to 350°C, which reveals that the materials have high thermal stability.
Optimization of imprinting conditions
The affinity between h-MIPs and glycosides is based on the typical boronate esterification reaction in which the boronic acid groups react with cis-diols of glycosides to form cyclic esters on the microspheres surface. The content of boronic acid groups embedded in the polymer shell is attributed to the enhancement of the adsorption efficiency of h-MIPs enriched glycosides. To achieve the best adsorption efficiency, the influence of the functional monomers (APBA) content was investigated in Table S1, which including different recipes with a changing mole ratio of functional monomers to dummy templates (D-glucose) and cross linkers (EGDMA) by considering the Q e of glycosides (daidzin, glycitin, rutin, and genistin) and the IF at the same conditions, and the results are shown in Fig. 3.
Obviously, the Q e and IF of h-NIPs had tiny changes with the increasing of the mole ratio of functional monomers. However, both the Q e and IF of h-MIPs increased obviously compared to that of the h-NIPs. As can be seen from Fig. 3a–d, the Q e and IF of h-MIPs increased along with increasing the ratio of APBA to D-glucose from 1:1 to 4:1. When the ratio reached 4:1, the Q e reached its peak value, and the IF reached its maximum at the same time. When the ratio was increased continuously, both the Q e and IF of h-MIPs showed a downward trend, and thus the ratio of APBA to D-glucose was chosen to be 4:1.
The sample pH value was also optimized. Respective data and figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) ratio between APBA and D-glucose of 4:1; (b) sample pH value of 8.5.
Evaluation of the adsorption selectivity of h-MIPs
The selective adsorption capacities of h-MIPs and h-NIPs toward four different glycosides (daidzin, glycitin, rutin, and genistin) and two reference compounds (cytidine and daidzein) were investigated by evaluating their Q e values in 2 μg·mL−1 of phosphate buffer (pH 8.5), and the results are shown in Fig. 4. The h-NIPs show the certain adsorption capacity toward the glycosides, which is due to the existence of cis-diol capturing by APBA linkage with D-glucose group. However, h-MIPs show superior adsorption capacity to the glycosides, because of the existence of imprinted cavities and tailored steric structures in the polymer shell compared to the h-NIPs, which presumably supported the glycosides from entering into the binding cavities due to steric effect [23]. In addition, h-MIPs and h-NIPs show very low adsorption capacities to the reference compounds. Cytidine, although contained cis-diol structure, is not selectively captured because of its different shape memory with D-glucose, not to say daidzein, non-containing cis-diol groups [24]. The results indicated that the adsorption selectivity of h-MIPs is not only related to the covalent bond interaction, but also depending on the shape memory effect of analyte screening.
Evaluation of the method
The analytical features of the present method under optimal experimental conditions, including linear range, regression coefficients of correlation (R 2), limit of detection (LOD), limit of quantitation (LOQ), and relative standard deviations (RSDs), are summarized in Table 1. All four glycosides had good linearity with R 2 values ranging from 0.9993 to 0.9999. LOD and LOQ, based on the signal-to-noise (S/N) ratios of 3 and 10, were calculated in the range of 3.5–10.8 ng·mL−1 and 11.5–36.2 ng·mL−1, respectively. The RSDs of intra-day and inter-day were in the ranges of 0.12–0.60% and 0.22–2.07%, respectively. Based on the results of RSDs, the precision and repeatability of the method were considered acceptable.
To illustrate the advantages of the h-MIPs as a novel extraction material, a comparative study of our method with other methods reported for the determination of different kind of glycosides was performed (Table 2). Regarding LODs, other analytical approaches had higher LODs than those in the present method, which use HPLC analysis [25,26,27,28]. Although LODs in the present study were higher than those using HPLC-MS/MS [29, 30], the expensive instruments required for this technique have limited its wide application. By comparison, our newly developed h-MIPs SPE-HPLC procedure is simple, effective and inexpensive.
Application in real samples analysis
To validate the feasibility of the present method for real samples, daidzin, glycitin, rutin, and genistin in three different kinds of soy product were determined by h-MIPs SPE-HPLC method. All the real samples were spiked with four glycoside standard solutions at two concentration levels to assess the matrix effects, Level 1 (0.5 μg·mL−1) and Level 2 (1.0 μg·mL−1), respectively. As representatives, the chromatograms of sample 1 extracted by boronate-affinity h-MIPs and standard addition in real samples are exhibited in Fig. 5. The recoveries were calculated and listed in Table 3. As shown in Table 3, the recoveries were in the range of 63.7–101.7%, implying that the present method was effective for the determination of daidzin, glycitin, rutin, and genistin in soy products, which can provide some guidance for the determination and separation of glycoside compounds with low concentrations in real samples.
Chromatograms of soy products obtained by the boronate-affinity h-MIPs SPE-HPLC method. a elution from h-MIPs of unspiked sample, b elution from h-MIPs of sample spiked with 0.5 μg·mL−1, c elution from h-MIPs of sample spiked with 1.0 μg·mL−1, peaks: (1) daidzin, (2) glycitin, (3) rutin, and (4) genistin
Conclusions
In summary, we have prepared boronate-affinity D-glucose dummy template h-MIPs. The h-MIPs displayed excellent characteristics, such as good thermal stability, high binding capacity, fast mass transfer, and specific recognition to glycosides, owing to the hollow structure, boronate affinity and dummy imprinted effect. In addition, the h-MIPs were applied to the selective extraction of four glycosides from soy products with good recovery and repeatability, and then the contents of them were directly determined by HPLC, the results of this paper provide new idea, new method to the determination of target glycosides from soy products. It should be noted that the hollow structure is easily prepared by etching the PS core with THF, which leads to the collapse of the polymer outside and changes the thickness of imprinting shell. However, the thick imprinting shell results a low mass transfer and low utilization ratio of the binding site. Further studies can be conducted focusing on the development of a solid and relatively thin h-MIPs shell for extensive applications in more complex samples.
References
Sakamoto S, Yusakul G, Pongkitwitoon B, Tanaka H, Morimoto S (2016) Colloidal gold-based indirect competitive immunochromatographic assay for rapid detection of bioactive isoflavone glycosides daidzin and genistin in soy products. Food Chem 194:191–195
Peng MJ, Xiang HY, Hu X, Shi SY, Chen XQ (2016) Boronate affinity-based surface molecularly imprinted polymers using glucose as fragment template for excellent recognition of glucosides. J Chromatogr A 1474:8–13
Magiera S, Sobik A (2017) Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods. J Food Compos Anal 57:94–101
Souza LM, Dartora N, Scoparo CT, Gorin PAJ, Iacomini M, Sassaki GL (2016) Differentiation of flavonol glucoside and galactoside isomers combining chemical isopropylidenation with liquid chromatography-mass spectrometry analysis. J Chromatogr A 1447:64–71
Zhang Z, Xu SF, Li JH, Xiong H, Peng HL, Chen LX (2012) Selective solid-phase extraction of sudan I in chilli sauce by single-hole hollow molecularly imprinted polymers. J Agric Food Chem 60:180–187
Klejdus B, Štěrbová DV, Kubáň V (2001) Identification of isoflavone conjugates in red clover (Trifolium Pratense) by liquid chromatography-mass spectrometry after two-dimensional solid-phase extraction. Anal Chim Acta 450:81–97
Chen W, Xue M, Xue F, Mu XR, Xu ZB, Meng ZH, Zhu GX, Shea KJ (2015) Molecularly imprinted hollow spheres for the solid phase extraction of estrogens. Talanta 140:68–72
Tang YW, Li M, Gao X, Liu XY, Ma Y, Li Y, Xu YX, Li JR (2016) Preconcentration of the antibiotic enrofloxacin using a hollow molecularly imprinted polymer, and its quantitation by HPLC. Microchim Acta 183:589–596
Zhao Q, Li HY, Xu Y, Zhang FS, Zhao JH, Wang L, Hou J, Ding H, Li Y, Jin HY, Ding L (2015) Determination of triazine pesticides in cereal samples based on single-hole hollow molecularly imprinted polymers. J Chromatogr A 1376:26–34
Hu Y, Huang W, Tong YK, Xia QF, Tian MM (2017) Boronate-affinity hollow molecularly imprinted polymers for the selective extraction of nucleosides. New J Chem 41:7133–7141
Song YP, Li N, Zhang HC, Wang GN, Liu JX, Liu J (2017) Dummy template molecularly imprinted polymer for solid phase extraction of phenothiazines in meat based on computational simulation. Food Chem 233:422–428
Jia WH, Ma XL, Zhang JH, Xie HK, Liu F, Wang X (2015) Preparation of the high purity gingerols from ginger by dummy molecularly imprinted polymers. J Chromatogr A 1387:24–31
Minami K, Ihara M, Kuroda S, Tsuzuki H, Ueda H (2012) Open-sandwich molecular imprinting: making a recognition matrix with antigen-imprinted antibody fragments. Bioconjugate Chem 23:1463–1469
Zhu FL, Wang J, Zhu LJ, Tan LL, Feng GL, Liu SM, Dai Y, Wang H (2016) Preparation of molecularly imprinted polymers using theanine as dummy template and its application as SPE sorbent for the determination of eighteen amino acids in tobacco. Talanta 150:388–398
Li HH, Zhu SQ, Cheng T, Wang SX, Zhu B, Liu XY, Zhang HX (2016) Binary boronic acid-functionalized attapulgite with high adsorption capacity for selective capture of nucleosides at acidic pH values. Microchim Acta 183:1779–1786
Qu YY, Liu JX, Yang KG, Liang Z, Zhang LH, Zhang YK (2012) Boronic acid functionalized core-shell polymer nanoparticles prepared by distillation precipitation polymerization for glycopeptide enrichment. Chem Eur J 18:9056–9062
Mayang YC, He XW, Chen LX, Zhang YK (2017) Detection of transferrin by using a surface plasmon resonance sensor functionalized with a boronic acid monolayer. Microchim Acta 184:2749–2757
Bie Z, Chen Y, Ye J, Wang S, Liu Z (2015) Boronate-affinity glycan-oriented surface imprinting: a new strategy to mimic lectins for the recognition of an intact glycoprotein and its characteristic fragments. Angew Chim Int Ed 54:10211–10215
Chen JH, Min XW, Li P, Chen W, Tian DW, Chen QH (2015) Sensitive determination of four camptothecins by solid-phase microextraction-HPLC based on a boronic acid contained polymer monolithic layer. Anal Chim Acta 879:41–47
Pan MR, Sun YF, Zheng J, Yang WL (2013) Boronic acid-functionalized core-shell-shell magnetic composite microspheres for the selective enrichment of glycoprotein. ACS Appl Mater Inter 5:8351–8358
Liu Y, Liu J, Liu J, Gan W, Ye BC, Li YC (2017) Highly sensitive and selective voltammetric determination of dopamine using a gold electrode modified with a molecularly imprinted polymeric film immobilized on flaked hollow nickel nanospheres. Microchim Acta 184:1285–1294
Guan GJ, Zhang ZP, Wang ZY, Liu BH, Gao DM, Xie CG (2007) Single-hole hollow polymer microspheres toward specific high-capacity uptake of target species. Adv Mater 19:2370–2374
Song YP, Zhang L, Wang GN, Liu JX, Liu J, Wang JP (2017) Dual-dummy-template molecularly imprinted polymer combining ultra performance liquid chromatography for determination of fluoroquinolones and sulfonamides in pork and chicken muscle. Food Control 82:233–242
Ma RT, Ha W, Chen J, Shi YP (2016) Highly dispersed magnetic molecularly imprinted nanoparticles with well-defined thin film for the selective extraction of glycoprotein. J Mater Chem B 4:2620–2627
Wei W, Fu YJ, Zu YG, Wang W, Luo M, Zhao CJ, Li CY, Zhang L, Wei ZF (2012) Ionic liquid-based microwave-assisted extraction for the determination of flavonoid glycosides in pigeon pea leaves by high performance liquid chromatography-diode array detector with pentafluorophenyl column. J Sep Sci 00:1–9
Ji WH, Wang T, Liu W, Liu F, Guo LP, Geng YL, Wang X (2017) Water-compatible micron-sized monodisperse molecularly imprinted beads for selective extraction of five iridoid glycosides from Cornus officinalis fructus. J Chromatogr A 1504:1–8
Ma RT, Ha W, Chen J, Shi YP (2016) Development of hydrophilic magnetic molecularly imprinted polymers by directly coating onto Fe3O4 with a water-miscible functional monomer and application in a solid-phase extraction procedure for iridoid glycosides. RSC Adv 6:50487–50496
Tu XJ, Ma SQ, Gao ZS, Wang J, Huang SK, Chen WB (2017) One-step extraction and hydrolysis of flavonoid glycosides in rape bee pollen based on soxhlet-assisted matrix solid phase dispersion. Phytochem Anal 28:505–511
Zhu JW, Xue BY, Ma B, Zhang Q, Liu M, Liu L, Yao D, Qi HH, Wang YL, Ying HJ, Wu ZD (2015) A pre-clinical pharmacokinetic study in rats of three naturally occurring iridoid glycosides, Picroside-I, II and III, using a validated simultaneous HPLC-MS/MS assay. J Chromatogr B 993:47–59
Ma J, Krynitsky AJ, Grundel E, Rader JI (2012) Quantitative determination of cucurbitane-type triterpenes and triterpene glycosides in dietary supplements containing bitter melon (momordica charantia) by HPLC-MS/MS. J AOAC Int 95:1597–1608
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 4396 kb)
Rights and permissions
About this article
Cite this article
Hu, Y., Xia, Q., Huang, W. et al. Boronate-modified hollow molecularly imprinted polymers for selective enrichment of glycosides. Microchim Acta 185, 46 (2018). https://doi.org/10.1007/s00604-017-2608-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2608-2