Abstract
An agent-free microwave-assisted method was developed for the preparation of a reduced graphene oxide/Fe3O4@gold nanocomposite. This material was used as an adsorbent for magnetic solid-phase extraction of organochlorine pesticides (OCPs) from water samples. The nanocomposite was characterized by transmission electron microscopy, scanning electron microscopy, Fourier transform infrared spectroscopy and energy-dispersive X-ray spectroscopy. The effects of sample volume, amount of sorbent, eluent volume, extraction and desorption time, and the effect of salt on the extraction efficiency were optimized. The linear response range of GC analysis extends from 0.05 to 500 μg L−1 of OCPs, the limits of detection range from 0.4 to 4.1 ng L−1, relative standard deviations from 1.7 to 7.3 %, and recoveries (from spiked seawater samples) from 69 to 114 %.

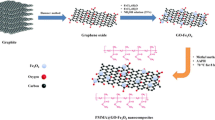
Oxygen functional groups in graphene oxide can bind to magnetic Fe3O4 nanoparticles through chemical bonds, and a magnetic reduced graphene oxide (RGO) is thus obtained. The interaction between magnetic RGO and HAuCl4 led to dispersion of the gold nanoparticles on the substrate. π-interaction of RGO and AuNPs with aromatic rings made this sorbent effective for extraction of organochlorine pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic solid-phase extraction (MSPE) is based on the adsorption of target analyte(s) on a magnetic adsorbent. Generally, in this procedure the extraction can be performed in an easy way, and it can save the time, without any need to the expensive equipment and large volume of the eluent. The adsorbed analyte(s) can be easily separated by an external magnetic field.
Until now different adsorbents have been reported. Carbon-based nanomaterials have been appeared as a strong coating due to their remarkable electronic properties and excellent mechanical, electrical and chemical properties [1]. Graphene recently is one of the most widely used carbon-based nanomaterials. Graphene has been attracted great attention as a sorbent due to its great properties such as large surface area, high adsorption capacity [2], and its usability in small quantities. The graphene-based MSPE have been used in various fields such as environmental analysis, food safety analysis and bio-analysis. Luo et al. [3] used magnetic graphene as a sorbent for extraction of sulfonamide antibiotics. Han et al. [4] reported a MSPE method based on the Fe3O4/graphene oxide (GO) for preconcentration of several polycyclic aromatic hydrocarbons in water samples.
Bare graphene sheets tend to agglomerate or restack to form graphite, if the sheets are not separated well [5]. It would reduce their surface area. Chemical modification of graphene can develop its properties. Graphene is usually non-polar and hydrophobic material. But graphene oxide contains much more polar moieties, and thus has a more polar and hydrophilic character than graphene [6]. In addition pristine magnetic nanoparticles (MNPs) can be aggregated, resulting in a reduction in the magnetic properties [7]. Graphene oxide sheets are negativlely charged when dispersed in aqueous solution. The composition of Iron (III) oxide and graphene can be formed through the electrostatic interaction, because Fe3O4 has a positively charged surface in aqueous solutions [8]. Functional groups, such as carboxyl (−COOH), carbonyl (−CO), and hydroxyl (−COH) groups, connect GO to magnetic Fe3O4 nanoparticles through chemical bonds [9]. Thus composite of Fe3O4/GO can overcome the above limitations. The presence of Fe3O4 NPs on the GO surface may reduce its effective surface area and effect on its adsorption capacity.
Gold nanoparticles (Au-NPs) have many important properties such as chemical stability, biocompatibility, magnetic properties and ease of chemical modification [10]. Application of Au-NPs as stationary phases obtained high separation efficiencies for a variety of analytes [11]. The combination of Fe and Au, such that iron or iron oxide as core and gold as shell, is attractive because of the fantastic properties of gold. A lot of studies focus on Fe–Au core–shell NPs [12]. Stoeva et al. [13] have used Fe3O4 and gold as the inner and outer shell for silica core. Several studies have also been used Au-NPs without any treatment or improvement for practical application [14]. The benefits of Fe3O4-Au composite increased its application such as using as probes for DNA [15], protein separation [16], catalysis [17], and adsorption [18]. Coating of gold nanoparticles as a novel metal onto graphene sheets can act as an operative sorbent for solid phase extraction. The aurum atom of gold nanoparticles can cause π interaction with the analytes through the π-conjugated system [19]. Also, they can bind with a large range of organic molecules such as molecules containing thiol and amino groups [20].
Organochlorine pesticides (OCPs) have been widely used in around the world [21]. Most of them are hydrophobic and remain in marine environment and can accumulate in biological organisms [22]. It has been reported that OCPs may influence the function of human and wildlife endocrine system [23]. There are several mechanisms that result in the deposition of OCPs in the environment such as urban runoff, industrial waste, chemical spills, outflow from agricultural areas and etc. [24].
In this work, the reduced graphene oxide/Fe3O4@ Au-NPs composite (RGO/Fe3O4@Au) was synthesized with a simple microwave-assisted method and used for extraction of OCPs, as model compounds, in the natural water samples.
Experimental
Chemicals and reagents
Iron (III) chloride hexahydrate (FeCl3· 6H2O), iron (II) chloride tetrahydrate (FeCl2· 4H2O), sulfuric acid (98 %), phosphoric acid (85 %), potassium permanganate (KMnO4), graphite powder (99.9 %) and ammonia (NH3) were purchased from Merck (Darmstadt, Germany, www.merckmillipore.com). Acetonitrile (HPLC grade) and hydrogen tetrachloroaurate (III) trihydrate (HAuCl4.3H2O) were purchased from Samchun Co. (Seoul, Korea, www.samchun.com) and Acros Organics (Noisy-le-Grand, France, www.acros.com), respectively.
The OCPs (Heptachlor, Aldrin, p,p’ - DDE, Dieldrin, p,p’ - DDD and p,p’ - DDT) standards were purchased from Sigma-Aldrich (Milan, Italy, www.sigmaaldrich.com). The stock standard solutions of OCPs were prepared in acetonitrile at a concentration of 100 μg mL−1. The working solutions were daily prepared by diluting the stock standard solution with deionized water.
Apparatus
An Agilent 6890 N gas chromatograph (Wilmington, DE, USA, www.agilent.com) equipped with a split/splitless inlet and micro-electron capture detector (μ-ECD) was used for the determination of OCPs. The injector and detector temperatures were 260 and 300 °C, respectively. The carrier gas was N2 (99.999 %, www.roham.com) with the flow rate of 1.2 mL min−1. Chromatographic separation was accomplished with a HP-5 (5 % biphenyl +95 % ploydimethylsiloxane) fused-silica column (30 m × 0.32 mm × 0.25 μm). The GC oven was programmed as follow: 100 °C, ramp to 220 °C at 20 °C min−1 and held for 0.5 min, after that ramp to 230 °C at 2 °C min−1 and held for 3 min, then ramp to 260 °C at 20 °C min−1 and then held for 2 min.
The gas chromatography–mass spectrometry (GC–MS) analysis was also performed using a model 6890 N network GC system (Agilent, USA) equipped with a 5973 mass selective detector (Agilent, USA) and HP-5MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm). The GC-MS oven temperature program was the same as the GC oven temperature program. He (99.999 %) was used as the carrier gas at a flow rate of 1.2 mL min−1. The injector and auxiliary temperatures were set at 260 and 280 °C, respectively. The acquisition mode of GC–MS was scan mode.
An ETHOS 1 Milestone (www.milestonesci.com) closed vessel microwave digestion was used for synthesis of RGO/Fe3O4@Au. The program was the microwave radiation at 150 °C for 1 h with 400 W powers.
Preparation of the sorbent
Preparation of graphene oxide
For preparation of graphene oxide (GO), 360 mL H2SO4 and 40 mL H3PO4 were mixed. The resulting solution was held on a stirrer and 3 g graphite powder was added into the mixture slowly. Then, 18 g KMnO4 was added into the mixture in the ice bucket very slowly. After that, the resulting product was placed in a flask under stirring while the temperature was held at 50 °C for 24 h. The mixture was washed, centrifuged and placed in an oven for 2 h and then GO was obtained [25].
Preparation of RGO/Fe3O4
For magnetization of GO, 40 mg GO was dissolved in distilled water under sonication for 30 min. Then the product was transferred into a round bottom flask under stirring and nitrogen purge. Next, 800 mg FeCl3· 6H2O and 300 mg FeCl2· 4H2O were added into the flask with 50 mL distilled water and the temperature was increased to 80 °C. 10 mL ammonia was added into the mixture and the resulting product was held under stirring for 30 min. Then the magnetic RGO was washed with distilled water and placed in an oven for 2 h and finally gathered by a magnetic field [26].
Preparation of RGO/Fe3O4@Au
When the initial amount of HAuCl4 reaches to 20 mg, the Au NPs loaded on the GO sheet tend to aggregate together [27]. Nevertheless, to increase the percentage of Au in the sorbent composition, more than 20 mg HAuCl4 was used in this work. One mL aqueous solution containing 36 mg HAuCl4.3H2O was added to 130 mg RGO/Fe3O4 in 75 mL distilled water. The mixture was transferred into a flask under stirring for 2 h and the temperature was held at 60 °C, during which time the Au seed crystals were deposited on the G sheets. Then 1 mL HAuCl4 solution was once again added into the mixture under stirring [28]. Afterward, the product was transferred into 60 mL Teflon-lined autoclave and heated in an ETHOS 1 closed vessel microwave digestion at 150 °C for 60 min with 400 W powers. After that, the autoclave was cooled to room temperature, then the precipitate was washed with distilled water and ethanol several times, and RGO/Fe3O4@Au was obtained by a magnetic field. Then the product was dried in an oven at 80 °C for 12 h.
MSPE procedure
20 mg RGO/Fe3O4@Au and 0.5 g NaCl was added to a container containing 10 mL water. The analytes adsorption was occurred on the sorbent under stirring for 10 min. After that, the water solution was separated with an external magnet (1.4 T). The analytes were eluted from the sorbent with 250 μL acetonitrile under stirring for 2 min. 1 μL of eluted solution was injected to the GC-μECD.
Sample collection
Sea water sample was collected from Caspian Sea (Mazandaran province, Iran). The water sample was filtered through a 0.45 μm membrane filter and stored in dark at 4 °C until used for the analysis.
Results and discussion
Choice of materials
Gold nanoparticles are known to be biocompatible and less toxic, and also chemically stable. The aurum atom of gold nanoparticles can cause π-interaction with the analytes through the π-conjugated system. Therefore, the gold coating of the substrate can lead to the high extraction efficiency for OCPs. Also, graphene has remarkable properties such as huge surface area (with a calculated value of 2630 m2 g−1), good chemical stability, and graphitized basal plane structure, which allow it to have strong π–interactions with the aromatic compounds [29]. Graphene tend to agglomerate and causes a great loss of effective surface area and consequently a lower adsorption capacity. Oxygen containing groups on GO surface can interact with Fe3O4 and result in reduced graphene oxide. RGO can act as a substrate for π-interactions with OCPs. SPE with magnetic nanoparticles causes simple extraction due to the magnetic properties of the sorbent. Sorbent is easily separated from the solution by an external magnetic field. The presence of Fe3O4 NPs on the GO surface may reduce its effective adsorption area and cause lower adsorption capacity for the sorbent due to the occupation of some of the active sites on the GO sheets by the Fe3O4 nanoparticles. The gold nanoparticles can compensate this reduction. Synergic effect of Au helped to extraction of OCPs due to π-interaction of gold and RGO with the analytes.
The extraction efficiencies of RGO/Fe3O4 and RGO/Fe3O4@Au were compared for the OCPs in the same extraction conditions (n = 3) include: 10 mL sample volume, 20 mg sorbent, 30 and 10 min extraction and desorption times, respectively, and 3 mL acetonitrile as eluent solvent which was finally concentrated to 0.5 mL. As the results shown in Fig. 1, RGO/Fe3O4@Au sorbent showed the higher extraction efficiency than RGO/Fe3O4.
Characterization of RGO/Fe3O4@Au
Figure 2 illustrated the FT-IR spectra of GO, RGO/Fe3O4 and RGO/Fe3O4@Au. The spectra of the sorbent showed the O-H stretching vibration at 3457.2 cm−1. The peaks at 1174.8 cm−1 and 1031.2 cm−1 can be attributed to the C-O stretching vibration. The C = C stretching vibration peak at 1622.0 cm−1, and Fe-O stretching vibration peak at 630.3 cm−1 [30] was observed in the Fig. 2b and Fig. 2c. Moreover, comparison of the spectra of a, b and c in Fig. 2 shows a dramatic decrease in intensity of the adsorption peaks of oxygen containing functional groups, which indicates GO was partially reduced after magnetization by Fe3O4.
The morphology of RGO/Fe3O4@Au was investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Figure 3 shows TEM and SEM images of RGO/Fe3O4@Au. Although the total structure is particulate, some aggregates can also be seen in SEM and TEM images. The size estimations from the SEM image showed that the mean diameter of particles were about 50 (±10) nm. As shown in Fig. 3a the prepared sorbent has the diameters in the range of 20–60 nm.
The EDX spectrum of the sorbent, shown in Fig. 4, indicates that Au nanoparticles anchored successfully onto the RGO/Fe3O4 particles. The EDX analyzes were also performed for three batches of the product and the standard deviations of its elemental composition were as ±0.2, ±1.0 and ±0.8 for Au, Fe and C, respectively. It shows the good reproducibility of the synthesis material from batch to batch.
Optimization of the MSPE conditions
The effect of sample volume was examined in the range of 10–50 mL. The extraction conditions were as: 10 mg sorbent, 30 and 10 min extraction and desorption times, respectively, and 3 mL acetonitrile as eluent solvent which was finally concentrated to 0.5 mL. The concentration of OCPs was 100 μg L−1 in working solution. As the results shown in the Figure S1a (Electronic Supplementary Material), high extraction recovery was observed in sample volume of 10 mL, and after that the extraction recoveries were reduced slowly. Therefore, 10 mL was selected for the subsequent experiments.
Different amounts of RGO/Fe3O4@Au ranging from 10 to 40 mg were exposed to 10 mL solution of OCPs. The extraction conditions include: 10 mL of sample (100 μg L−1), 30 and 10 min extraction and desorption times, respectively, and 3 mL of acetonitrile as eluent solvent which was finally concentrated to 0.5 mL. The highest recoveries were obtained at 20 mg sorbent for all of the analytes (Online Resource 1b).
The influence of eluent volume was studied in the range of 0.1–0.5 mL. The extraction conditions include: 10 mL sample solution, 20 mg sorbent, and 30 and 10 min for extraction and desorption times, respectively. It was found that the highest extraction efficiency was obtained at 0.1 mL eluent solvent for most of the compounds (Online Resource 1c), but because of the practical problem of using this volume, 250 μL was used for the subsequent procedures.
Desorption times were also studied in the range of 2–15 min. The extraction conditions include: 10 mL sample solution, 20 mg sorbent, 30 min extraction time, and 250 μL acetonitrile as eluent. As can be seen in Figure S1d, 2 min was enough to elute the OCPs from the sorbent.
The extraction times were studied in the range of 2–30 min. The extraction conditions include: 10 mL sample solution, 20 mg sorbent, 2 min desorption time, and 250 μL acetonitrile as eluent. As shown in Figure S1e, the extraction recovery increased to the highest level at 10 min.
To investigate the salt effect on the extraction recovery, different amounts of NaCl in the concentration range of 0–30 % (w/v) were added into the sample solutions. The extraction conditions include: 10 mL sample solution (100 μg L−1), 20 mg sorbent, 2 and 10 min desorption and extraction times, respectively, and 250 μL acetonitrile as eluent. The highest extraction recovery was obtained by adding 5 % NaCl into the sample for most of the compounds. According to the results (Online Resource 1f), the addition of salt would decrease the solubility of OCPs in the aqueous phase and increase the hydrophobic interaction between the OCPs and the extraction phase, therefore salt addition increase the efficiency of extraction (salting-out). On the other hand, more addition of salt decreased the extraction recoveries due to weaken of interaction between OCPs and G/Fe3O4@Au (salting-in).
Reusability
To investigate the reusability of RGO/Fe3O4@Au, the sorbent was washed with 2 mL acetonitrile and then reused for next extraction under the optimized condition. The adsorption-desorption cycles were repeated seven times without any significant loss of the extraction efficiency.
Method validation
The figures of merit of the introduced method including linearity, limit of detections (LODs), limit of quantitations (LOQs), relative standard deviations (RSDs) and preconcentration factors (PFs) were studied for the extraction of OCPs (Table 1). The analytes exhibited good linearities in the range of 0.05–500 μg L−1 with the proper correlation coefficients (R2) from 0.991 to 0.999. The LODs and LOQs were in the range of 0.4–4.1 and 1.3–13.6 ngL−1, respectively. Preconcentration factors (PFs) were calculated based on the concentration ratio of the analytes in the extraction phase that was injected (Cinj) and that in the sample solution (Cs):
The PFs of the analytes were among 32–40, while the maximum theoretical PF value can be 40.
Table 2 compares the performance data of the introduced method and other methods reported in the literatures for analysis of the OCPs. The results show that the LODs and RSDs of the present method are better or comparable with the other works.
Analysis of real sample
To evaluate the performance of the method in real sample, it was applied for analyzing of the OCPs in sea water sample, collected from Caspian Sea. The detected concentration of the analytes were 2.89, 1.31, 1.55, 0.68, 3.75 μg L−1 for aldrin, p, p’ DDE, dieldrin, p, p’ DDD, p, p’ DDT, respectively. Heptachlor was not detected in the real sample. The recoveries for the spiked samples were ranged from 69 % to 114 % (Table 3). The chromatograms of the non-spiked and spiked seawater samples at two concentration levels (5 and 50 μg L−1) were shown in Fig. 5.
To confirm the gas chromatographic results in the real sample, GC–MS analysis was also performed for the non-spiked sample. As shown in Fig. 6, the analytes have been attended in real sample and the result confirms the presence of OCPs in the real sample.
Conclusion
The introduced sorbent exhibited excellent extraction efficiency for the OCPs, and it is expected that to have the potential to use as MSPE sorbent for other organic and inorganic analytes, especially for aromatic compounds. The use of Au showed considerable improvement in the adsorption and led to producing an adsorbent with the high extraction efficiency due to its π- interaction with the analytes and its high surface area. The introduced method showed good accuracy for determining OCPs in the environmental water samples. It was compatible with the environment, because of the low organic solvent consumption. It was also a quick, easy and sensitive method for determination of the OCPs. Low reusability, stability, selectivity are among the disadvantages of the introduced sorbent that functionalization of the composite material may overcome these limitations.
References
Mehdinia A, Khani H, Mozaffari S (2014) Fibers coated with a graphene-polyaniline nanocomposite for the headspace solid-phase microextraction of organochlorine pesticides from seawater samples. Microchim Acta 181:89–95. doi:10.1007/s00604-013-1071-y
Jiang X, Huang K, Deng D, Xia H, Hou X, Zheng C (2012) Nanomaterials in analytical atomic spectrometry. TrAC Trends Anal Chem 39:38–59. doi:10.1016/j.trac.2012.06.002
Luo YB, Shi ZG, GAO QA, Feng YQ (2011) Magnetic retrieval of graphene: extraction of sulfonamide antibiotics from environmental water samples. J. Chromatogr. A 1218:1353–1358. doi:10.1016/j.chroma.2011.01.022
Han Q, Wang ZH, Xia JF, Chen S, Zhang XQ, Ding MY (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395. doi:10.1016/j.talanta.2012.09.046
Yan S, Qi TT, Chen DW, Li Z, Li XJ, Pan SY (2014) Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A 1347:30–38. doi:10.1016/j.chroma.2014.04.073
Wu J, Xiao D, Zhao H, He H, Peng J, Wang C, Zhang C, He J (2015) A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim Acta 182:2299–2306. doi:10.1007/s00604-015-1575-8
Liu Q, Shi J, Wang T, Guo F, Liu L, Jiang G (2012) Hemimicelles/admicelles supported on magnetic graphene sheets for enhanced magnetic solid-phase extraction. J Chromatogr A 1257:1–8. doi:10.1016/j.chroma.2012.08.028
Han Q, Wang Z, Xia J, Chen S, Zhang X, Ding M (2012) Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 101:388–395. doi:10.1016/j.talanta.2012.09.046
Junyong C, Yongmei H, Yan L, JiaJia G (2013) Magnetic graphene oxides highly effective adsorbents for rapid removal of a cationic dye rhodamine B from aqueous solutions. RSC adv 3:7254–7258. doi:10.1039/c3ra22599b
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. doi:10.1021/cr030698+
Liu FK (2009) Analysis and applications of nanoparticles in the separation sciences: a case of gold nanoparticles. J Chromatogr A 1216:9034–9047. doi:10.1016/j.chroma.2009.07.026
Cho SJ, Kauzlarich SM, Olamit J, Liu K, Grandjean F, Rebbouh L, Long GJ (2004) Characterization and magnetic properties of core/shell structured Fe/Au nanoparticles J. Appl Phys 95:6804–6806. doi:10.1063/1.1676033
Stoeva SI, Huo F, Lee JS, Mirkin CA (2005) Three-layer composite magnetic nanoparticle probes for DNA. J Am Chem Soc 127:15362–15363. doi:10.1021/ja055056d
Deng X, Lin K, Chen X, Guo Q, Yao P (2013) Preparation of magnetic Fe3O4/Au composites for extraction of benzo[a]pyrene from aqueous solution. Chem Eng J 225:656–663. doi:10.1016/j.cej.2013.04.004
Stoeva SI, Huo FW, Lee JS, Mirkin CA (2005) Three-layer composite magnetic nanoparticle probes for DNA. J Am Chem Soc 127:15362–15363. doi:10.1021/ja055056d
Bao J, Chen W, Liu T, Zhu Y, Jin P, Wang L, Liu J, Wei Y, Li Y (2007) Bifunctional Au-Fe3O4 nanoparticles for protein separation. ACS Nano 1:293–298. doi:10.1021/nn700189h
Xuan S, Wang Y, Yu JC, Leung KC (2009) Preparation, characterization, and catalytic activity of core/shell Fe3O4@polyaniline@Au nanocomposites. Langmuir 25:11835–11843. doi:10.1021/la901462t
Zhao X, Cai Y, Wang T, Shi Y, Jiang G (2008) Preparation of alkanethiolate-functionalized core/shell Fe3O4@Au nanoparticles and its interaction with several typical target molecules. Anal Chem 80:9091–9096. doi:10.1021/ac801581m
Mehdinia A, Khojasteh E, Baradaran Kayyal T, Jabbari A (2014) Magnetic solid phase extraction using gold immobilized magnetic mesoporous silica nanoparticles coupled with dispersive liquid–liquid microextraction for determination of polycyclic aromatic hydrocarbons. J. Chromatogr. A 1364:20–27. doi:10.1016/j.chroma.2014.08.063
Ulman A (1991) An introduction to ultrathin organic films. Academic Press, Boston
Cortada C, Vidal L, Pastor R, Santiago N, Canals A (2009) Determination of organochlorine pesticides in water samples by dispersive liquid–liquid microextraction coupled to gas chromatography–mass spectrometry. Anal Chim Acta 649:218–221. doi:10.1016/j.aca.2009.07.041
Thomann RV (1989) Bioaccumulation model of organic chemical distribution in aquatic food chains. Environ Sci Technol 23:699–707. doi:10.1021/es00064a008
Nakata H, Kawazoe M, Arizono K, Abe S, Kitano T, Shimada H, Li W, Ding X (2002) Organochlorine pesticides and polychlorinated biphenyl residues in foodstuffs and human tissues from China: status of contamination, historical trend, and human dietary exposure. Arch Environ Contam Toxicol 43:473–480. doi:10.1007/s00244-002-1254-8
Vallack HW, Bakker DJ, Brandt I, Lundén EB, Brouwer A, Bull KR, Gough C, Guardans R, Holoubek I, Jansson B, Koch R, Kuylenstierna J, Lecloux A, Mackay D, McCutcheon P, Mocarelli P, Taalman RDF (1998) Controlling persistent organic pollutants–what next? Environ Toxicol Pharmacol 6:143–175. doi:10.1016/S1382-6689(98)00036-2
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. doi:10.1021/nn1006368
Kassaee MZ, Motamedi E, Majdi M (2011) Magnetic Fe3O4-graphene oxide/polystyrene: fabrication and characterization of a promising nanocomposite. Chem Eng J 172:540–549. doi:10.1016/j.cej.2011.05.093
Song J, Xu L, Xing R, Li Q, Zhou C, Liu D, Song H (2014) Synthesis of Au/graphene oxide composites for selective and sensitive electrochemical detection of ascorbic acid. Sci Report 4:7515–7521. doi:10.1038/srep07515
Liu H, Su X, Duan C, Dong X, Zhou S, Zhu Z (2014) Microwave-assisted hydrothermal synthesis of Au NPs–graphene composites for H2O2 detection. J Electroanal Chem 731:36–42. doi:10.1016/j.jelechem.2014.08.013
Sun H, Cao L, Lu L (2011) Magnetite/reduced graphene oxide nanocomposites:one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Res 4:550–562. doi:10.1007/s12274-011-0111-3
Verdonck L, Hoste S, Roelandt FF, Van der Kelen GP (1982) Normal coordinate analysis of α-FeOOH - a molecular approach. J Mol Struct 79:273–279. doi:10.1016/0022-2860(82)85065-5
Ratola N, Santos L, Herbert P, Alves A (2006) Uncertainty associated to the analysis of organochlorine pesticides in water by solid-phase microextraction/gas chromatography–electron capture detection—evaluation using two different approaches. Anal Chim Acta 573–574:202–208. doi:10.1016/j.aca.2006.03.065
Zambonin CG, Aresta A, Nilsson T (2002) Analysis of organochlorine pesticides by solid-phase microextraction followed by gas chromatography-mass spectrometry. Int J Environ Anal Chem 82:651–657. doi:10.1080/0306731021000075375
Leong MI, Huang SD (2008) Dispersive liquid–liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J Chromatogr A 1211:8–12. doi:10.1016/j.chroma.2008.09.111
Jiang X, Wu M, Wu W, Cheng J, Zhou H, Cheng M (2014) A novel dispersive micro-solid phase extraction method combined with gas chromatography for analysis of organochlorine pesticides in aqueous samples. Anal Methods 6:9712–9717. doi:10.1039/C4AY02302A
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOC 472 kb)
Rights and permissions
About this article
Cite this article
Mehdinia, A., Rouhani, S. & Mozaffari, S. Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim Acta 183, 1177–1185 (2016). https://doi.org/10.1007/s00604-015-1691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1691-5