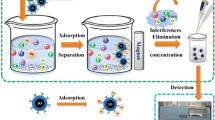
Abstract
The article describes a method for preconcentration and speciation of Cr(III) and Cr(VI) in river waters. It is based on ultrasound-assisted magnetic solid phase extraction employing a nanocomposite prepared from magnetite nanoparticles, manganese oxide and alumina oxide, and then functionalized with [3-(2-aminoethylamino)propyl] trimethoxysilane (AAPTMS). By taking advantage of the oxidative properties of MnO2, the Fe3O4@MnO2,Al2O3 nanocomposite was used for the preconcentration of total chromium. The AAPTMS-modified nanocomposite, in turn, is selective for Cr(VI). The concentration of Cr(III) can be calculated as the difference between total chromium and Cr(VI). The preconcentrated chromium species were quantified using ICP-OES. The method was optimized using response surface methodology. Under optimal conditions, the limit of detection and quantification are 20 and 50 pg ⋅ mL−1, respectively. The intraday and interday precisions of the method, expressed in terms of relative standard deviation, are 2.6 and 4.9%, respectively. In addition, the effects of potentially interfering ions were examined. The method was successfully applied to the speciation of chromium in spiked river water and a certified reference material.

The magnetic Fe3O4@MnO2,Al2O3 was used as an adsorbent for preconcentration of total chromium in aqueous samples, while Fe3O4@MnO2,Al2O3 modified with [3-(2-aminoethylamino)propyl] trimethoxysilane (AAPTMS) was used for speciation of Cr(VI). The concentrations of Cr(III) was calculated from total Cr and Cr(VI).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chromium is a known carcinogen that also causes a variety of adverse health effects [1]. However, these adverse effects depend on the oxidation state at which chromium is present within the environmental matrices. Chromium exists in water predominantly in two oxidation states such as Cr(III) and Cr(VI) [2]. Trivalent chromium is an essential element that plays a significant role in regulating the blood sugar levels by improving the way human bodies use insulin. Therefore, its present in our diet is required in ultra-trace levels [3]. Conversely, hexavalent chromium has no known health benefits and is therefore classified as a class 1 carcinogen [4]. Due to this, the world health organization (WHO) implemented a guideline value of 0.05 mg L−1 for total chromium which governs the recommended maximum concentrations acceptable in water [5]. Quantification of chromium in water is therefore essential for environmental assessment and remediation purposes. Usually chromium is quantified in water by determining the total concentration levels. However, total chromium concentration in water does not give satisfying results regarding the bioavailability, bioaccumulation and toxicity of chromium [6]. Due to this, the speciation of chromium is therefore necessary when evaluating the risk imposed by this trace metal in water bodies. This will give conclusive results as to whether the water under investigation is risky to both aquatic and terrestrial organisms.
There have been significant advancements on the analytical techniques used to quantify trace metals in water [7, 8]. However, these techniques can only quantify the total concentrations of trace metals present in water samples unless they are coupled with separation techniques that enable the speciation of trace metals [9]. One major drawback that limits the use of these coupled analytical techniques is cost since coupling techniques is highly expensive. Therefore, sample preparation methods that can separate different species of the same metal have been developed [2, 10–12]. These methods do not only separate different species of the analyte but also preconcentrate the analyte to isolate it from the entire matrix. These methods include; solid phase microextraction (SPME) [13], capillary microextraction (CME) [10], dispersive liquid-liquid microextraction [14] and magnetic solid phase extraction (MSPE) [11]. Although these conventional sample preparation methods suffer from various drawbacks that can limit their use in the speciation of trace metals, they can be easily manipulated such that they perform according to a particular objective. Amongst the aforementioned methods, MSPE is one of the sample preparation techniques that can be manipulated to ensure that it performs according to the stipulated objective. Thus, MSPE has been selected as the sample preparation method to be used based on its selectivity, specificity, cost effectiveness and simplicity [15]. In addition, the adsorbent used in MSPE technique is one major component that is usually manipulated to fit a particular objective.
Nanomaterials and polymers have been frequently used as adsorbents for the MSPE technique [16, 17]. However, most researchers have adopted the use of nanocomposites as adsorbent in MSPE as they are more effective than the previously used adsorbents. Nanocomposites are a combination of nanomaterials with different or the same dimensions, i.e. 0D, 1D and 2D nanomaterials [18]. Nanocomposites are much more effective adsorbents since they provide various adsorption sites based on their morphologies [19]. For instance, Nabid et al. [20] employed the use of multiwalled carbon nanotubes/poly(2-amino thiophenol) nanocomposites as an effective sorbents in solid-phase extraction for separation and preconcentration of Cd(II) and Pb(II) trace levels in environmental samples. The separated species were quantified using flame atomic absorption spectroscopy. The results indicate that the novel nanocomposite presents a high affinity for heavy metals due to the presence of several good extractive sites (S and N) which were introduced to the synthesized nanocomposite. Khan et al. [21] synthesized and used the magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) (MGO-DVB-VA) nanocomposite for magnetic solid phase extraction of Pb(II), Cd(II), Cu(II), Ni(II) and Co(II) prior to their determination by flame atomic absorption spectroscopy. The results for the analysis of certified reference materials showed that the percent recoveries in the range of 94–107% and the percent recoveries of water and food samples ranged from 94 to 108%. Metal oxides nanocomposites have also been reported for the preconcentration of trace metals in water via solid phase extraction [22, 23]. Most metal oxide nanocomposites are known to be ineffective in the speciation of trace metals in water; instead they are suitable for total content [23]. However, their modification with organic functional groups yields to adsorbents that are suitable separation and speciation of trace metals [12, 24].
Thus, the study aims on the (i) synthesis and characterization of Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite, (ii) application of the synthesized nanocomposite as a sorbent in UA-MSPE for speciation of chromium in river water samples and validation using the certified reference materials, (iii) ICP-OES quantification of the preconcentrated and separated Cr species (Cr(III) and Cr(VI)). The Fe3O4@MnO2,Al2O3 nanocomposite was influenced by the oxidative properties of MnO2 to give the total chromium in the water samples, whereas the Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was specific to Cr(VI) due to the presence of AAPTMS. To the best of our knowledge, the speciation of chromium using Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite has never been previously reported.
Experimental
Reagents and solutions
All reagents used were of analytical grade unless stated otherwise and ultra-pure (type 1 with 18 MΩ cm−1 resistivity) deionized water from a Milli-Q water purification system (Millipore, Bedford, MA, USA, www.merckmillipore.com) was used to prepare all solutions. Iron(III) chloride hexahydrate (FeCl3.6H2O) and iron(II) chloride tetrahydrate (FeCl2.4H2O) salts purchased from Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com) were used as precursors for the preparation of Fe3O4 nanoparticles. Ammonia solution (25% v/v) used all the way through was purchased from Associated Chemical Enterprises, (Pty) Ltd. (Johannesburg, South Africa, www.acechem.co.za). Manganese(II) chloride tetrahydrate (MnCl2.4H2O), Absolute ethanol and Aluminum chloride (AlCl3) were all purchased from Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com) and then used for the preparation of manganese oxide and alumina oxide. [3-(2-Aminoethylamino)propyl] trimethoxysilane (AAPTMS), Nitric acid (69% v/v) and toluene purchased from Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com) were used to functionalize the prepared nanocomposite. Cr(III) standard (PROLAB, VWR international LTD, Leicestershire, England, uk.vwr.com) and Cr(VI) standard (Spectrascan, Teknolab AB, Kungsbacka, Sweden, www.teknolab.se) were used to prepare all chromium model and calibration standard solutions. The certified reference material (Environmental express, South Carolina, USA, www.envexp.com) for trace metal in drinking water standard was used for validation of the UA-MSPE method. The ICP quality control multi element standard (PROLAB, VWR international LTD, Leicestershire, England, www.envexp.com) was used in the interferences studies.
Instrumentation
An ICP-OES (iCAP 6500 Duo, Thermo Scientific, UK, www.thermofisher.com) equipped with a charge injection device (CID) detector was used for the determination of Cr species. The instrument operating conditions used were recommended by the manufacturer and are specified in Table 1. All pH measurements were done using H1 9811–5, (HANNA Instruments, Smithfield, Rhode Island, USA, www.hannainst.com) pH meter supplied with a combined electrode. Branson 5800 Ultrasonic Cleaner (Danbury, CT, USA, www.hilsonic.co.uk) was used for the speciation analysis studies. An Infrared spectra (4000–400 cm−1) using a KBr disc technique were recorded using a PerkinElmer spectrum 100 Fourier transform infrared spectrometer (Waltham, MA, USA, www.perkinelmer.com). The XRD measurements were done on a PANalytical X’Pert X-ray Diffractometer (PANalytical BV, Netherlands, www.panalytical.com) using a Cu Kα radiation (λ = 0.15406 nm) in the 2θ range 4–90 ° at room temperature. The morphology of the nanocomposite was evaluated using the scanning electron microscopy (SEM, TESCAN VEGA 3 XMU, LMH instrument (Czech Republic, www.tescan.com) coupled with energy dispersive x-ray spectroscopy (EDS) for elemental composition analysis at an accelerating voltage of 20 kV. The specific surface area value was determined from adsorption isotherms by the Brunauer, Emmett and Teller (BET) multipoint method using Surface Area and Porosity Analyzer (ASAP2020 V3. 00H, Micrometrics Instrument Corporation, Norcross, USA, www.micromeritics.com) after degassing the samples at 77 K for 10 h. The magnetic properties of the material were done using a vibrating sample magnetometer (Limited PPMS, London-UK, www.cryogenic.co.uk).
Sample collection and preparation
Surface water samples from rivers and a dam were collected from different sampling sites in Thohoyandou and Makhado (Limpopo, South Africa) into the pre-cleaned polyethylene sample bottles that were rinsed overnight with 1% nitric acid and three times with deionized water before collection. The samples were kept in a cooler box with ice before they were stored in the refrigerator until they were analyzed. The sampling sites included Mutshindudi river located in Thohoyandou, Luvuvhu river located in Makhado and Nandoni dam located in Thohoyandou. It should be noted that two rivers of Mutshindudi and Luvuvhu connect in the Nandoni dam. Each of the two rivers is located near a wood preservation industry that uses Chromated Copper Arsenate for wood preservation. The samples were collected upstream (LV1, ND1 and MT1) and downstream (LV2, ND2 and MT2). The sample collection was carried out a day before analysis. Prior to analysis, the samples were filtered through the 0.22 pore size membrane filters (Millipore Corporation, Bedford, MA, USA, www.merckmillipore.com).
Synthesis of magnetic Fe3O4 nanoparticles
The magnetic Fe3O4 nanoparticles were prepared by the conventional co-precipitation method previously published with minor modifications [12]. In this method, a mixture containing 20.21 g of FeCl3.6H2O and 10.05 g of FeCl2.4H2O was dissolved in 300 mL deionized water under nitrogen atmosphere with vigorous stirring at 85 °C. Then, 50 mL of ammonia solution (25% v/v) was added quickly into the solution and the color of the solution immediately changed from orange to black. The mixture was stirred for another 15 min under the same conditions before it was cooled down at room temperature. The black magnetic precipitate (Fe3O4) was separated from the supernatant by magnetic decantation and then washed with deionized water to the final pH of 10. The Fe3O4 nanoparticles were dried in an oven at 60 °C for 10 h and then grinded to fine particles using a pulvirisette.
Synthesis of ferrous-ferric oxide@manganese oxide, alumina oxide (Fe3O4@MnO2,Al2O3) nanocomposite
The Fe3O4@MnO2,Al2O3 nanocomposite was prepared by the sol gel method. Briefly, 6.06 g of anhydrous AlCl3 and 6.04 g MnCl2.4H2O on a 1:1 ratio were dissolved in 100 mL of ethanol with vigorous stirring. Then, 12.34 g of the synthesized magnetic Fe3O4 nanoparticles was added into the solution and was followed by the dropwise addition of 60 mL of ammonia solution (25% v/v). The addition of the latter was done in order to form a gel to ensure the initiation for the formation of MnO2 and Al2O3 shell around the Fe3O4 core. The formed gel was left to maturate for 24 h at room temperature before it was dried at 60 °C in an oven for 12 h. The dark brown powder was then grinded into a fine powder using a pulvirisette. To ensure the complete formation of MnO2 and Al2O3 shell around the Fe3O4 core, the powder was calcined at 500 °C and 750 °C for 2 h.
Functionalization of Fe3O4@MnO2,Al2O3 nanocomposite with [3-(2-aminoethylamino)propyl] trimethoxysilane
The Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was prepared based on the modified method reported by Nomngongo and Ngila [25]. Prior to functionalization, Fe3O4@MnO2,Al2O3 nanocomposite synthesized previously was activated. Briefly, 12.34 g Fe3O4@MnO2,Al2O3 was placed in 5.00 mol L−1 HNO3 solution and refluxed for 12 h at 60 °C with vigorous stirring with a magnetic stirrer. The activated Fe3O4@MnO2,Al2O3 was filtered by vacuum filtration and washed with ultrapure water and ethanol several times and dried in an oven at 60 °C. Typically, 10.02 g of the activated Fe3O4@MnO2,Al2O3 was then mixed with 10 mL AAPTMS in 20 mL toluene solvent. The reaction was heated at 100 °C for 24 h under nitrogen atmosphere and then continuously stirred using a stirrer heater. The mixture was filtered and washed with toluene and ethanol. The final product was then dried in an oven at 60 °C for 12 h.
Ultrasound assisted magnetic solid phase extraction (UA-MSPE) procedure
In the optimization of the preconcentration method, Cr(III) and Cr(VI) model solutions at the concentration of 10 μg L−1 (pH 2.0–9.0) were prepared by serial dilutions from 1000 mg L−1 stock solutions of Cr(III) and Cr(VI), respectively. Each of the prepared model solution was then mixed with about 10–100 mg of the sorbent material in a polypropylene sample bottle and the solutions were sonicated for 5–15 min to extract Cr(III) or Cr(VI) from the liquid phase (solution) into the solid phase (sorbent material). The supernatant was separated from the sorbent material inside the sample bottle by magnetic decantation. In the sample bottle containing the sorbent material, about 5.0 mL of the eluent (1.00–3.00 mol L−1 HNO3) was added and the mixture was sonicated for 1–5 min to elute the adsorbed metal ions from the sorbent material into the solution. The supernatant was separated from the sorbent material by magnetic decantation and filtered into a pre-washed ICP-OES vial. The eluted analytes were quantified with ICP-OES. After the attainment of the optimum conditions from the optimization procedure described here, the same procedure was repeated for the certified reference material and river water samples to validate the UA-MSPE method.
Chemometric optimization of UA-MSPE preconcentration method
Optimization of methodologies is known to be one significant factor used to determine the best conditions that can be used to achieve the best possible results. A multivariate optimization approach was employed in this study to simultaneous investigate the effect imposed by certain variables on the preconcentration method. The variables investigated included; sample pH, amount of the sorbent (AS), extraction time (ET), eluent concentration (EC) and desorption time (DT) as shown in Table 2. Using STATISTICA version 13 software, a small central composite design (SCCD) with two central points was used in the optimization of factors that are influential on the performance of the analytical procedure. A total of 24 experiments were done and the percentage recoveries of the analyte of interest were used to find the optimum conditions to be used on the UA-MSPE preconcentration method.
Results and discussion
Choice of material
Various solid phase extraction adsorbents have been applied significantly for the preconcentration and speciation of trace metals in aqueous samples. This includes multi walled carbon nanotubes [26], activated carbon [27], ionic liquid [2], amongst others. However, these materials require complexing agents or chelating agents to ensure their effectiveness towards adsorption of trace metals is effective. In addition, the nanometer sized metal oxides such as Fe3O4/Fe2O3, TiO2, Al2O3, ZrO2 and GO-SiO2 [11–13, 28] have been confirmed as alternative adsorbent for preconcentration and speciation of trace metals in aqueous samples. However, most studies have indicated that these nanometer sized metal oxides are not selective and are not suitable for samples with complicated matrices. In order to improve their selectivity and adsorption capacities, the modification of the metal oxide sorbent is required [11, 18, 28]. In addition, combining various metal oxides can lead to higher adsorption capacities. This is due to the availability of various active sites presented by the combination of different metal oxides. For instance, Fe3O4 possesses magnetic properties that enable it to be separated from solution by applying an external magnetic field as well as excellent adsorption properties. In addition, Al2O3 has been proven to be a good adsorbent of trace metals from aqueous matrices and MnO2 is a strong oxidant that oxidizes Cr(III) to Cr(VI) to ensure that total chromium is adsorbed on the adsorbent completely. In view of the above, it is necessary to develop adsorbent that combines all the above-mentioned properties. Therefore, Fe3O4@MnO2,Al2O3@AAPTMS was synthesized for selective adsorption of Cr(VI) thus enabling speciation of Cr.
Characterization of the prepared nanocomposites
X-ray diffraction spectroscopy (XRD)
To evaluate the crystal structure of the prepared Fe3O4@MnO2,Al2O3 nanocomposite, XRD measurements were carried out. Fig. S1 shows the crystal structure of Fe3O4@MnO2,Al2O3 nanocomposite prepared by the sol gel method and calcined for 3 h at 500 °C. The characteristic peaks at 23.9° and 41.1° which were indexed as 111 and 310, respectively were attributed to MnO2 [29]. In addition, the characteristic peaks at 49.4°, 64.2° and 78.0° indexed as 331, 440 and 622, respectively were assigned to Al2O3 [25]. Based on the results, both MnO2 and Al2O3 were embedded on the crystal lattice of the core Fe3O4 nanoparticles thus forming a shell around Fe3O4 nanoparticles. There was no major shift on the magnetite peaks from the nanocomposite when compared to that of the magnetite nanoparticles; however the crystal structure changed from cubic to tetragonal. The change of structure is due to MnO2 and Al2O3 being embedded on the crystal lattice of magnetite. These results can be further confirmed with the TEM and FTIR. The Debye-Scherrer equation was employed in order to find the average particle size (D) was calculated as 21 nm at the most intense peak.
Scanning electron microscopy/ energy dispersive x-ray spectroscopy (SEM/EDS)
The surface morphology of Fe3O4@MnO2,Al2O3 nanocomposite was then studied using SEM (Fig. S2). The Fe3O4@MnO2,Al2O3 nanocomposite shows a variety of shapes and size of particles that are responsible for the adsorption properties of the material. The shapes of the particles included nanorods (dominant), semi-spheres (Fig. S2a) and flowers (Fig. S2b);. The presence of particles with different shapes and sizes shows that the nanocomposite possesses many adsorption sites that will lead to higher adsorption capacities [19]. These results are in agreement with the tetragonal crystal structure acquired from the XRD analysis. Elemental analysis was studied using EDS (Fig. S2c) to confirm the elements present in the nanocomposite. It was found that the nanocomposite synthesized composed of Fe, Mn, Al and O with weight percentages of 49.7, 9.0, 2.7 and 26.0, respectively. This confirms that the nanocomposite was free from impurities since no foreign elements were detected except for carbon which was used to coat the material prior to analysis.
High resolution-transmission electron microscopy (HR-TEM)
To further investigate the morphology of Fe3O4@MnO2,Al2O3 nanocomposite, HR-TEM measurements were performed (Fig. S3). A variety of shapes can be observed in Fig. S3 including spheres and tetragonal shaped particles with an average particle size of 24 nm and these results are in agreement with the XRD and SEM observations. Employing the sol-gel synthesis method, the Fe3O4 nanoparticles were coated with MnO2 and Al2O3 simultaneously (Fig. S3 (a-d)) and these results agree with the XRD analysis.
Nitrogen adsorption studies using Brunauer-Emmett-teller (BET)
The surface area properties of Fe3O4@MnO2,Al2O3 nanocomposite were examined using nitrogen adsorption/desorption experiments. The specific surface area, pore size, and the total pore volume were found to be 36.4 m2 g−1, 7.71 nm, and 0.12 cm3 g−1, respectively.
Magnetic characteristics analysis
A vibrating sample magnetometer (VSM) was used to evaluate the magnetic properties of the Fe3O4@MnO2,Al2O3 nanocomposite (Fig. S4). The magnetic properties give an idea as to whether the nanoadsorbent can be separated in solution by applying an external magnetic field [30]. The remanence and coercivity is almost zero, suggesting that the samples are superparamagnetic [31]. Although the Fe3O4@MnO2,Al2O3 nanocomposite has a low saturation magnetization when compared to that of Fe3O4 nanoparticles (Fig. S4), the nanocomposite possesses enough magnetic response and thus it can be separated in solution by applying an external magnetic field.
Fourier transform infrared spectroscopy (FTIR)
To establish the successful functionalization of Fe3O4@MnO2,Al2O3 nanocomposite with AAPTMS, FTIR spectroscopy was employed to characterize both Fe3O4@MnO2,Al2O3 and Fe3O4@MnO2,Al2O3@AAPTMS nanocomposites (Fig. S5). The detailed assignment of the characteristic peaks can be found in Electronic Supplementary Material. The FTIR spectra confirmed that the Fe3O4@MnO2,Al2O3 nanocomposite was successfully modified with AAPTMS.
Optimization of the UA-MSPE method
The following parameters were optimized: (a) Sample pH value; (b) Amount of adsorbent (AS); (c) Extraction time; (d) Eluent concentration and (e) Desorption time. Respective data and Figures are given in the Electronic Supporting Material (ESM) Fig. S6, Fig. S7 and Table S1. Based on the results from the optimization, an equation (not shown) was then used to calculate the AS and pH value that would yield the highest recovery. Based on the calculations using the equation, the optimum conditions were found as 6.0, 250 mg, 10 min, 2.00 mol L−1 and 3 min for the pH, amount of sorbent, extraction time, eluent concentration and desorption time, respectively. These optimum conditions were then applied on the preconcentration of river water samples and validation with a certified reference material.
Analytical performance of the UA-MSPE/ICP-OES
Under optimized conditions, the analytical performance of the UA-MSPE method was investigated. A calibration curve was prepared by preconcentrating standard solutions of Cr(VI) at a concentration level of 0–500 μg L−1. The correlation coefficient (r2) of the prepared calibration curve was found to be 0.9980 after preconcentration. The enrichment (preconcentration) factor (EF or PF), defined as the ratio of the sensitivity of UA-MSPE/ICP-OES method to the sensitivity of the direct ICP-OES analysis of the liquid sample was found to be 94. The limit of detection (LOD) and quantification (LOQ) were defined according to IUPAC definition, which are the lowest concentration of an analyte giving signals equal to three or ten times, respectively, the standard deviation (SD) of blank signal (n = 10) divided by the slope of the calibration curve [14]. Under optimum experimental conditions, the LOD and LOQ, were found to be 0.02 and 0.05 μg L−1, respectively. Precision of the UA-MSPE method was determined by repeatability (intraday precision, n = 10) and reproducibility (interday precision, n = 7), expressed in terms of relative standard deviation (% RSD), were 2.6 and 4.9%, respectively.
Comparison of UA-MSPE/ICP-OES method with other sample preparation procedures
A comparison of the analytical performance data of the UA-MSPE/ICP-OES method with other sample preparation methods for preconcentration of Cr(III)/Cr(VI) or total content in environmental matrices was performed. The comparative results are summarized in Table 3. The analytical performances revealed that the UA-MSPE/ICP-OES method has relatively lower LOD, comparable and better preconcentration factors when compared with those reported in the literature [11, 24, 33]. However, preconcentration factor for the UA-MSPE/ICP-OES method was lower than those reported by Refs [32, 33]. In addition, the LOD was higher than those reported by Ref [32]. Furthermore, the precision was found to be higher compared to that reported by Jiang et al. [32] and Cui et al. [33].
Reusability and adsorption capacity
The reusability of Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was evaluated according to the modified method reported by Nyaba et al. [36]. This was done to investigate as to how many times the prepared nanoadsorbent can be used in the preconcentration method while maintaining optimal recoveries. Briefly, the nanoadsorbent already used in the preconcentration procedure was dispersed in 20 mL of a 2.00 mol L−1 HNO3 solution. The mixture was sonicated for 30 min to ensure that all traces of metals that were adsorbed are removed from the sorbent to the solution. The nanoadsorbent was separated from solution by magnetic decantation and the supernatant was discarded. The nanoadsorbent was then washed three times with ultrapure water to clean the adsorbent. Finally, the nanoadsorbent was separated from the supernatant by magnetic decantation and dried at 60 °C prior to being reused. This procedure was employed successively before the nanoadsorbent was reused. The nanoadsorbent was reused for more than 10 times with an average RSD of 2.8% whilst maintaining the high recoveries of the studied metal ions.
The adsorption capacity of the nanoadsorbent employed in this study was performed according to Nomngongo and Ngila [25]. Cr(VI) solutions were prepared from 5 to 500 mg L−1 and preconcentrated following the previously used preconcentration method. Briefly, 0.25 g of Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was equilibrated in 10 mL of Cr(VI) solutions with concentrations varied at 5–500 mg L−1 by sonication for 10 min at pH 6.0. The unadsorbed Cr(VI) was quantified with ICP-OES. The results were fitted into the general linearized equation of the Langmuir and Freundlich isotherm models in Eqs. 1 and 2, respectively.
Where Ce is the equilibrium concentration of the adsorbate (mg L−1), qe is adsorption capacity adsorbed at equilibrium (mg g−1), qmax is the maximum adsorption capacity (mg g−1), KL is the Langmuir’s adsorption constant, K and n are constants for a given adsorbate and adsorbent.
These equations were applied in order to determine the adsorption capacity of Cr(VI). The correlation coefficient from the Langmuir and Freundlich linear equations were used to establish the fitness of the data. The data was fitted into the Langmuir and Freundlich linear equations and the correlation coefficients were 0.9985 and 0.5550, respectively. Therefore, the data fitted well with the Langmuir isotherm model and the maximum adsorption capacity was therefore found to be 30.1 mg g−1.
Influence of potentially interfering ions
The influence of potentially interfering ions on the preconcentration of Cr(VI) under optimized conditions was investigated. The interferences may be due to the existence of some common alkali, alkaline and transition metals that may compete with the analyte during preconcentration using the UA-MSPE method. Solutions containing fixed concentrations of 10.0 μg L−1 Cr(VI) and varied concentrations (0.10 mg L−1, 0.50 mg L−1 and 1.00 mg L−1) of potentially interfering ions were preconcentrated following the UA-MSPE procedure. The tolerance limit of the potentially interfering ions was defined as the interfering ions concentration that causes a relative error smaller than or equal to 5% with respect to the concentration on the analytes [37]. The analytical results (Table 4) showed that the presence of potentially interfering ions had no significant influence on the recovery of Cr(VI). Thus, the UA-MSPE method had high tolerance for the potentially interfering ions and therefore would be suitable for the analysis of river water samples and the certified reference material. In addition, the presence of Mo(VI), Sb(V) and V(V) which have similar properties to the target analyte did interfere with the quantitative preconcentration and recovery of C(VI), this might be due the availability of active sites for the target analyte and other oxyanions (Mo(VI), Sb(V) and V(V)). In view of the above, the method reported in this was found to more suitable for speciation of Cr in water samples.
Validation and application of UA-MSPE/ICP-OES
Speciation of chromium in water is essential when evaluating the risk imposed by trace metals within the environment. For this reason, the UA-MSPE/OES procedure was employed for the preconcentration and speciation of chromium in river water samples. From the synthetic samples, it was discovered that the Fe3O4@MnO2,Al2O3 nanocomposite oxidizes Cr(III) to Cr(VI) at the optimal conditions; whereas Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was only selective to Cr(VI). Therefore, Fe3O4@MnO2,Al2O3 nanocomposite was used to determine the total chromium content and the Fe3O4@MnO2,Al2O3@AAPTMS nanocomposite was used to determine Cr(VI) content in river water samples. The concentrations of Cr(III) were then estimated as the difference between the total chromium concentration and Cr(VI) concentration.
The analytical results are shown in Table 5. From the analytical results, it can be observed that Cr(VI) existed in higher concentrations than Cr(III). This may be due to the waste discharge from the wood preservative area since the chromated copper arsenate (CCA) mixture used for wood preservation contains excessive amounts of Cr(VI). The results were lower than the maximum permitted chromium levels of 0.05 mg L−1 set by the world health organization (WHO). However, one might need to investigate both water and the sediments as some concentration of the analyte might be associated with the sediments. The UA-MSPE method was validated by analyzing the certified reference material (CRM) for trace metals in drinking water to evaluate the accuracy of the method. This was done by preparing CRM solutions containing 10.0 μg L−1 of Cr(VI) and preconcentrating the solution using the UA-MSPE method. The recoveries of Cr(VI) from the CRM solutions were found to be 99.7% ± 1.4 which proves the validity of the UA-MSPE method. In addition, the results for the total Cr concentration using the UA-MSPE method were compared with those obtained using the reference method, ICP-MS (Table 5). According to the Student paired t-test, the results were in agreement with the data from the comparative method at 95% confidence level.
Reference method = ICP-MS analysis.
Conclusion
In this study, the AAPTMS functionalized magnetic Fe3O4@MnO2,Al2O3 nanocomposite was as an adsorbent for the speciation of Cr(VI) in river water samples. Due to the availability of active sites, the prepared nanocomposite may have the potentiality of being used for simultaneous preconcentration and separation of various trace metals. The UA-MSPE showed high tolerance of coexisting ions, relatively good precision and accuracy. Besides its cost effectiveness, re-usability, simplicity and rapidness, the dUA-MSPE method also showed to possess high adsorption capacities. In addition, the UA-MSPE method revealed that is that it is applicable at sample pHs values that are within range of typical environmental sample. However, the main disadvantage of the current method is the use of functionalized adsorbent because the ligand may leach out with time, thus affecting the selectivity of the solid material.
References
Singh A, Pal R, Gangwar C, Gupta A, Tripathi A (2015) Release of heavy metals from industrial waste and E-waste burning and its effect on human health and environment. IJERMT 4(12):51–56
Zeeb M, Ganjali MR, Norouzi P (2013) Preconcentration and trace determination of chromium using modified ionic liquid cold-induced aggregation dispersive liquid–liquid microextraction: application to different water and food samples. Food Anal Method 6(5):1398–1406
Khan MY, Aziz I, Bihari B, Kumar H, Roy M, Kumar V (2014) A review-Phytomedicines used in treatment of diabetes. Diabetes 101:126
Seidler A, Jähnichen S, Hegewald J, Fishta A, Krug O, Rüter L, Strik C, Hallier E, Straube S (2013) Systematic review and quantification of respiratory cancer risk for occupational exposure to hexavalent chromium. Int Arch Occ Env Hea 86(8):943–955
de San Miguel ER, Vital X, de Gyves J (2014) Cr(VI) transport via a supported ionic liquid membrane containing CYPHOS IL101 as carrier: system analysis and optimization through experimental design strategies. J Hazard Mater 273:253–262
Remaili TM, Simpson SL, Amato ED, Spadaro DA, Jarolimek CV, Jolley DF (2016) The impact of sediment bioturbation by secondary organisms on metal bioavailability, bioaccumulation and toxicity to target organisms in benthic bioassays: implications for sediment quality assessment. Environ Pollut 208:590–599
Limbeck A, Rupp GM, Kubicek M, Téllez H, Druce J, Ishihara T, Kilner JA, Fleig J (2016) Dynamic etching of soluble surface layers with on-line inductively coupled plasma mass spectrometry detection–a novel approach for determination of complex metal oxide surface cation stoichiometry. J Anal Atom Spectrom 31(8):1638–1646
Kmiecik E, Tomaszewska B, Wątor K, Bodzek M (2016) Selected problems with boron determination in water treatment processes. Part I: comparison of the reference methods for ICP-MS and ICP-OES determinations. Environ Sci Pollut R 23(12):11658–11667
Franze B, Engelhard C (2014) Fast separation, characterization, and speciation of gold and silver nanoparticles and their ionic counterparts with micellar electrokinetic chromatography coupled to ICP-MS. Anal Chem 86(12):5713–5720
Hu B, He M, Chen B, Xia L (2013) Liquid phase microextraction for the analysis of trace elements and their speciation. Spectrochim Acta B 86:14–30
Wu YW, Zhang J, Liu JF, Chen L, Deng ZL, Han MX, Wei XS, Yu AM, Zhang HL (2012) Fe3O4@ZrO2 nanoparticles magnetic solid phase extraction coupled with flame atomic absorption spectrometry for chromium (III) speciation in environmental and biological samples. Appl Surf Sci 258(18):6772–6776
Pourghazi K, Amoli-Diva M, Beiraghi A (2015) Speciation of ultra-trace amounts of inorganic arsenic in water and rice samples by electrothermal atomic absorption spectrometry after solid-phase extraction with modified Fe3O4 nanoparticles. Int J environ analChem 95(4):324–338
Su S, Chen B, He M, Hu B (2014) Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 123:1–9
Yang F, Li J, Lu W, Wen Y, Cai X, You J, Ma J, Ding Y, Chen L (2014) Speciation analysis of mercury in water samples by dispersive liquid–liquid microextraction coupled to capillary electrophoresis. Electrophoresis 35(4):474–481
Płotka-Wasylka J, Szczepańska N, de la Guardia M, Namieśnik J (2015) Miniaturized solid-phase extraction techniques. TrAC-Trend Anal Chem 73:19–38
Huang D, Deng C, Zhang X (2014) Functionalized magnetic nanomaterials as solid-phase extraction adsorbents for organic pollutants in environmental analysis. Anal Method 6(18):7130–7141
Augusto F, Hantao LW, Mogollon NG, Braga SC (2013) New materials and trends in sorbents for solid-phase extraction. TrAC-Trend Anal Chem 43:14–23
Rajendran R, Shrestha LK, Minami K, Subramanian M, Jayavel R, Ariga K (2014) Dimensionally integrated nanoarchitectonics for a novel composite from 0D, 1D, and 2D nanomaterials: RGO/CNT/CeO2 ternary nanocomposites with electrochemical performance. J Mater Chem A 2(43):18480–18487
Wang Z, Ma Y, He H, Pei C, He P (2015) A novel reusable nanocomposite: FeOOH/CBC and its adsorptive property for methyl orange. Appl Surf Sci 332:456–462
Nabid MR, Sedghi R, Bagheri A, Behbahani M, Taghizadeh M, Oskooie HA, Heravi MM (2012) Preparation and application of poly(2-amino thiophenol)/MWCNTs nanocomposite for adsorption and separation of cadmium and lead ions via solid phase extraction. J Hazard Mater 203:93–100
Khan M, Yilmaz E, Sevinc B, Sahmetlioglu E, Shah J, Jan MR, Soylak M (2016) Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinyl acetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta 146:130–137
Zhang N, Peng H, Hu B (2012) Light-induced pH change and its application to solid phase extraction of trace heavy metals by high-magnetization Fe3O4@SiO2@TiO2 nanoparticles followed by inductively coupled plasma mass spectrometry detection. Talanta 94:278–283
Magoda C, Nomngongo PN, Mabuba N (2016) Magnetic iron–cobalt/silica nanocomposite as adsorbent in micro solid-phase extraction for preconcentration of arsenic in environmental samples. Microchem J 128:242–247
Peng H, Zhang N, He M, Chen B, Hu B (2015) Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino) propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 131:266–272
Nomngongo PN, Ngila JC (2014) Functionalized nanometer-sized alumina supported micro-solid phase extraction coupled to inductively coupled plasma mass spectrometry for preconcentration and determination of trace metal ions in gasoline samples. RSC Adv 4(86):46257–46264
Duran A, Tuzen M, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169(1):466–471
Barfi B, Rajabi M, Zadeh MM, Ghaedi M, Salavati-Niasari M, Sahraei R (2015) Extraction of ultra-traces of lead, chromium and copper using ruthenium nanoparticles loaded on activated carbon and modified with N, N-bis-(α-methylsalicylidene)-2, 2-dimethylpropane-1, 3-diamine. Microchim Acta 182(5–6):1187–1196
Hu B, He M (2012) Pre-concentration and sample treatment techniques for trace element analysis. comprehensive sampling and sample preparation 3(3.19): 365–394.
Shu Z, Wang S (2008) Synthesis and characterization of magnetic Nanosized Fe3O4/MnO2 composite particles. J Nanomater 2009:1–5
Konicki W, Sibera D, Mijowska E, Lendzion-Bieluń Z, Narkiewicz U (2013) Equilibrium and kinetic studies on acid dye acid red 88 adsorption by magnetic ZnFe2O4 spinel ferrite nanoparticles. J Colloid Interface Sci 398:152–160
Gao G, Du G, Cheng Y, Fu J (2014) Specific recognition of bovine serum albumin using superparamagnetic molecularly imprinted nanomaterials prepared by two-stage core–shell sol–gel polymerization. J Mater Chem B 2(7):783–792
Jiang HM, Yang T, Wang YH, Lian HZ, Hu X (2013) Magnetic solid-phase extraction combined with graphite furnace atomic absorption spectrometry for speciation of Cr(III) and Cr(VI) in environmental waters. Talanta 116:361–367
Cui C, He M, Chen B, Hu B (2014) Chitosan modified magnetic nanoparticles based solid phase extraction combined with ICP-OES for the speciation of Cr(III) and Cr(VI). Anal Method 6(21):8577–8583
Islam A, Ahmad H, Zaidi N, Kumar S (2016) A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim Acta 183(1):289–296
Chang MM, Ginjom IR, Ngu-Schwemlein M, Ng SM (2016) Synthesis of yellow fluorescent carbon dots and their application to the determination of chromium (III) with selectivity improved by pH tuning. Microchim Acta 183(6):1899–1907
Nyaba L, Matong JM, Nomngongo PN (2016) Nanoparticles consisting of magnetite and Al2O3 for ligandless ultrasound-assisted dispersive solid phase microextraction of Sb, Mo and V prior to their determination by ICP-OES. Microchim Acta 183(4):1289–1297
Nomngongo PN, Ngila JC, Msagati TA, Moodley B (2014) Chemometric optimization of hollow fiber-liquid phase microextraction for preconcentration of trace elements in diesel and gasoline prior to their ICP-OES determination. Microchem J 114:141–147
Acknowledgements
This work was financially sponsored by the Department of Science and Technology (DST, South Africa) / National Nanoscience Postgraduate Teaching and Training Programme (NNPTTP) and the authors wish to thank the University of Johannesburg (UJ) for providing their laboratory facilities. The physics department of the University of Johannesburg is kindly acknowledged for assistance with their vibrating sample magnetometer (VSM) instrument.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 1219 kb)
Rights and permissions
About this article
Cite this article
Munonde, T.S., Maxakato, N.W. & Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3 . Microchim Acta 184, 1223–1232 (2017). https://doi.org/10.1007/s00604-017-2126-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2126-2