Abstract
A metal-organic framework (MOF) was designed and prepared from luminescent Tb(III), adenosine diphosphate (ADP) and bipyridyl (Bipy). Its green fluorescence at 545 nm is shown to enable the fluorometric detection of cyanide ion based on the principle of π-conjugation-induced fluorescence enhancement. The fluorescence of the probe is strongly increased by cyanide due to extended π-conjugation between probe MOF and cyanide which sensitizes the fluorescence of Tb(III). This effect can be used to quantify cyanide at levels as low as 30 nM in aqueous solution. The method was applied to the determination of cyanide in saliva samples. The lack of interference by acetate and fluoride is a specific feature of this method. The method based on the principle of π-conjugation-induced fluorescence enhancement provides a new sensing way for widely used fluorescence assays.

A cyanide-selective Tb-ADP-Bipy MOF was designed and synthesized for the detection of cyanide based on the principle of π-conjugation-induced fluorescence enhancement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanide is a vital inorganic material in industry sector including gold mining, electroplating, metallurgy, synthetic fibers, and synthesis of nylon and acrylic polymers as well [1]. However, it is very noxious to the human health and environment [2]. Cyanide of 0.5–3.5 mg·kg−1 of body weight will cause emesis, losing consciousness and eventually death because it interferes with the electron transport of cytochrome c oxidase following hypoxia. The highest allowable level of cyanide in drinking water is only 0.05 mg·L−1 (1.9 μM) according to the World Health Organization (WHO) [3]. Chinese National Standard stipulated that the content of cyanide in drinking water should be less than 0.05 mg·L−1 [4], and in surface water of Class І and П should be less than 0.005 and 0.05 mg·L−1 [5], respectively. Thus, it is very important to monitor the level of cyanide in the production processes and environment.
General approaches used for the assay of cyanide including electrochemistry [6], hydrogen-bond interaction [7], supramolecular self-assembly [8, 9], nucleophilic addition reactions [10, 11], and spectroscopy [12, 13]. Quite a number of cyanide probes were designed on the basis of the interaction of hydrogen-bonding between the receptor unit and cyanide [14,15,16]. However, F− and CH3COO− (Ac−) are also easily to form hydrogen bound with the receptor unit, which weakens the specificity of determination of these probes. The cyanide probes using organic dye molecule, although usually possess high specificity, are limited in the assays of aqueous biological samples because most organic dye molecules are water-insoluble [17]. Some cyanide probes have to use an enhancement regent to meet the requirement of specificity and solubility [18]. In additions, many of these probes are slowing responsive and time-consuming [19, 20]. Despite many research works, the properties of highly sensitivity, selectivity, capable of being used in aqueous solutions and quick response to cyanide are rarely all present in a single molecule probe.
Newly emerged metal-organic frameworks consisting of metal ions and organic bridging ligands have attracted great attention in designing functional materials. The composition and structure of MOFs are flexible and rich, and it is easy to be assembled into desired functional structures according to the design objective. The MOFs containing lanthanide ions are of excellent photostability against photobleaching [21] and unique long fluorescence lifetimes which allows the use of the time-resolved fluorescence techniques [22]. However, the luminescence of lanthanide ions is weak, and often needs an emission enhancement by antenna molecules.
In this work, we designed and synthesized a metal-organic framework (MOF) material based on the principle of π-conjugation-induced fluorescence enhancement (Scheme 1). This MOF material is composed of luminescent Tb(III) ion, biomolecule adenosine diphosphate (ADP) and dipyridyl (Bipy), denoted as Tb-ADP-Bipy MOF. The ADP and Bipy with π-conjugation structure are used as the ligands of Tb3+ by p-π conjugation. In the presence of linearly structural cyanide, an extended π-conjugation can be formed between cyanide and Tb-ADP-Bipy MOF. As a result, the fluorescence of Tb-ADP-Bipy MOF will be greatly increased.
Experimental section
Chemicals and solutions
Tb(NO3)3·6H2O (99.99%) was purchased from Baotou Rewin Rare Earth Metal Materials Co., Ltd. (http://www.btrew.com/); 2–2′-Bipyridyl (Bipy) (99.0%), Adenosine-5′-diphosphate disodium salt (ADP) (>98%), D-Phenylalanine (Phe) (98%), Dopamine hydrochloride (DA) (98%), 1,10-Phenanthroline Monohydrate (Phen) (98%) and Nicotinic acid (VB3) (99%) were purchased from Aladdin (http://www.aladdin-e.com/); Cyanide standard (50 μg·mL−1) was from the center of Chinese reference materials (http://www.gbw-china.com/); The ultrapure water (resistivity 18 MΩ·cm) purified with a Millipore filtration system was used to prepare all aqueous solutions. The stock solutions of interference ions (1 mM) were prepared by dissolving Na3PO4, Na2CO3, NaAc, NaF, NaCl, NaBr, NaNO3, NaNO2, Na2SO3, Na2SO4, FeCl3, FeCl2, Co(NO3)2, CuCl2, Zn(NO3)2, CaCl2, Ni(NO3)2, MnCl2, Pb(NO3)2, Cd(NO3)2, HgCl2 and AgNO3 in ultrapure water, respectively. Unless otherwise stated, all chemicals were analytical reagent grade and used without further purification.
Instruments
The morphology and size of MOF materials were observed by Ultra Plus scanning electron microscope (SEM) (Zeiss, Germany). The fluorescence spectra were recorded at excitation wavelength of 230 nm by an LS55 luminescence spectrometer (PerkinElmer, UK). The detection solution was placed in a quartz micro cuvette with 1 mL capacity and 2 mm light path. A delay time of 0.05 ms and a gate time of 2 ms were used. Excitation spectra were recorded by observing the emission intensity of Tb3+ at 545 nm. The Fourier transform infrared (FT-IR) were recorded with an Avatar 360 FT-IR spectrometer (Nicolet, USA). UV-visible absorption spectra were recorded with a UV-1800 spectrophotometer (Shimadzu, Japan).
Preparation of Tb-ADP-Bipy MOF
Typically, 1 mL of Tb(NO3)3 solution (10 mM) was added to 1 mL of 2, 2′- Bipyridyl solution (20 mM), after this mixture was stirred for 30 min, 1 mL of adenosine diphosphate aqueous solution (10 mM) was added. The reaction was allowed to continue for 1.5 h under stirring. Then, the mixture was centrifuged at 14000 rpm for 10 min. To remove unreacted reactants, we washed the precipitate three times. Finally, the precipitate was dried, weighed (approximately 4.01 mg) and dispersed in 2 mL of ultrapure water to form a Tb-ADP-Bipy suspension of 2.00 mg·mL−1 for the subsequent experiments. As controls, Tb-Phe-Bipy, Tb-DA-Bipy, Tb-VB3-Bipy and Tb-ADP-Phen were prepared in the same way.
Fluorescence response of Tb-ADP-Bipy MOF to cyanide
For fluorescence response of Tb-ADP-Bipy to cyanide, 10 μL of cyanide solution with the different concentrations (0, 0.30, 0.60, 1.20, 2.40, 4.80, 9.60, 19.20 μM) were added to 100 μL of Tb-ADP-Bipy suspension, and ultrapure water was added until the total volume reached to 1 mL. After adequately mixing, the solution was placed in a sample cell of the luminescence spectrometer. The fluorescence intensities at 545 nm were recorded under a 230-nm excitation wavelength. Other materials such as Tb-GMP-Bipy were done in the same way.
Selectivity of Tb-ADP-Bipy MOF to cyanide
For the selectivity of the assay of cyanide, 20 μL of interference ion (anion: PO4 3−, CO3 2−, Ac−, F−, Cl−, Br−, NO3 −, NO2 −, SO3 2− and SO4 2−; cation: Fe3+, Fe2+, Co2+, Cu2+, Zn2+, Ca2+, Ni+, Mn2+, Pb2+, Cd2+, Hg2+ and Ag+) aqueous solution (1 mM) were added to 100 μL of Tb-ADP-Bipy suspension (2.00 mg·mL−1), respectively, and the ultrapure water was added until the total volume reached to 1 mL. The fluorescence intensities at 545 nm were measured on the luminescence spectrometer with an excitation wavelength of 230 nm.
Detection of cyanide in saliva sample
Fresh saliva was collected from volunteers. These saliva samples were diluted 20-fold with ultrapure water and centrifuged at a speed of 10,000 rpm for 10 min for the removing of possible oral epithelium. The supernatant was further filtrated with 0.22 μm filter membrane (whatman). The saliva samples containing 1, 5 and 10 μM cyanide were prepared by spiking standard cyanide solution. For the measurement of cyanide in the saliva samples, 100 μL of Tb-ADP-Bipy suspension (2.00 mg·mL−1) was added to 100 μL of saliva samples, and the ultrapure water was added until the total volume reached to 1 mL. These solutions were incubated for 5 min, and their fluorescence spectra were recorded under a 230-nm excitation wavelength.
Results and discussion
The scanning electron microscopy (SEM) image of Tb-ADP-Bipy MOF is shown in Fig. 1. The selected area electron diffraction and XRD show that the structure of Tb-ADP-Bipy is amorphous (Fig. S1 and S2). In the presence of cyanide, the morphology of Tb-ADP-Bipy does not obviously change. The chemical elements of Tb-ADP-Bipy MOF was determined via energy dispersive X-ray (EDX), and the result shows the existence of the element C, N, O, Tb and P (Fig. S3). ADP is the only P source, suggesting that ADP is included in Tb-ADP-Bipy.
To understand the mutual bonding of ADP, Bipy and Tb3+, we measured the FTIR spectra of Tb-ADP-Bipy. As shown in Fig. 2, the asymmetrical stretching vibration peaks of PO3 group (υas(PO3)) of ADP at 1131 cm−1, Tb-ADP at 1120 cm−1, Tb-ADP-Bipy at 1116 cm−1 and Tb-ADP-Bipy in the presence of cyanide at 1112 cm−1 show a gradual blue shift as the conjugation effect enhanced. It implies that a decrease in the absorbed energy caused by the formation of an extended π-conjugation. There are several differences between the ADP and Tb-ADP in FTIR spectra. Compared with ADP, the peaks of Tb-ADP at 1581 cm−1 for υ(C2-N1) shifted to 1577 cm−1, at 1650 cm−1 for δ(N-H) shifted to 1646 cm−1, at 1210 cm−1 for υas(PO2) shifted to 1221 cm−1, and at 1131 cm−1 for υas(PO3) shifted to 1120 cm−1 [23]. These results show that the N1, amido and phosphate group of ADP may have participated in the coordination with Tb3+. A band at 1577 cm−1, which is assigned to the stretching vibration peak of Bipy of Tb-ADP-Bipy (υ(C=N)) is observed, suggesting that Bipy also coordinated with Tb3+. Furthermore, compared with Tb-ADP, Tb-ADP-Bipy displays a blue shift of UV absorption and the hyperchromic effect is observed because -C≡N group is chromophore (Fig. S4). After the addition of cyanide, Tb-ADP-Bipy was thoroughly washed. We found that the band of Tb-ADP-Bipy at 2360 cm−1 for υ(C≡N) is still observed, indicating that cyanide ion coordinated with Tb3+, and was bonded to the surface of Tb-ADP-Bipy MOF.
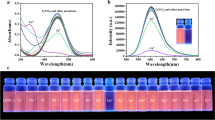
The results of fluorescence spectra are shown in Fig. 3. Neither Tb-Bipy complex nor Tb-Bipy complex in the presence of cyanide has obvious emission at 545 nm that is the characteristic emission of Tb3+. It indicates that single -Bipy or single -Bipy-cyanide is profitless to the sensitization of emission of Tb3+. Compared with Tb-ADP, the fluorescence of Tb-ADP in the presence of cyanide only has a tiny increase. This indicates that cyanide itself did not sensitize the emission of Tb3+ signally.
Excitation and fluorescence spectra of Tb-Bipy complex solution, Tb-Bipy + cyanide solution, Tb-ADP suspension, Tb-ADP suspension + cyanide, Tb-ADP-Bipy suspension and Tb-ADP-Bipy suspension + cyanide. The concentrations of Tb3+ and cyanide ion are 10 and 2 μM, respectively. Emission wavelength for excitation spectra and excitation wavelength for fluorescence spectra are 545 and 230 nm, respectively
The fluorescence intensity of Tb-ADP-Bipy is stronger than that of Tb-ADP. As the second ligand, Bipy may improve the energy transfer from ADP to Tb3+, which causes an increase in the emission of Tb3+. Bipy itself cannot obvious sensitize the emission of Tb3+. However, in the presence of cyanide, the fluorescence of Tb-ADP-Bipy is enhanced intensively. As anticipated, this intensive enhancement is a result of the formation of an extended π-conjugation between Tb-ADP-Bipy and cyanide that greatly sensitized the fluorescence of Tb3+.
The emission of Tb-ADP-Bipy is gradually increased with the addition of cyanide (Fig. 4). There is a linear relationship between the fluorescence and the concentration of cyanide in the range of 30 nM to 3.84 μM (Fig. 4 inset), and the detection limit is as low as 30 nM (Fig. S5). This limit of detection is much lower than the highest level of cyanide of 0.05 mg·L−1 (1.9 μM) in drinking water permitted by the WHO. In the presence of excess cyanide, the fluorescence of Tb-ADP-Bipy does not increase or even decrease with the increasing concentration of cyanide. Probably, other ligands such as ADP can be replaced by cyanide due to stronger coordination ability of cyanide.
The response time of Tb-ADP-Bipy to cyanide is very fast, and the fluorescence of Tb-ADP-Bipy reaches its maximum in about 10 s (Fig. S6).The stability of fluorescence of Tb-ADP-Bipy in water solution is excellent. No obvious changes in fluorescence intensity of Tb-ADP-Bipy are observed at least in 30 days (Fig. S7).
Further, the selectivity of the assay using Tb-ADP-Bipy MOF was measured. The anions including PO4 3−, CO3 2−, Ac−, F−, Cl−, Br−, NO3 −, NO2 −, SO3 2− and SO4 2− were used as the interfering ions. As shown in Fig. 5a, some competitive species such as PO4 3–, CO3 2−, Ac− and F−, which often show strong interference to cyanide detection, do not make any significant fluorescence changes with the concentration 10 times higher than cyanide. The anions of PO4 3−, CO3 2−, NO3 −, NO2 −, SO3 2− and SO4 2− with more complex structures also does not interfere with the assay due to the fact that they cannot form a stable conjugated structure. The influence of metal ions on the fluorescence of Tb-ADP-Bipy MOF was also tested (Fig. 5b). Their influences are negligible within the error allowed. Potentially interfering hydrogen peroxide (H2O2) did not show interference for the assay.
The selectivity of cyanide assay using Tb-ADP-Bipy MOF material. The concentrations of interfering ion, cyanide and Tb-ADP-Bipy were 20 μM, 1.92 μM and 2.00 mg·mL−1 respectively. I0: the fluorescent intensity of Tb-ADP-Bipy. I: the fluorescent intensity of Tb-ADP-Bipy in the presence of interfering ions
The fluorescence enhancement of Tb-ADP-Bipy is a result of an extended π-conjugation caused by cyanide. In order to prove this mechanism, we further selected and tested other ligands capable to form a π-conjugation with cyanide including Phenanthroline (Phen), GMP, Phenylalanine (Phe), Dopamine hydrochloride (DA) and Nicotinic acid (VB3). Phen possessing P electron was chosen to instead of Bipy. As shown in Fig. S8, the fluorescence of Tb-ADP-Phen is enhanced in the presence of cyanide due to an extended π-conjugation among Tb3+, ADP, Phen and cyanide. Similarly, the fluorescence enhancement is observed as GMP, Phe, DA and VB3 replaced ADP, and the fluorescence of Tb-GMP-Bipy, Tb-Phe-Bipy, Tb-DA-Bipy and Tb-VB3-Bipy increases with the concentration of cyanide (Fig. S9, S10, S11 and S12).
We compared other fluorescent methods for the measurement of cyanide (Table 1). The present method shows a comparable detection limit and wider detection range.
Cyanides are widespread chemicals found in industrial waste, biological sources even in vegetables [31]. Cyanide has great possibility causing poisoning to human via oral cavity approach. We used Tb-ADP-Bipy MOF to determinate cyanide in saliva according to the strategy of π-conjugation-induced fluorescence enhancement mentioned above. The results of detecting cyanide in saliva samples are shown in Table 2. The recoveries and relative standard deviations fell in the range of 99.88–102.85% and 0.53–0.76, respectively, indicating a good precision and satisfactory reproducibility for the assay of cyanide.
In conclusion, a new π-conjugation-induced fluorescence enhancement assay for cyanide was established. This assay is based on the principle that an extended π-conjugation is formed between Tb-ADP-Bipy MOF and cyanide which greatly sensitized the fluorescence of Tb3+. Similarly structural Tb-Phe-Bipy, Tb-DA-Bipy and Tb-ADP-Phen were synthesized and used to further validate this mechanism. Tb-ADP-Bipy MOF-based assay showed excellent selectivity and high sensitivity with a detection limit as low as 30 nM, and can satisfactorily detect cyanide in saliva samples, which probably provides an alternative means for the forensic investigation of cyanide. A specific feature of this method is lack of the disadvantage of common assays for cyanide that are subject to the interference of acetate and fluoride. To the best of our knowledge, this is the first time an extended π-conjugation-induced fluorescence enhancement was applied to an assay. This strategy opened a new way to the sensing of other small molecules/ions capable of forming an extended π-conjugation.
References
Dutra AJB, Rocha GP, Pombo FR (2008) Copper recovery and cyanide oxidation by electro winning from a spent copper-cyanide electroplating electrolyte. J Hazard Mater 152:648–655
Shifrin NS, Beck BD, Gauthier TD, Chapnick SD, Goodman G (1996) Chemistry, toxicology, and human health risk of cyanide compounds in soils at former manufactured gas plant sites. Regul Toxicol Pharmacol 23:106–116
World Health Organization (1996) Guidelines for drinking-water quality, Geneva
Ministry of Health of the People's Republic of China & China National Standardization Management Committee (2007) Standards for drinking water quality. China
State Environmental Protection Administration & State Administration for Quality Supervision and Inspection and Quarantine (2002) Environmental quality standard for surface water. China
Bohrer D, Do Nascimento PC, Garcia Pomblum S, Seibert E, Machado de Carvalho L (1998) Polarographic determination of cyanide as nickelcyano complex in blood plasma after selective extraction in a methylene blue impregnated polyethylene column. Fresenius J Anal Chem 361:780–783
Manivannan R, Satheshkumar A, Elango KP (2013) Tuning of the H-bonding ability of imidazole N-H towards the colorimetric sensing of fluoride and cyanide ions as their sodium salts in water. New J Chem 37:3152–3160
Yao L, Zhou J, Liu J, Feng W, Li F (2012) Iridium-complex-modified upconversion nanophosphors for effective LRET detection of cyanide anions in pure water. Adv Funct Mater 22:2667–2672
Shi B, Zhang P, Wei T, Yao H, Lin Q, Zhang Y (2013) Highly selective fluorescent sensing for CN− in water: utilization of the supramolecular self-assembly. Chem Commun 49:7812–7814
Fillaut JL, Akdas-Kilig H, Dean E, Latouche C, Boucekkine A (2013) Switching of reverse charge transfers for a rational design of an off-on phosphorescent chemodosimeter of cyanide anions. Inorg Chem 52:4890–4897
Khatua S, Samanta D, Bats JW, Schmittel M (2012) Rapid and highly sensitive dual-channel detection of cyanide by bis-heteroleptic ruthenium (II) complexes. Inorg Chem 51:7075–7086
Afkhami A, Sarlak N (2007) A novel cyanide sensing phase based on immobilization of methyl violet on a triacetylcellulose membrane. Sensors Actuators B Chem 122:437–441
Park IS, Heo EJ, Kim JM (2011) A photochromic phenoxyquinone based cyanide ion sensor. Tetrahedron Lett 52:2454–2457
Anzenbacher P, Tyson DS, Jursíková K, Castellano FN (2002) Luminescence lifetime-based sensor for cyanide and related anions. J Am Chem Soc 124:6232–6233
Ding Y, Li T, Zhu W, Xie Y (2012) Highly selective colorimetric sensing of cyanide based on formation of dipyrrin adducts. Org Biomol Chem 10:4201–4207
Gimeno N, Li X, Durrant JR, Vilar R (2008) Cyanide sensing with organic dyes: studies in solution and on nanostructured Al2O3 surfaces. Chem Eur J 14:3006–3012
Lv X, Liu J, Liu Y, Zhao Y, Sun YQ, Wang P, Guo W (2011) Ratiometric fluorescence detection of cyanide based on a hybrid coumarin-hemicyanine dye: the large emission shift and the high selectivity. Chem Commun 47:12843–12845
Xie Y, Ding Y, Li X, Wang C, Hill JP, Ariga K, Zhu W (2012) Selective, sensitive and reversible “turn-on” fluorescent cyanide probes based on 2, 2′-dipyridylaminoanthracene-Cu2+ ensembles. Chem Commun 48:11513–11515
Männel-Croisé C, Zelder F (2012) Complex samples cyanide detection with immobilized corrinoids. ACS Appl Mater Interfaces 4:725–729
Lee S, Nam YS, Choi SH, Lee Y, Lee KB (2016) Highly sensitive photometric determination of cyanide based on selective etching of gold nanorods. Microchim Acta 183(11):3035–3041
Liao T, Yuan F, Shi C, He CX, Li Z (2016) Lanthanide chelate-encapsulated polystyrene nanoparticles for rapid and quantitative immunochromategraphic assay of procalcitonin. RSC Adv 6:103463–103470
Liu B, Chen Y (2013) Responsive lanthanide coordination polymer for hydrogen sulfide. Anal Chem 85(22):11020–11025
Barth A, Mäntele W (1998) ATP-induced phosphorylation of the sarcoplasmic reticulum Ca2+ ATPase: molecular interpretation of infrared difference spectra. Biophys J 75:538–544
Yang Y, Yin C, Huo F, Chao J, Zhang Y, Cheng F (2014) A new highly selective and turn-on fluorescence probe for detection of cyanide. Sensors Actuators B Chem 193:220–224
Li Q, Zhang JH, Cai Y, Qu WJ, Gao GY, Lin Q, Wei TB (2015) A facile colorimetric and fluorescent cyanide chemosensor: utilization of the nucleophilic addition induced by resonance-assisted hydrogen bond. Tetrahedron 71:857–862
Mu J, Feng Q, Chen X, Li J, Wang H, Li M (2015) Silica nanoparticles doped with an iridium (III) complex for rapid and fluorometric detection of cyanide. Microchim Acta 182:2561–2566
Razi SS, Ali R, Srivastava P, Misra A (2014) Simple Michael acceptor type coumarin derived turn-on fluorescence probes to detect cyanide in pure water. Tetrahedron Lett 55(18):2936–2941
Zhai Y, Jin L, Wang P, Dong S (2011) Dual-functional Au-Fe3O4 dumbbell nanoparticles for sensitive and selective turn-on fluorescent detection of cyanide based on the inner filter effect. Chem Commun 47:8268–8270
Dong Y, Wang R, Tian W, Chi Y, Chen G (2014) “Turn-on” fluorescent detection of cyanide based on polyamine- functionalized carbon quantum dots. RSC Adv 4:3701–3705
Shang L, Zhang L, Dong S (2009) Turn-on fluorescent cyanide sensor based on copper ion-modified CdTe quantum dots. Analyst 134:107–113
Xu Z, Chen X, Kim HN, Yoon J (2010) Sensors for the optical detection of cyanide ion. Chem Soc Rev 39:127–137
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 21575023), the Natural Science Foundation of Jiangsu Province (Grant No. BK20161414) and the Postgraduate Scientific Research Innovation Program of Jiangsu Province (Grant No. KYLX15_0162).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 474 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Wang, S. & Chen, Y. Detection of cyanide via extended π-conjugation-induced fluorescence enhancement of a metal organic framework composed of terbium(III), bipyridyl and adenosine diphosphate. Microchim Acta 184, 4597–4602 (2017). https://doi.org/10.1007/s00604-017-2505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2505-8