Abstract
The authors have developed a rapid and efficient preconcentration method for speciation of Mo(VI), Sb(V) and V(V) that is based on ligandless ultrasound-assisted dispersive solid phase microextraction (UDSPME) using Fe3O4@Al2O3 nanoparticles (NPs) as a sorbent. The NPs were characterized using XRD, SEM, EDX and surface area (BET). The experimental parameters affecting the preconcetration system were optimized using fractional factorial design. The target analytes were preconcetrated, eluted with dilute nitric acid, and then determined by ICP-OES. Under optimum conditions, the limits of detection (for n = 20) for the analytes range from 0.16 to 0.18 ng L−1 and the limits of quantification range from 0.5 to 0.6 ng L−1. The repeatability (intra-day precision; for n = 15) and reproducibility (inter-day precision; for n = 7) ranged from 2.1 to 3.9 % and 4.5 to5.3 %, respectively. The accuracy of the UDSPME method was checked by analysing certified reference material (CRM) and standard reference materials (SRM). Finally, a non-chromatographic method was developed for speciation of Mo, Sb and V at trace levels and successfully applied for the determination of analytes in environmental water samples.

Fe3O4@Al2O3 nanoparticles were used as sorbent in ligandless ultrasound-assisted dispersive solid phase microextraction and speciation of Mo(VI), Sb(V) and V(V) in environmental water samples prior to inductively coupled plasma optical emission spectrometry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trace metals in environmental matrices may provide benefit or risk to living organisms. This is because some trace elements are toxic, non-biodegradable and tend to accumulate in vital human organs, where they can act progressively over a long period through food chains [1]. Therefore, proper sampling and sample preparation procedures are essential for preconcentration and speciation of trace metal analysis is required to provide reliable data [2]. This is because the latter will help in addressing the issues around human and environmental health.
The speciation of trace metals using analytical techniques in the environmental samples is important [3, 4]. However, in normal conditions, conventional analytical detection techniques such as FAAS, GFAAS, ICP-OES and ICP-MS, do not have selectivity to different valent state of an element [5]. In cases where direct chromatographic method is not applied, selective non-chromatographic procedure combined with detection techniques becomes the most effective strategy for speciation of trace elements [5].
Sample preparation techniques that are commonly employed for preconcentration and speciation of metal ions are reported in the literature. These include dispersive liquid-liquid micro-extraction (DLLME) [6] solid phase extraction [7] and ultrasound dispersive solid-phase extraction (UDSPE) [8]), among others. UDSPE has escalated as an alternative to conventional solid phase extraction. This preconcentration method is considered as quick, easy, effective, and safe sample preparation method [8, 9]. It employs the SPE methodology, but the sorbent is added to extract without conditioning, and the dispersion is carried out assisted by an external energy [9]. Sorbent dispersion results in increase of its active surface, thus to an improving the extraction kinetics. In addition, this method allows the use of smaller amounts of sorbent compared to the conventional extraction approaches resulting in the saving of solid phase material [9]. Suitable adsorbent materials for speciation of trace elements need to be carefully chosen. They should possess good characteristics such as large surface area and surfaces that have different charges at pHs lower or higher than its point of zero charge (PZC). These properties provide more sorption sites and lead to speciation of trace elements based on the pH of the solution.
The use of nanometer-sized metal oxides as adsorbents has become an active area of research in the field of analytical science due to their special properties [10]. The latter include small diameter, large specific surface area, corrosion-resistance, non-toxicity, low cost, high chemical stability, unique electrical properties and the resultant superior mechanical properties [11]. For this reason, researchers have applied metal oxide nanoadsorbents for speciation of trace elements. Wu et al. [12] synthesized zirconium coated magnetic NPs (Fe3O4@ZrO2) by sol–gel method. The prepared nanomaterials were applied for the speciation of chromium species in environmental water. The authors discovered that the pH played a vital role in the adsorption of chromium species on the surface of the magnetic material. This was because of the different adsorption characteristics of Fe3O4@ZrO2 NPs toward Cr(III) and Cr(VI) at a wide pH range. In addition, Afkhami et al. [13] reported the application of Ni0.5Zn0.5Fe2O4 nanocomposite for the removal and preconcentration of Cr(VI), Mo(VI), V(V) and W(VI) species from water and wastewater samples. Other methods are reported in the literature [5, 8, 14, 15], among others. Among various nanometer-sized metal oxides the iron and aluminium oxides have been widely used due to their superior advantages such as low cost, extensive availability, thermal stability and remarkable adsorption capacity [12].
The objective of the study was to synthesized using sol-gel method Fe3O4@Al2O3 NPs and characterized by scanning electron microscopy (SEM), energy dispersive X-ray (EDX), nitrogen adsorption-desorption, and zeta potential measurements. The synthesised NPs were applied as solid phase material for ultrasound-assisted dispersive solid phase microextraction (UDSPME). The latter was used for ligandless preconcetration and speciation of Mo, Sb and V species in environmental water samples. The speciation of Mo, Sb and V species was found to be pH dependent. The experimental parameters affecting the preconcentration and speciation method were optimized by two level fractional factorial design. To the best of our knowledge, the ligandless UDSPME using Fe3O4@Al2O3 NPs combined with ICP-OES for speciation of target elements has not been reported in the literature.
Experimental
Instrumentation
The quantification of the analytes (Sb, Mo and V) was performed using a ICP-OES spectrometer (iCAP 6500 Duo, Thermo Scientific, UK, www.thermofisher.com) equipped with equipped with a charge injection device (CID) detector. The samples were introduced with a concentric nebulizer and a cyclonic spray chamber. The operating parameters of the instruments are presented in Table S1.
Branson 5800 Ultrasonic Cleaner (UK, www.hilsonic.co.uk) and Eppendorf 5702 Centrifuge (Germany, www.eppendorf.com), were used for ultrasonic assisted extraction and centrifugation, respectively. The morphology Al2O3, Fe3O4 and Al2O3/Fe3O4 nanomaterials was observed using scanning electron microscope (SEM) (VEGAS-TESCAN, USA, www.tescan-usa.com) after gold coating and the diameter of the mixed metal oxide was measured by image processing software. The size distribution of NPs was investigated using dynamic light scattering (DLS) on a Zetasizer Nano ZS instrument (Malvern Instruments Ltd., Worcestershire, UK, www.malvern.com/ZetasizerNanoZS). The specific surface area value was determined from adsorption isotherms by the Brunauer, Emmett and Teller (BET) multipoint method using Surface Area and Porosity Analyzer (ASAP2020 V3. 00 H, Micromeritics Instrument Corporation, Norcross, USA, www.micromeritics.com). All the gases used for analysis were of instrument grade. X-ray powder diffraction (XRD, www.panalytical.com) measurements were carried out with a Philips X-ray generator model PW 3710/31 a diffractometer with automatic sample changer model PW 1775 (scintillation counter, Cu-target tube and Ni-filter at 40 kV and 40 mA).
Regents and standard
All reagents were of analytical grade unless stated otherwise and double distilled deionized water was used throughout the experiments. Absolute ethanol (99.9 %), iron oxide NPs, anhydrous aluminium chloride (99.999 % trace metals basis) ammonium hydroxide solution (28 %) and ultrapure nitric acid (69 %) were purchased form Sigma-Aldrich (St. Loius, MO, USA, www.sigmaaldrich.com). Spectrascan single element standard (1000 mg L−1) of Sb, Mo and V species (Teknolab, Norway, spectrascan.se/about-spectrascan) were used to prepare the working multi-element solutions for UDSPE at concentrations of 50 μg L−1 for all metal ions. A Spectrascan multi-element standard solution at a concentration of 100 mg L−1 (Teknolab, Norway, spectrascan.se/about-spectrascan) was used to prepare working standard solutions for quantification of analyte concentrations in model and sample solutions.
Preparation of Fe3O4@Al2O3 composite nanoparticles
Appropriate amount of Fe3O4 (1.0 g) was weighed into a beaker and dispersed in 100 mL ethanol, then 2.66 g AlCl3 was added to the mixture and stirred continuously using magnetic stirrer bar. Ammonium hydroxide (28 %) solution was added drop-wise to the mixture until sol-gel formed. The resulting sol-gel was left to mature at room temperature for 30 h, and then dried for 24 h at 100 °C. Finally the sol-gel was calcined by heating at 1000 °C in the furnace.
Ultrasonic dispersive solid phase extraction general procedure
A mass (accurately weighed 50–150 mg) of Fe3O4@Al2O3 nanocomposite was placed in 100 mL polypropylene sample bottles. The latter were then placed in a sample rack and dipped into an ultrasonic water bath at 25 °C. An aliquot of 50 mL model (synthetic sample) solution (pH 2–9) containing Mo(VI), Sb(V) and V(V) ions was cautiously added to the sample bottle and the latter were was covered with cap. The extraction of the target analytes was carried out by sonicating the sample bottles for 5–30 min. After a certain extraction time, the samples were immediately transferred to 50 mL centrifuge tubes. Then the adsorbent and model sample solutions were separated by centrifugation at 3000 rcf for 5 min. The target analytes adsorbed/by the solid phase material were transferred to an aqueous phase by adding 5.0 mL of elution solution (0.5–3.0 mol L−1 HNO3). The mixture was sonicated for 5 min and the two phases were separated by centrifugation at 3000 rcf for 5 min. The nitric acid phase was collected and the metal concentration adsorbed was determined using ICP-OES.
Optimization strategy
Multivariate optimization was performed considering four influential variables or factors. The latter include sample pH, eluent concentration (EC), amount of sorbent (AS) and extraction time (ET). A two-level (24–1) fractional factorial design with a central point was used for the optimization of factors that affect the preconcetration and extraction of target analytes. The upper and lower values given to each factor are shown in Table 1. The purpose of using multivariate optimization strategy is to obtain the optimum conditions and to minimise analysis time. The design of experiments was performed using Minitab 17 statistical software.
Results and discussion
Characterization of alumina-iron oxide nanoparticles
Figure 1 shows the XRD patterns for alumina, iron oxide and iron oxide/alumina NPs. The XRD pattern of alumina NPs showed peaks at 43.3°, 60.9° and 67.1°. These peaks were assigned to the two crystallization phases, that is, α-Al2O3 and γ-Al2O3 [7]. The XRD pattern of Fe3O4 particles showed characteristics peaks at 30.4°, 35.6°, 43.3°, 57.3°, and 62.8°. The XRD pattern for the binary metal oxide nanomaterial in show the main peaks at 43.1°, 62.8° and 66.1° corresponding to alumina and 35.8°, 57.2° and 62.7° corresponding to Fe3O4 [16, 17]. These results revealed that the metal oxides formed a single composite.
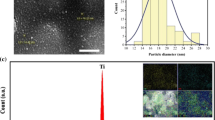
The SEM image (Fig. 2a) of Fe3O4@Al2O3 NPs demonstrates that the NPs are pseudo-spherical shaped. The DLS measurement demonstrated that the particle size of the NPs the size range of 80–100 nm. The mean size was found to be 90 ± 14 nm. Energy dispersive X-ray (EDX) analysis data (Fig. 2b) show that the main compositions of the sample are Fe, Al, and O. It can be seen from this figure that there are no foreign metals present as an impurities and the atomic percentages of Al and Fe were 36.4 and 31.7 %, respectively. In addition, the EDX spectra demonstrated that the Al2O3 phase was relatively abundant on the surface of nanocomposite. This was deduced from the high atomic percentage of aluminium. Furthermore, the EDX results confirmed the formation of alumina coat on the surface of Fe3O4 NPs. These results and the XRD results confirm that the prepared nanoadsorbent is composed of alumina and iron oxide.
The surface area and the pore structure of Al2O3/Fe3O4 NPs were examined using nitrogen adsorption/desorption experiments. The specific surface area, pore size, and the total pore volume were found to be 288 m2 g−1, 24.2 Å and 0.28 mL·g−1, respectively. The obtained results were comparable those reported by El-Latif et al. [17].
The zeta potentials of the Fe3O4@Al2O3 nanoadsorbent were determined at varied pH. The point of zero charge (PZC), also known as isoelectric point (IEP), for the synthesized nanoadsorbent was located at 5.4. The obtained results were in agreement with those reported in the literature [16, 18]). This means that the surface charge of the nanoadsorbent material remains negative above the PZC and positive below this value [19].
Choice of materials
Various low-polarity adsorbents materials including have been used for the preconcetration or speciation of trace elements in different in aqueous samples prior to their quantification. These include Amberlite XAD resins [20], biosorbent [21] polyurethane foams [22] and ion exchange resins [23], among others. However, some of these adsorbents need to be anchored by organo-functional groups. This is done in order to achieve selective preconcetration or speciation of trace elements. Due to the leaching behaviour of some sample matrices, the main drawback of using a modified sorbent is the difficulty in maintaining the OFG attached to the solid phase [23]. Due to the abovementioned limitation, high polar inorganic metallic oxides, such as titania [24], alumina [25], zirconia [26], tin oxide [27] and iron oxide [28], among others have been preconcentration for trace elements and their speciation analysis. In addition, these nanometer metal oxides provide high specific surface area and affinity for heavy metals in aqueous systems, high chemical and thermal stability as well as amphoteric properties (29). However, some of these nanoadsorbents are unusable in fixed beds or flow systems because of the excessive pressure drops [29, 30]. Conversely, the use of magnetic nanocomposite materials attract increasing attention because they can easily be separated from water under a magnetic field and can be recycled or regenerated [29]. Among these nanoadsorbents, Fe3O4 NPs are the most frequently used magnetic materials in sample preparation due to their easy preparation, surface modification and good recoverability [31]. Unmodified Fe3O4 NPs can be directly used for speciation and preconcentration of trace elements. However, they are prone to the formation of large aggregates resulting in changes of their magnetic properties. Therefore, alumina was chosen in order to provide an inorganic coating to the magnetic Fe3O4 NPs and to avoid their dissolution in acidic solutions. In addition, alumina was used to lengthen their utilization without losing their magnetic properties [32]. Hence, due to the above attraction properties and limited reports on Fe3O4@Al2O3 nanocomposite, it was chosen as solid material for speciation of the selected trace elements.
Optimization of ligandless ultrasound-assisted dispersive solid phase microextraction
Factors that influenced the performance of the UDSPME were optimised using multivariate strategy. The influential factors include selected sample pH, amount of sorbent (AS), eluent concentration (EC) and extraction time (ET). The percentage recoveries obtained for each metal ion were used as an analytical response. Preliminary studies showed that the lower oxidations of the studied elements are not retained at pH levels lower than 5. The experimental design matrix and the results derived from each run for Sb, Mo and V species, respectively (Table S2). The ANOVA results presented in terms of Pareto charts (Fig. S1), were used to evaluate the main effects and their interactions. The length of the Pareto chart bar is proportional to the absolute value of the estimated effects and helps in comparing the relative importance of effects (Nomngongo et al. [7].
The results showed that that sample pH was more significant for both antimony and molybdenum, except for vanadium. Other factors such as amount of sorbent, eluent concentration and extraction time had little statistical outcome on the extraction of Sb and V. The amount of sorbent was found to be significant for preconcetration of Mo(VI). In view of the above, it can been seen from the data collected in Table S2 and ANOVA results in Fig. 1 that Sb(V), Mo(VI) and V(V) can be pre-concentrated simultaneously due to the fact that they all had highest percentage recoveries at the same experimental conditions which are experiments 3 and 7. The decrease in the adsorption of target analytes at pH > PZC can be due to the electrostatic repulsion between the metal ions and the adsorbent surface. In addition, the change in the adsorption behaviour of the investigated metal species with pH might also be due to the effect of pH on the elemental speciation [13]. Therefore, the optimum sample pH, amounts of sorbent, eluent concentration and extraction time were chosen to be 2, 150 mg, 1.75 mol L−1 and 5 min, receptively.
Analytical figures of merit
Under optimum condition, the analytical figures of merit for the UDSPME/ICP-OES method were evaluated. The linearity was examined by preconcentrating multi-element species [Mo(VI), Sb(V) and V(V)] standards at concentrations in the range of 0.0001 to 150 μg L1and analysed by ICP-OES. The calibration curves were linear (r2 = 0.9987–0.9991) for all target analytes. The limits of detection (LOD) and limits of quantification (LOQ) were defined as the lowest concentration of an analyte giving signals equal to three or ten times, respectively, the standard deviation (SD) of blank signal divided by the slope of the calibration curve [7].
Under the optimum conditions, the LOD and LOQ (n = 20) were found to be 0.17, 0.16 and 0.18 ng L−1 and 0.57, 0.52 and 0.59 ng L−1for Mo, Sb and V, respectively. The repeatability (intra-day precision, n = 15) and reproducibility (inter-day precision, n = 7), expressed in terms of relative standard deviation (% RSD; n = 15), ranged from 2.1–3.9 % and 4.5–4.3 %, respectively. The enrichment factors (EF) calculated according to Meng et al. [33], were 270, 254 and 215 for Mo(VI), Sb(V) and V(V), respectively. The time required for preconcentration of 50 mL of sample (extraction time was 5 min; centrifusion time was 5 min and elution was 5 min) was about 15 min. It should be noted that the ultrasonic system used can hold up to 18 samples. Therefore, the overall time for processing all samples was approximately 20 min. Hence, the throughput sample was approximately 54 samples h−1.
A comparison of the method with others reported for preconcentration is summarized in Table 2. Our UDSPME has a low LOD and relatively high precision and enrichment factors. The latter were comparable with refs [13, 34, 40, 41] and lower than those reported by ref [35]. Overall, the analytical figures of merit for the target indicate that UDSPME/ICP-OES method is a reproducible, sensitive rapid and simple. Therefore, it is suitable for speciation and preconcentration of metal ion species in water samples.
Reusability and adsorption capacity
Reusability is one of the important factors for performance evaluation of an adsorbent. The reusability of Fe3O4@Al2O3 was investigated by dispersing the already used nanoadsorbent in 20 mL of a 2.0 mol L−1 HNO3 solution. The mixture was then sonicated for 10 min and washed three times with deionised water. The nanoadsorbent was separated by centrifugation. The Fe3O4@Al2O3 nanoadsorbent was dried in oven at 70 °C and reused. The RSD % of the obtained recovery indicated that the prepared nanoadsorbent was stable and can be re-used up to 30 times (RSD = 3.8 %) without an obvious decrease in the recoveries of studied elemental species, after desorption/wash/dry procedures.
The adsorption capacity determines how much of sorbent is required to quantitatively preconcentrate the analytes of interest from a given solution [42, 43]. Therefore, the adsorption capacity of the Fe3O4@Al2O3 as an adsorbent was studied and the experimental data were fitted into the general equation of the modified Langmuir model (equation not included). The adsorption capacities obtained for each analyte were different; this might be due to the variation in their size, degree of hydration and the value of their binding constant with the adsorbent [30]. The maximum adsorption capacities were found to be 151, 194, 189 mg g−1 for Mo(VI), Sb(V) and V(V), respectively.
Effect of interfering ions
Inorganic anions and cations are common components of different water samples. For this reason, interferences may occur due to the competition of these ions for the adsorbents sites, thus affecting the extraction of the target analytes. Therefore, the effect of potential interfering ions was evaluated in order to examine the possibility of selective recovery of the target analytes in the presence of some anions and cations in wastewater samples. The tolerance limit was set as the concentration of the interfering ion required to cause the relative error to be more than or equal to 5 % [7]). The results obtained are presented in Table 3. The results show that most of the investigated ions do not significantly interfere with the extraction of the target analyte and the recoveries quantitative (≤ 95 %). This is because Mo(VI), Sb(V) and V(V) exists as oxyanions in water samples. Therefore, their preconcentration was selective at pH 3. In addition, due to the large surface area, As(V) and Cr(VI) did not interfere.
Validation and application of the method
The accuracy of the UDSPME method was evaluated by analysing certified reference materials (CRMs) and standard reference materials (SRMs). A suitable aliquot of CRM-TWDM, CRM-TWDM-A, CWW-TM-A and CWW-TM-B were diluted 10-fold and their pH was adjusted to 2 with 1.0 mol L−1 nitric and NH3 solutions. The certified value and the analytical results (mean value ± standard deviation n = 7) determined by the current method are presented in Table 4. It can be seen that the values obtained are in good agreement with the certified and added values for all target analytes. The recoveries of target analytes ranged from 98 to 103 % for the CRMs and SRMs.
The applicability of the method was evaluated for the preconcetration and determination of Mo, Sb and V species in real environmental samples. The latter include tap water, wastewater and river water samples collected from selected areas in Johannesburg and results are presented in Table 5. It can be seen from the results that Mo and V were detected in all the samples and Sb was not detected in tap water samples. Antimony compounds such as Sb2O3 are normally used in the production of glassware and ceramics as a clarifying reagent. In addition, Sb2O3 is employed as a pigment in dyes and paints as well as in the textile industry [36]. Therefore, the relative high concentration of Sb(III) in wastewater and river water 1 sample might be due to residues of Sb(III) leached into waste by industrial activities. The high concentrations of V and Mo in river water 1 might due to the contamination from the mining activities that take place near this river.
The accuracy of the UDSPME/ICP-OES method was assessed by analysing the samples for total metal ion content using ICP-MS. And the results obtained were compared with those obtained by the USDSPME/ICP-OES method. According to Student paired t-test, the results were not significantly different at 95 % confidence level. These findings demonstrated the reliability and accuracy of the current method.
Conclusions
The Fe3O4@Al2O3 NPs were synthesized and successfully applied as solid phase sorbent in ligandless UDSPME method for preconcentration of V(V), Mo (VI) and Sb (V) species in water samples prior to ICP-OES detection, has been reported. The UDSPME preconcetration method demonstrated excellent qualities such as high sensitivity, low cost, high sample throughput, relatively low detection limits, high enrichment factors as well as high precision and accuracy. Due to high adsorption capacity and potential reusability, Fe3O4@Al2O3 NPs can be used as an effective adsorbent in the water and wastewater treatment for removal preconcentration of the trace amount of oxyanions. However, the main disadvantage of the UDSPME method is that it is applicable at sample pHs lower than the pH of the typical environmental samples (pH in a range of 6–8.5).
References
Ojeda CB, Rojas FSN, Pavon JMC (2012) Determination of cobalt in food, environmental and water samples with preconcentration by dispersive liquid-liquid microextraction. Am J Anal Chem 3:125–130
Picó Y (2012) Chemical Analysis of Food: Techniques and Applications: Techniques and Applications. Press, Academic
Uluozlu OD, Tuzen M, Mendil D, Soylak M (2010) Determination of As (III) and As (V) species in some natural water and food samples by solid-phase extraction on Streptococcus pyogenes immobilized on Sepabeads SP 70 and hydride generation atomic absorption spectrometry. Food Chem Toxicol 48(5):1393–1398
Vieira MA, Grinberg P, Bobeda CR, Reyes MN, Campos RC (2009) Non-chromatographic atomic spectrometric methods in speciation analysis: a review. Spectrochim Acta Part B 64(6):459–476
Jiang HM, Yan ZP, Zhao Y, Hu X, Lian HZ (2012) Zincon-immobilized silica-coated magnetic Fe3O4 nanoparticles for solid-phase extraction and determination of trace lead in natural and drinking waters by graphite furnace atomic absorption spectrometry. Talanta 94:251–256
Kazi TG, Tuzen M (2015) Magnetic stirrer induced dispersive ionic-liquid microextraction for the determination of vanadium in water and food samples prior to graphite furnace atomic absorption spectrometry. Food Chem 172:161–165
Nomngongo PN, Ngila JC (2014) Functionalized nanometer-sized alumina supported micro-solid phase extraction coupled to inductively coupled plasma mass spectrometry for preconcentration and determination of trace metal ions in gasoline samples. RSC Adv 4:46257–46264
Diniz KM, Tarley CRT (2015) Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction. Microchem J 123:185–195
Jamali MR, Firouzjah A, Rahnama R (2013) Solvent-assisted dispersive solid phase extraction. Talanta 116:454–459
Shishehbore M, Afkhami A, Bagheri H (2011) Salicylic acid functionalized silica-coated magnetite nanoparticles for solid phase extraction and preconcentration of some heavy metal ions from various real samples. Chem Cent J 5:1–10
Chen S, Zhu S, D L (2013) Titanium dioxide nanotubes as solid-phase extraction adsorbent for on-line preconcentration and determination of trace rare earth elements by inductively coupled plasma mass spectrometry. Microchem J 110:89–93
Wu H, Wang X, Liu B, Liu Y, Li S, Lu J, Yang Z (2011) Simultaneous speciation of inorganic arsenic and antimony in water samples by hydride generation-double channel atomic fluorescence spectrometry with on-line solid-phase extraction using single-walled carbon nanotubes micro-column. Spectrochim Acta Part B 66(1):74–80
Afkhami A, Aghajani S, Mohseni M, Madrakian T (2015) Effectiveness of Ni0.5Zn0.5Fe2O4 for the removal and preconcentration of Cr (VI), Mo (VI), V (V) and W (VI) oxyanions from water and wastewater samples. J Iran Chem Soc 12(11):2007–2013
Yan L, Deng B, Shen C, Long C, Deng Q, Tao C (2015) Selenium speciation using capillary electrophoresis coupled with modified electrothermal atomic absorption spectrometry after selective extraction with 5-sulfosalicylic acid functionalized magnetic nanoparticles. J. Chromatogr A 1395:173–179
Li Y, Wang Q, Li Q, Zhang Z, Zhang L, Liu X (2015) Simultaneous speciation of inorganic rhenium and molybdenum in the industrial wastewater by amino-functionalized nano-SiO2. J Taiwan Inst Chem 55:126–132
Mahapatra A (2013) Fabrication and Characterization of Novel Iron Oxide/Alumina Nanomaterials for Environmental Applications. Doctoral dissertation, National Institute of Technology, Rourkela
El-Latif MMA, Ibrahim AM, Showman MS, Hamide RRA (2013) Alumina/iron oxide nano composite for cadmium ions removal from aqueous solutions. Int. J. Nonferrous Metall 2:47–62
Sujana MG, Soma G, Vasumathi N, Anand S (2009) Studies on fluoride adsorption capacities of amorphous Fe/Al mixed hydroxides from aqueous solutions. J Fluor Chem 130(8):749–754
Chai L, Wang Y, Zhao N, Yang W, You X (2013) Sulfate-doped Fe3O4@Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res 47:4040–4049
Tiwari S, Sharma N, Saxena R (2016) On-line speciation of chromium using modified chelating resin and determination in industrial water samples by flame atomic absorption spectrometry. New J Chem. doi:10.1039/C5NJ02283E
Anagnostopoulos V, Symeopoulos B, Bourikas K, Bekatorou A (2015) Biosorption of U (VI) from aqueous systems by malt spent rootlets. Kinetic, equilibrium and speciation studies. Int J Environ Sci and Technol 1–12 doi:10.1007/s13762-015-0872-4
El-Shahawi MS, Al-Sibaai AA, Bashammakh AS, Alwael H, Al-Saidi HM (2015) Ion pairing based polyurethane foam sorbent packed column combined with inductively coupled plasma–optical emission spectrometry for sensitive determination and chemical speciation of bismuth (III & V) in water. J Ind Eng Chem 28:377–383
Nomngongo PN, Ngila JC, Kamau JN, Msagati TA, Moodley B (2013) Preconcentration of molybdenum, antimony and vanadium in gasolsine samples using Dowex 1-×8 resin and their determination with inductively coupled plasma–optical emission spectrometry. Talanta 110:153–159
Deng D, Zhou J, Ai X, Yang L, Hou X, Zheng C (2012) Ultrasensitive determination of selenium by atomic fluorescence spectrometry using nano-TiO 2 pre-concentration and in situ hydride generation. J Anal Atom Spectrom 27(2):270–275
Afkhami A, Saber-Tehrani M, Bagheri H, Madrakian T (2011) Flame atomic absorption spectrometric determination of trace amounts of Pb (II) and Cr (III) in biological, food and environmental samples after preconcentration by modified nano-alumina. Microchim Acta 172(1–2):125–136
Shakerian F, Dadfarnia S, Shabani AMH, Esfahani GS (2013) Preconcentration and determination of lead (II) by microextraction based on suspended cadion covered zirconia nanoparticles in a surfactant media. Microchim Acta 180(13–14):1225–1232
Nilchi A, Dehaghan TS, Garmarodi SR (2013) Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 321:67–71
Zhai Y, He Q, Yang X, Han Q (2010) Solid phase extraction and preconcentration of trace mercury (II) from aqueous solution using magnetic nanoparticles doped with 1, 5-diphenylcarbazide. Microchim Acta 169(3–4):353–360
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Dados A, Paparizou E, Eleftheriou P, Papastephanou C, Stalikas CD (2014) Nanometer-sized ceria-coated silica–iron oxide for the reagentless microextraction/preconcentration of heavy metals in environmental and biological samples followed by slurry introduction to ICP-OES. Talanta 121:127–135
Li XS, Zhu GT, Luo YB, Yuan BF, Feng YQ (2013) Synthesis and applications of functionalized magnetic materials in sample preparation. TrAC Trend Anal Chem 45:233–247
Giakisikli G, Anthemidis AN (2013) Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal Chim Acta 789:1–16
Meng L, Chen C, Yang Y (2015) Suspension Dispersive Solid Phase Extraction for Preconcentration and Determination of Cobalt, Copper, and Nickel in Environmental Water by Flame Atomic Absorption Spectrometry. Anal Lett 48(3):453–463
Pekiner OZ, Tüzen M (2014) Preconcentration and speciation of vanadium by three phases liquid–liquid microextraction prior to electrothermal atomic absorption spectrometry. J Ind Eng Chem 20:1825–1829
Bazán C, Gil R, Smichowski P, Pacheco P (2014) Multivariate optimization of a solid phase extraction system employing l-tyrosine immobilized on carbon nanotubes applied to molybdenum analysis by inductively coupled plasma optical emission spectrometry with ultrasound nebulization. Microchem J117:40–45
Jiang X, Wen S, Xiang G (2010) Cloud point extraction combined with electrothermal atomic absorption spectrometry for the speciation of antimony (III) and antimony (V) in food packaging materials. J Hazard Mater 175(1):146–150
Mulugeta M, Wibetoe G, Engelsen CJ, Lund W (2010) Speciation analysis of As, Sb and Se in leachates of cementitious construction materials using selective solid phase extraction and ICP-MS. J Anal Atom Spectrom 25:169–177
Erdem A, Shahwan T, Çağır A, Eroğlu AE (2011) Synthesis of aminopropyl triethoxysilane-functionalized silica and its application in speciation studies of vanadium (IV) and vanadium (V). Chem Eng J 174:76–85
Łukaszczyk L, Żyrnicki W (2010) Speciation analysis of Sb (III) and Sb (V) in antileishmaniotic drug using Dowex 1× 4 resin from hydrochloric acid solution. J Pharm Biomed Anal 52:747–751
López-García I, Rivas RE, Hernández-Córdoba M (2011) Use of carbon nanotubes and electrothermal atomic absorption spectrometry for the speciation of very low amounts of arsenic and antimony in waters. Talanta 86:52–57
Samadi-Maybodi A, Rezaei V (2012) A cloud point extraction for spectrophotometric determination of ultra-trace antimony without chelating agent in environmental and biological samples. Microchim Acta 178:399–404
Huang C, Hu B (2011) Synthesis and characterization of titania hollow fiber and its application to the microextraction of trace metals. Analyst 136(7):1425–1432
Nomngongo PN, Ngila JC (2015) Alumina–titania (Al2O3–TiO2) hollow fiber sorptive microextraction coupled to inductively coupled plasma mass spectrometry for determination of trace elements in diesel and gasoline samples. RSC Adv 5(89):72500–72507
Acknowledgments
The authors wish to thank University of Johannesburg (Applied Chemistry, Centre for Nanomaterial Science Research Centre) and National Research Foundation (NRF, South Africa, grant no. 98745) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 71.5 kb)
Rights and permissions
About this article
Cite this article
Nyaba, L., Matong, J.M. & Nomngongo, P.N. Nanoparticles consisting of magnetite and Al2O3 for ligandless ultrasound-assisted dispersive solid phase microextraction of Sb, Mo and V prior to their determination by ICP-OES. Microchim Acta 183, 1289–1297 (2016). https://doi.org/10.1007/s00604-016-1766-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1766-y