Abstract
The authors describe the synthesis of a multifunctional nanocomposite with an architecture of type Fe3O4@SiO2@graphene quantum dots with an average diameter of about 22 nm. The graphene quantum dots (GQDs) were covalently immobilized on the surface of silica-coated magnetite nanospheres via covalent linkage to surface amino groups. The nanocomposite displays a strong fluorescence (with excitation/emission peaks at 330/420 nm) that is fairly selectively quenched by Hg2+ ions, presumably due to nonradiative electron/hole recombination annihilation. Under the optimized experimental conditions, the linear response to Hg2+ covers the 0.1 to 70 μM concentration range, with a 30 nM lower detection limit. The high specific surface area and abundant binding sites of the GQDs result in a good adsorption capacity for Hg2+ (68 mg⋅g−1). The material, due to its superparamagnetism, can be separated by using a magnet and also is recyclable with EDTA so that it can be repeatedly used for simultaneous detection and removal of Hg2+ from contaminated water.

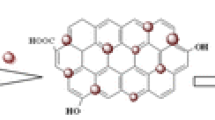
A schematic view of preparation process for the Fe3O4@SiO2@graphene quantum dots nanocomposite (denoted as Fe3O4@SiO2@GQDs). The graphene quantum dots were covalently immobilized on the surface of silica-coated magnetite nanospheres (Fe3O4@SiO2) via covalent linkage to surface amino groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury is considered as the most toxic nonradioactive metal, and leads to adverse environmental and health problems such as bioaccumulation by numerous organisms and severe physiological problems, including neurological, neuromuscular, or nephritic disorders originating from its presence in water resources [1]. As a consequence, mercury-indicating methodologies, which are developed to provide critical information for mercury hazard assessment and mercury pollution management, have received much attention. The fluorescence assays display unique advantages of high sensitivity, great simplicity, easy monitoring, and rapid response, providing a better choice for the detection of Hg2+ [2]. The typical fluorescent sensing system based on organic dyes exhibits high sensitivity and relative versatility. However, these organic fluorescent probes are mostly synthesized through several steps as well as the structures of the probes are complicated; limit their reliable application in the real sample assay [3]. Therefore, new nanomaterial-based fluorescent probes with a few synthesis steps, high sensitivity and selectivity for Hg2+ measurement is of vital importance.
Graphene quantum dots (GQDs), as a member of carbon-based photoluminescent nanomaterial, have increasingly drawn research interests on account of their fascinating physical and chemical properties, chemical inertness, low toxicity, excellent biocompatibility, good dispersibility in water, and stable photoluminescence (PL) [4]. GQDs are graphene nanosheets in the form of one, two or more layers all less than 10 nm thick and 100 nm in lateral size. Compared to graphene sheets with the zero band gap electronic state, GQDs have discrete band-gaps and show typical semiconducting properties [5]. Their band gap and optical properties can be manipulated by reducing their size to nano-level [6]. GQDs are attracting considerable attention as they may gradually replace traditional semiconductor quantum dots due to their superiority in chemical inertness, low toxicity, biocompatibility, high fluorescent activity and excellent photostability [7]. Moreover, compared to the common carbon-based photoluminescent nanomaterials (carbon dots), GQDs exhibit some excellent characteristics such as higher surface area and better surface grafting by using the π–π conjugated network or abundant surface functional groups (carboxyl, hydroxyl, carbonyl, epoxide) [8, 9]. By taking advantages of their unique chemical and optical properties, various GQDs-based fluorescent probes for the detection of metal ions (Fe3+, Cu2+, Pb2+ and Hg2+) [10–15], anions (S2−) [16], organic molecules (paranitrophenol) [17] and biomolecules (urea, glucose) [18, 19] have been explored. However, most of these reports have been focused on homogeneous systems as the solution state, which is not recyclable and separable. Since, GQDs have ultrahigh specific surface area due to the nature of nano-sized single layer graphene sheets, they have the potential not only for sensitive detection of pollutants, but also for effective separation and removal of them from environments. It means that novel sensing structures based on GQDs which can firstly detect and then remove pollutants from samples should be developed.
It seems that multifunctional magnetic composite materials, which combines magnetic sorting feature and additional characters from each component, can satisfy above requirement. Fe3O4 nanoparticles have been proved promising in site-specific targeting, sample sorting and isolating [20, 21] To improve their performance, Fe3O4 nanoparticles are usually covered by silica shell to prevent aggregation and provide sites for surface modification [22].
Bearing the above statement in mind, we synthesized a multifunctional nanocomposite material (Fe3O4@SiO2@GQDs) (Scheme 1). This nanocomposite was successfully prepared by coating of the GQDs onto the surface of amine functionalized Fe3O4@SiO2 nanospheres through acylamide binding in the presence of EDC as the activator. The synthesized nanocomposite exhibited high performance not only for detection, but also the removal of Hg2+ from aqueous solution. Additionally, the mercury deposited magnetic nanocomposite can also be recovered and reused without significant loss of its activity. The Fe3O4@SiO2@GQDs nanocomposite was easily separated from solutions by adding an external magnetic field, so the nanospheres with Hg2+ were quickly removed. The successful application of the Fe3O4@SiO2@GQDs in the detection of Hg2+ ions in real water samples is also demonstrated. The dual application of Fe3O4@SiO2@GQDs as sensing platform and adsorbent can be potentially extended to other environmental systems and biological samples.
Experimental
Reagents
Citric acid, ferric chloride hexahydrate (FeCl3.6H2O, 97%), ferrous chloride tetrahydrate (FeCl2.4H2O, 98%), ammonium hydroxide (NH4OH, 25%), 1-(3-dimethylaminopropyl)3-ethylcarbodiimide hydrochloride (EDC) and Hg(NO3)2.H2O, were purchased from Sigma-Aldrich (Chemical Co., Milwauke, WI, USA, www.sigmaaldrich.com). Tetraethyl orthosilicate (TEOS) and 3-aminopropyltriethoxysilane (APTES), was obtained from Merck (Darmstadt, Germany, www.merck.de). 10 mM phosphate buffer solutions were prepared by mixing stock standard solutions of NaH2PO4 and Na2HPO4. All abovementioned materials were used without any further purification. The deionized water was used in all procedures.
Apparatus
The Fourier transform infrared (FT-IR) spectra of samples were recorded using a Bruker Equinox 55 apparatus (www.bruker.com). Transmission electron microscopy (TEM) was performed using a Zeiss EM10C instrument (www.zeiss.com) at an accelerating voltage of 80 kV. X-ray diffraction (XRD) measurements were carried out using a Bruker, D8-advance diffractometer (www.bruker.com) with Cu Kα radiation (λ = 1.541874 Å) at a generator voltage of 40 kV and a generator current of 30 mA. Raman spectrum was recorded using an Almega Thermo Nicolet Dispersive Raman Spectrometer (USA) with a 532 nm laser. The magnetic property was analyzed by using a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co., Kashan, Iran). Fluorescence spectra were determined by using a Varian Cary Eclipse Fluorescence Spectrometer (www.varianinc.com). Absorption spectrum was determined on a Perkin Elmer, Lambda 25 Spectrophotometer. The concentration of mercury was analyzed by a Varian 710ES Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) (www.varianinc.com).
Preparation of core–shell structured magnetic nanospheres
Synthesis of the GQDs
The GQDs were produced with the use of a simple “bottom-up” method in which citric acid was incompletely pyrolyzed [23]. Briefly, 2 g of citric acid transferred to a 25 mL round bottom flask, and heated to 200 °C by a heating mantle for about 30 min, until the citric acid changed to an orange liquid. This liquid was then dissolved by dropwise addition of a sodium hydroxide solution (10 mg mL−1 NaOH) and vigorous stirring until the pH of the GQD solution was neutral (pH = 7.0). This solution was stored at 4 °C.
Synthesis of Fe3O4@SiO2
Fe3O4 nanoparticles were synthesized via the co-precipitation of Fe2+ and Fe3+ ions (molar ratio 1:2) in alkali solution. FeCl3.6H2O (3.7 mmol) and FeCl2.4H2O (1.85 mmol) were dissolved in 30 mL deionized water and the resulting solution was dropped to a 25% NH4OH solution (10 mL) with precisely constant drop rate under nitrogen gas and vigorous mechanical stirring (800 rpm) to obtain small and uniform particles. A black precipitate of Fe3O4 was continuously stirred for 1 h at room temperature and then heated to 80 °C for 2 h. The synthesized Fe3O4 was collected by a permanent magnet after washing three times with deionized water and ethanol. Then, it was dried at 100 °C in vacuum for 24 h. A sample of Fe3O4 (1 g) was suspended thoroughly in methanol (80 mL) by ultrasonic bath for 1 h at 40 °C. Then, concentrated ammonia solution was added to the resulting mixture and stirred at 40 °C for 30 min. Afterward, TEOS (1.0 mL) was introduced to the reaction vessel, and continuously stirred at 40 °C for 12 h. The silica-coated magnetic nanoparticles were collected simply in few seconds by a permanent magnet and were thoroughly redispersed and washed with deionized water three times and then dried at 60 °C in vacuum for 24 h.
Preparation of Fe3O4@SiO2@GQDs
For the preparation of Fe3O4@SiO2@GQDs nanocomposites, 0.1 g of the prepared Fe3O4@SiO2 was dispersed in 1.5 mL APTES solution (4% v/v in dry toluene). The mixture was stirred under reflux condition at 110 °C for 24 h. After it is cooled to room temperature, the sample was separated from the solution by an external magnet, and washed thoroughly twice with toluene and once with distilled water and the amino-functionalized Fe3O4@SiO2 nanospheres were obtained. Then, the amino-functionalized Fe3O4@SiO2 nanospheres were dispersed in a mixture of 1.0 mL of the prepared GQDs and 1.0 mL of EDC (10 mg mL−1). The mixed suspension was stirred at 4 °C for 12 h. Finally, the Fe3O4@SiO2@GQDs nanocomposites were collected simply in few seconds by a permanent magnet and were thoroughly washed with deionized water to remove unbound GQDs.
General procedure for fluorescent detection
All the fluorescence detections were conducted under the same conditions and the excitation wavelength was set at 330 nm. First, 3 mL phosphate buffered solution of the nanomaterial (4 μg mL−1, pH = 6.0) was added in a quartz cuvette, followed by the addition of a certain volume of Hg2+ solution (1 × 10−2 M). The solution was mixed thoroughly and left at room temperature for 1 min for the reaction to complete. The PL spectra of the mixed solutions were recorded and all experiments were performed at room temperature. The concentrations of Hg2+ used in the sensitivity experiments were 0, 0.1, 5.0, 10.0, 20.0, 30.0, 40.0, 50.0, 60.0, 70.0, 80.0, 90.0, 100.0, 110.0, 120.0 and 130.0 μM, respectively. 100.0 μM of Hg2+ was used in the optimization experiments. Various other metal ions of 100.0 μM were used in the selectivity experiments. The other metal ions used in the selectivity experiments are as follows: Na+, K+, Mg2+, Al3+, Cr3+, Ca2+, Mn2+, Ni2+, Fe2+, Fe3+, Pb2+, Cu2+, Co2+, Cd2+, Zn2+.
Adsorption studies
To measure the adsorption kinetics of Hg2+ ions onto the Fe3O4@SiO2@GQDs nanocomposite, 10 ml of mercury solution with an initial concentration of 50 mg L−1 was introduced into the flask and mixed with 5 mg of Fe3O4@SiO2@GQDs. The solution was stirred continuously at 25 °C. The Hg2+ loaded composite nanospheres were then isolated from the solution by adding an external magnetic field at different time intervals. To investigate the equilibrium adsorption isotherms for Hg2+, 5 mg of Fe3O4@SiO2@GQDs was added into 10 mL Hg2+ solution with the concentration ranging from 10 to 50 mg L−1 and collected until the adsorption equilibrium was reached. The pH values of the above systems were maintained at 6.0. The adsorbent was separated by an external magnetic field. The amount of the target ion in the solution was determined by ICP/AES.
Results and discussion
Structural characterization
Figure 1a, b exhibits the FTIR spectra of GQDs and Fe3O4@SiO2@GQDs nanocomposite. The broad intense peaks at 3300 cm−1 are assigned to −OH and −NH groups stretching vibration. Additionally, the peak at 2939 cm−1 in spectra shows the asymmetric stretching and symmetric vibrations of C − H. The well-defined peak at 1574 cm−1 is related to bending vibrations of C = C group. The peaks at 1668, 1412, and 1049 cm−1 were assigned to the C = O, C − O (carboxy), and C − O (alkoxy) functional groups, respectively. For the Fe3O4@SiO2@GQDs nanocomposite (Fig. 1b), the characteristic peak at 1070 cm−1 corresponds to the stretching vibration of Si–O and the peak at 583 cm−1 is due to the vibrations of Fe–O. The spectrum of the nanocomposite includes the main characteristic peaks of GQDs (beside peaks of Fe3O4@SiO2) that the successful coating of the GQDs onto the surface of Fe3O4@SiO2 nanospheres.
The X-ray diffraction (XRD) patterns of GQDs and Fe3O4@SiO2@GQDs samples are shown in Fig. 1c, d. As shown in Fig. 1c, GQDs exhibited a wide (002) peak at 22.6°, which was in good agreement with the interlayer distance of graphene sheets, demonstrating the graphitic nature of the crystalline dots [24]. The XRD pattern of the Fe3O4@SiO2@GQDs nanocomposite (Fig. 1d) shows diffraction peaks at 2θ = 30.2, 35.5, 43.1, 54.3, 57.1 and 62.8, which can be assigned to the (220), (311), (400), (422), (511) and (440) planes of Fe3O4, respectively, indicating that the Fe3O4 nanoparticles in the Fe3O4@SiO2@GQDs nanocomposite were pure Fe3O4 with a cubic spinel structure; these match well with the standard Fe3O4 sample (JCPDS card no. 85–1436). As shown in Fig. 1d, no diffraction peaks corresponding to SiO2 were observed because the prepared SiO2 is amorphous. Moreover, no signal about GQDs can be detected, which is explained by the small amounts, high dispersion and low crystallinity of GQDs in Fe3O4@SiO2@GQDs nanocomposite.
Transmission electron microscopy (TEM) was performed to investigate the structure and morphology of the GQDs and Fe3O4@SiO2@GQDs nanocomposite (Fig. 2). From the TEM image (Fig. 2a), it can be seen that the diameters of GQDs were 3–7 nm. Coating of the GQDs onto the surface of Fe3O4@SiO2 nanospheres was achieved through acylamide binding in the presence of EDC as the activator. As shown in Fig. 2b, the resulting composites were spherical nanoparticles with a diameter of about 20 nm and GQDs are present at the periphery of nanospheres.
The magnetic property of the Fe3O4@SiO2@GQDs nanocomposite was investigated by VSM (Fig. S1 (Electronic Supplementary Material, ESM)). The Raman spectrum for the GQDs is shown in Fig. S2 (ESM). More details are placed in the Electronic Supplementary Material (ESM).
Optical characterization
The UV-Vis absorption spectrum of the GQDs showed that there was a shoulder peak at 230 nm and a typical absorption peak at 335 nm (Fig. S3 (ESM)). The peak at 230 nm was attributed to the π-π* transitions of aromatic C = C bonds. The peak at 335 nm corresponds to the n-π* transition of the C = O bond [25]. The fluorescence intensity of Fe3O4@SiO2@GQDs nanocomposite is slightly lower than the free GQDs (Fig. S3 (ESM)). The drop in the fluorescence intensity of the nanocomposite can be attributed to the covalent functionalization of the GQDs with silica-coated magnetite nanospheres containing NH2 groups, which modifies the original moieties in the GQDs. The GQDs aqueous solution exhibited bright blue emission under excitation of 365 nm UV light as shown in Fig. S4a (ESM) inset. The emission wavelength of GQDs is excitation-independent, with the maximum excitation wavelength and the maximum emission wavelength at 335 and 450 nm, respectively (Fig. S4a (ESM)). The excitation-independent emission of the GQDs, implies that both the size and the surface state of those sp2 clusters contained in GQDs should be uniform, which may contribute to their strong fluorescence emission [23, 26]. In order to confirm that GQDs have been immobilized onto the surface of Fe3O4@SiO2 nanospheres successfully, the fluorescence spectra of Fe3O4@SiO2@GQDs nanocomposite at different excitation wavelengths and photograph under UV light are shown in Fig. S4b (ESM).
Optimization of conditions for Hg2+ detection
To improve the performance of the prepared assay for Hg2+, the following parameters were optimized: (a) sample solution pH value; (b) reaction time. Respective data and Figures are given in the Electronic Supplementary Material (ESM). The following experimental conditions were found to give best results: (a) sample solution pH value of 6.0 (Fig. S5, ESM); (b) reaction time of 1 min (Fig. S6, ESM).
Sensitivity of the Fe3O4@SiO2@GQDs for Hg2+ detection
The sensitivity of the synthesized Fe3O4@SiO2@GQDs towards the sensing of Hg2+ ions was analyzed by fluorescence titrations. From Fig. 3, it can be seen that the fluorescence intensity of Fe3O4@SiO2@GQDs is gradually quenched as the concentration of Hg2+ gradually increases from 0 to 130 μM. We found that the fluorescence quenching in this system followed the Stern-Volmer equation [27].
Emission spectra of the Fe3O4@SiO2@GQDs (4 μg mL−1) in the presence of increasingconcentrations of Hg2+ in phosphate buffer (pH = 6.0), excitated at 330 nm (a) and Stern–Volmer plot for thefluorescence quenching of Fe3O4@SiO2@GQDs by Hg2+ ions (inset: linear relationship between I0/I within the Hg2+ concentrations in the range from 0.1 to 70 μM. I0 and I are the emission fluorescence intensities of Fe3O4@SiO2@GQDs at 420 nm in the absence and presence of metal ions, respectively) (b)
Where I0 and I are the fluorescence intensity in the absence and presence of Hg2+ ions, respectively, KSV is the Stern-Volmer constant, and [Q] is the quencher concentration. Quenching data are usually presented as plots of I0/I versus [Q]. The Stern-Volmer plot shown in Fig. 3b does not fit a linear calibration plots over the whole Hg2+ concentration range of 0.1 to 130 μM. In contrast, inset of Fig. 3b reveals that a linear calibration plot for the quantitative analysis of Hg2+ can be fitted between the I0/I and the concentration of Hg2+ at the range from 0.1 to 70 μM, with a linear regression equation of I0/I-1 = 0.0101 [Hg2+] + 0.0006 (μM) (correlation coefficient R2 = 0.996, n = 10). At higher concentrations of Hg2+ there is a deviation from linearity (Fig. 3b), probably due to the presence of both static and dynamic mechanisms as observed by other researchers [28]. The error bars represent variations among three separate measurements. The detection limit of 30 nM was obtained based on a 3δ/m (δ is the standard deviation of the blank Fe3O4@SiO2@GQDs sample signal (n = 10) and m is the slope of the linear calibration plot), which was lower than most of the previous reported assays for Hg2+ detection.
A performance comparison of the methods for the analysis of Hg2+ from this work with that from some other studies was investigated (Table 1). It reveals that this method has high sensitivity, good linear response range and comparable detection limit to that found in other studies. The exception are some functionalized semiconductor quantum dots, which are highly sensitive. However, these nanomaterials suffer from intrinsic limitations such as heavy metals potential toxicity and environmental hazards and are being replaced by less toxic nanomaterials such as GQDs and carbon dots.
Selectivity of Fe3O4@SiO2@GQDs for Hg2+ detection
To evaluate the selectivity of Fe3O4@SiO2@GQDs for metal ions, the relative fluorescence intensity changes to different metal ions were measured in water (Fig. 4, blue bars). The results indicated that the fluorescence of Fe3O4@SiO2@GQDs was highly quenched by Hg2+ and slightly quenched by Fe2+ and Fe3+ ions, whereas the other tested metal ions induce no obvious changes in the fluorescence intensity ratio. These results clearly demonstrated that the method was highly selective for Hg2+ over a number of other metal ions (Fig. 4, blue bar). However, the probe may be unsuitable to use for complicated sample where Fe2+ (100 fold) or Fe3+ (1000 fold over Hg2+) are exist. Therefore, Fe3O4@SiO2@GQDs can be an efficient probe for Hg2+ detection in real water samples with undetectable concentration of Fe2+ and Fe3+ or after removal of these ions with an appropriate masking agent. Furthermore, competition experiments were conducted in the presence of Hg2+ and other metal ions (Fig. 4, green bars). It can be seen that the fluorescence of Fe3O4@SiO2@GQDs upon binding to Hg2+ did not significantly change in the presence of other metal ions. The outstanding Hg2+ ion selectivity can be ascribed to the strong affinity between Hg2+ and the carboxylic (−COOH) and hydroxyl (−OH) functionalities on the GQDs surface over other metal ions as reported in the literature [37]. The selective fluorescence quenching of the nanocomposite by Hg2+ is presumably due to the facilitating nonradiative electron/hole recombination annihilation via an effective electron transfer from the GQDs to Hg2+ ions [38], i.e. the excited-state electrons transfer to the LUMO of the Hg2+ cation from the GQDs, and then, the electrons return to the ground state of the GQDs in a radiationless transfer. Some other metal ions may also adsorb on the surface of GQDs, but the ion binding is weaker and the binding affinity is not as strong as that of Hg2+. Thus, the electron transfer and fluorescence quenching will be weaker [39].
Analytical application
To monitor the possible application of the fluorescence assay for real samples, three different water samples, river water (Babolrud river), well water (Tehran) and tap water (Tehran) were analyzed and the results were summarizes in Table 2. River water was filtered using a 0.45 μm pore size membrane filter to remove suspended particular solids. After dispersing Fe3O4@SiO2@GQDs in each sample, the fluorescence intensity spectra of the prepared samples were recorded to explore possible fluorescence quenching. Table 2 shows the results derived from the recorded fluorescence spectra after the addition of Hg2+ with the help of calibration curve. For each sample, five parallel experiments were conducted, and the recoveries from the water samples were excellent and varied from 97.8% to 105.0% with the relative standard deviation (RSD) ranging from 1.2% to 2.2%. These results indicated that the fluorescence analytical assay based on the core–shell Fe3O4@SiO2@GQDs nanospheres has the potential applicability for mercury detection in real samples.
Adsorption performance
Besides the sensing performance, we also investigated the use of the Fe3O4@SiO2@GQDs nanocomposite as an adsorbent for the magnetically guided removal of Hg2+ from water samples. The amount of Hg2+ adsorption at equilibrium qe (mg g−1) was calculated by Eq. (2):
Where C0 and Ce (mg L−1) are the liquid phase concentrations of Hg2+ at initial and equilibrium, respectively, V (L) the volume of the solution, and W (g) is the mass of adsorbent used. Fig. S7 (ESM) shows the adsorption kinetic curve of Fe3O4@SiO2@GQDs to Hg2+. It can be found that the uptake of Hg2+ by the nanospheres is a very fast process, which finishes in about 1.5 min. This fast process is attributed to the strong affinity of the fluorescent probe towards Hg2+. To optimize the design of an adsorption system for the adsorption of Hg2+, two equilibrium models, i.e., Langmuir and Freundlich isotherms, have been used to describe the equilibrium characteristics of the adsorption [40]. These adsorption models give a representation of the adsorption equilibrium between the analyte in solution and the active sites of the adsorbent. The Langmuir isotherm is based on the monolayer adsorption model with the homogeneous binding sites on the adsorbent surface. The linear form of Langmuir isotherm can be expressed as;
Where Ce (mg L−1) is the equilibrium solute concentration, qe (mg g−1) the equilibrium adsorption capacity, and qmax (mg g−1) and b (L mg−1) are Langmuir constants related to maximum monolayer adsorption capacity and energy change in adsorption, respectively. The Langmuir constants qmax and b were calculated from the slope and intercept of the linear plot of Ce/qe versus Ce, and their values are listed in Table S1 (ESM).
The Freundlich isotherm describes the multilayered adsorption on a heterogeneous surface and is expressed in the linear form as;
Where Kf and n are the Freundlich constants which represent the adsorption capacity (mg1–1/n L1/n g−1) and adsorption intensity, respectively. Kf and n values were calculated from the intercept and slope of the linear plot between ln qe and ln Ce, and their values are listed in Table S1. The fit of a model to the experimental data are usually evaluated in terms of linear regression analysis where the R2 value is used as an indication for the goodness of model fit. With respect to R2 values (Table S1, ESM), the adsorption of Hg2+ on the Fe3O4@SiO2@GQDs can be evaluated as a process that follows the Langmuir model. Therefore, the maximum adsorption capacity of mercury ions is found to be 68.027 mg per gram of Fe3O4@SiO2@GQDs adsorbent.
Recycling sensing performance
An important requirement for the solid multifunctional probe for detection and removal of Hg2+ ions is its reusability. To investigate its reusability, 5 mg of Fe3O4@SiO2@GQDs nanosphere was used to complex Hg2+ in a repeated adsorption–desorption fashion. In this test, EDTA solution (in deionized water) was used as a stripping agent for removal of Hg2+ from the composite nanospheres. Fig. S8 (ESM) indicates the change of fluorescence intensity of Fe3O4@SiO2@GQDs in five repeated experiments. This process involves the following steps: (1) washing Fe3O4@SiO2@GQDs with EDTA (2 mL of 0.01 M EDTA for 5 mg of the nanocomposite) for 2 min, (2) washing Fe3O4@SiO2@GQDs with water and ethanol for 3 times, respectively, (3) fluorescent detection of Hg2+. As shown in Fig. S8 (ESM), the recovered nanospheres was successfully used for at least four consecutive cycles and a very negligible loss of sensing ability was observed (which might be due to the loss of Fe3O4@SiO2@GQDs in the regeneration experiment).
Conclusions
In summary, we designed and synthesized a multifunctional fluorescent probe based on Fe3O4@SiO2@GQDs nanospheres for simultaneously detecting and removing of Hg2+. These multifunctional nanospheres exhibited high selectivity and sensitivity for targeting Hg2+ over a number of other metal ions. Since, fluorescence was slightly quenched by Fe2+ and Fe3+ ions, the Fe3O4@SiO2@GQDs can be an efficient probe for Hg2+ detection in real water samples with undetectable concentration of Fe2+ and Fe3+ or after removal of these ions. Moreover, by the method of ICP-AES, the used nanocomposite proved that they can efficiently remove Hg2+ in aqueous solution and be easily separated from the mixture by adding an external magnetic field. More importantly, the Fe3O4@SiO2@GQDs can also be regenerated by treating with EDTA solution and maintain high efficiency upon repeated use for at least four times. This means that the Fe3O4@SiO2@GQDs can be reused and will cause little additional environmental pollution each use compared to the amount of pollution caused by using non-renewable materials. The sensing method has been successfully applied for the detection of Hg2+ in real water samples.
References
Amini MK, Khezri B, Firooz AR (2008) Development of a highly sensitive and selective optical chemical sensor for batch and flow-through determination of mercury ion. Sensors Actuators B Chem 131:470–478
Wang ZX, Ding SN (2014) One-pot green synthesis of high quantum yield oxygen-doped, nitrogen-rich, photoluminescent polymer carbon nanoribbons as an effective fluorescent sensing platform for sensitive and selective detection of silver(I) and mercury(II) ions. Anal Chem 86:7436–7445
Ding Y, Wang S, Li J, Chen L (2016) Nanomaterial-based optical sensors for mercury ions. Trends Anal Chem 82:175–190
Hallaj T, Amjadi M, Manzoori JL, Shokri R (2015) Chemiluminescence reaction of glucose-derived graphene quantum dots with hypochlorite, and its application to the determination of free chlorine. Microchim Acta 182:789–796
Li Y, Hu Y, Zhao Y, Shi G, Deng L, Hou Y, Qu L (2011) An electrochemical avenue to green luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv Mater 23:776–780
Sun R, Wang Y, Ni Y, Kokot S (2014) Graphene quantum dots and the resonance light scattering technique for trace analysis of phenol in different water samples. Talanta 125:341–346
Bacon M, Bradley SJ, Nann T (2014) Graphene quantum dots. Part Part Syst Charact 31:415–428
Li L, Wu G, Yang G, Peng J, Zhao J, Zhu JJ (2013) Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 5:4015–4039
Xu J, Wang Y, Hu S (2017) Nanocomposites of graphene and graphene oxides: synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim Acta 184:1–44
Zhang C, Cui Y, Song L, Liu X, Hu Z (2016) Microwave assisted one-pot synthesis of graphene quantum dots as highly sensitive fluorescent probes for detection of iron ions and pH value. Talanta 150:54–60
Wang FX, Gu ZY, Lei W, Wang WJ, Xia XF, Hao QL (2014) Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sensors Actuators B Chem 190:516–522
Qi YX, Zhang M, Fu QQ, Liu R, Shi GY (2013) Highly sensitive and selective fluorescent detection of cerebral lead(II) based on graphene quantum dot conjugates. Chem Commun 49:10599–10601
Chakraborti H, Sinha S, Ghosh S, Pal SK (2013) Interfacing water soluble nanomaterials with fluorescence chemosensing: graphene quantum dot to detect Hg2+ in 100% aqueous solution. Mater Lett 97:78–80
Wang B, Zhuo S, Chen L, Zhang Y (2014) Fluorescent graphene quantum dot nanoprobes for the sensitive and selective detection of mercury ions. Spectrochim Acta A Mol Biomol Spectrosc 131:384–387
Li Z, Wang Y, Ni Y, Kokot S (2015) A rapid and label-free dual detection of Hg (II) and cysteine with the use of fluorescence switching of graphene quantum dots. Sensors Actuators B Chem 207:490–497
Yu N, Peng H, Xiong H, Wu X, Wang X, Li Y, Chen L (2015) Graphene quantum dots combined with copper(II) ions as a fluorescent probe for turn-on detection of sulfide ions. Microchim Acta 182:2139–2146
Zhou Y, Qu ZB, Zeng Y, Zhou T, Shi G (2014) A novel composite of graphene quantum dots and molecularly imprinted polymer for fluorescent detection of paranitrophenol. Biosens Bioelectron 52:317–323
Shao T, Zhang P, Tang L, Zhuo S, Zhu C (2015) Highly sensitive enzymatic determination of urea based on the pH-dependence of the fluorescence of graphene quantum dots. Microchim Acta 182:1431–1437
He YZ, Wang XX, Sun J, Jiao SF, Chen HQ, Gao F, Wang L (2014) Fluorescent blood glucose monitor by hemin-functionalized grapheme quantum dots based sensing system. Anal Chim Acta 810:71–78
Xu Y, Zhou Y, Ma W, Wang S, Li S (2013) Functionalized magnetic core–shell Fe3O4@SiO2 nanoparticles for sensitive detection and removal of Hg2+. J Nanopart Res 15:1716–1724
Xiao D, Lu T, Zeng R, Bi Y (2016) Preparation and highlighted applications of magnetic microparticles and nanoparticles: a review on recent advances. Microchim Acta 183:2655–2675
Lu D, Yang L, Tian Z, Wang L, Zhang J (2012) Core-shell mesoporous silica nanospheres used as Zn2+ ratiometric fluorescent sensor and adsorbent. RSC Adv 2:2783–2789
Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, Lin X, Chen G (2012) Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 50:4738–4743
Liu Y, Liu CY, Zhang ZY (2011) Synthesis and surface photochemistry of graphitized carbon quantum dots. J Colloid Interface Sci 356:416–421
Dai Y, Long H, Wang X, Wang Y, Gu Q, Jiang W, Wang Y, Li C, Zeng TH, Sun Y, Zeng J (2014) Versatile graphene quantum dots with tunable nitrogen doping. Part Part Syst Charact 31:597–604
Fan LS, Hu YW, Wang X, Zhang LL, Li FH, Han DX, Li ZG, Zhang QX, Wang ZX, Niu L (2012) Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 101:192–197
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, New York
Mohapatra S, Sahu S, Sinha N, Bhutia SK (2015) Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 140:1221–1228
Lu Y, Yu J, Ye W, Yao X, Zhou P, Zhang H, Zhao S, Jia L (2016) Spectrophotometric determination of mercury(II) ions based on their stimulation effect on the peroxidase-like activity of molybdenum disulfide nanosheets. Microchim Acta 183:2481–2489
Kamali KZ, Pandikumar A, Jayabal S, Ramaraj R, Lim HN, Ong BH, Bien CSD, Kee YY, Huang NM (2016) Amalgamation based optical and colorimetric sensing of mercury(II) ions with silver@graphene oxide nanocomposite materials. Microchim Acta 183:369–377
Chen ZB, Zhang CM, Tan Y, Zhou TH, Ma H, Wan CQ, Lin YQ, Li K (2015) Chitosan-functionalized gold nanoparticles for colorimetric detection of mercury ions based on chelation-induced aggregation. Microchim Acta 182:611–616
Shamsipur M, Safavi A, Mohammadpour Z, Ahmadi R (2016) Highly selective aggregation assay for visual detection of mercury ion based on competitive binding of sulfur-doped carbon nanodots to gold nanoparticles and mercury ions. Microchim Acta 183:2327–2335
Yan F, Kong D, Luo Y, Ye Q, He J, Guo X, Chen L (2016) Carbon dots serve as an effective probe for the quantitative determination and for intracellular imaging of mercury(II). Microchim Acta 183:1611–1618
Yang R, Ding X, Zhou Y, Li J, Qu L, Zhang K (2015) A novel fluorescent sensor for mercury (II) ion using self-assembly of poly(diallyl dimethyl ammonium)chloride functionalized CdTe quantum dots. Anal Methods 7:436–442
Ding X, Qu L, Yang R, Zhou Y, Yang J, Li J (2015) A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Luminescence 30:465–471
Zhang J, Yu SH (2014) Highly photoluminescent silicon nanocrystals for rapid, label-free and recyclable detection of mercuric ions. Nanoscale 6:4096–4101
Lu W, Qin X, Liu S, Chang G, Zhang Y, Luo Y, Asiri AM, Alyoubi AO, Sun X (2012) Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem 84:5351–5357
Wu ZZ, Li WY, Chen J, Yu C (2014) A graphene quantum dot-based method for the highly sensitive and selective fluorescence turn on detection of biothiols. Talanta 119:538–543
Xia YS, Zhu CQ (2008) Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 75:215–221
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Acknowledgements
We gratefully acknowledge financial support of this investigation by The Research Council of University of Tehran through Grant (Grant no.94009890).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 946 kb)
Rights and permissions
About this article
Cite this article
Alvand, M., Shemirani, F. A Fe3O4@SiO2@graphene quantum dot core-shell structured nanomaterial as a fluorescent probe and for magnetic removal of mercury(II) ion. Microchim Acta 184, 1621–1629 (2017). https://doi.org/10.1007/s00604-017-2134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2134-2