Abstract
A simple, sensitive and accurate method was developed for solid-phase extraction and preconcentration of trace levels of gold in various samples. It is based on the adsorption of gold on modified oxidized multi-walled carbon nanotubes prior to its determination by graphite furnace atomic absorption spectrometry. The type and volume of eluent solution, sample pH value, flow rates of sample and eluent, sorption capacity and breakthrough volume were optimized. Under these conditions, the method showed linearity in the range of 0.2–6.0 ng L−1 with coefficients of determination of >0.99 in the sample. The relative standard deviation for seven replicate determinations of gold (at a level of 0.6 ng L−1) is ±3.8 %, the detection limit is 31 pg L−1 (in the initial solution and at an S/N ratio of 3; for n = 8), and the enrichment factor is 200. The sorption capacity of the modified MWCNTs for gold(III) is 4.15 mg g−1. The procedure was successfully applied to the determination of gold in (spiked) water samples, human hair, human urine and standard reference material with recoveries ranging from 97.0 to 104.2 %.

A sorbent based on modified carbon nanotubes was prepared and used to extract gold ion from various samples prior to its determination by graphite furnace atomic absorption spectrometry
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold is used in a wide range of industrial, economical and biological applications, and the separation of this noble metal from different matrices has a great importance [1, 2]. However, the level of gold in environmental samples is too low to be determined directly by conventional techniques [3]. Therefore, an effective preconcentration and separation method is usually necessary prior to determination. Methods such as liquid-liquid extraction [4], liquid-phase microextraction [5], electrodeposition [6], coprecipitation [7] and solid-phase extraction (SPE) [8] have been applied. Among them, SPE has become increasingly attention because of its advantages of high enrichment factor, high recovery, rapid phase separation, ease regeneration of solid-phase, low cost and low consumption of organic solvents [9]. The mechanism of sorption in this method depends on the nature of the sorbent and can include simple adsorption, chelation or ion-exchange. Adsorption occurs through van der Waals forces or hydrophobic interactions while in ion-exchange or chelation, appropriate functional groups in solid matrices is needed [9]. Numerous materials such as thiol cotton fiber [10], bonded-silica [11], polymers [12, 13], biosorbents [14] and carbon-based sorbents (carbon black, activated carbon and carbon nanotubes) [15, 16] were used in SPE for preconcentration of gold.
Carbon nanotubes (CNTs), as one of the members of carbon family, can be visualized as a sheet of graphite that has been rolled into a tube. Based on the number of layers of graphene sheets, they are divided into three types: (1) single-walled CNTs (SWCNTs), (2) double-walled CNTs (DWCNTs) and (3) multi-walled CNTs (MWCNTs) [17]. Since they were first prepared in 1991 by Ijima [18], CNTs have become attractive materials for their structure characteristics. Their large surface areas, hydrophobicity and high adsorption capacities make them a promising solid sorbent for preconcentration procedures [19–21]. It has been proven that CNTs have great potential as superior adsorbents for removing many kinds of organic and inorganic materials [22–24]. Recent applications for metal ions extraction have been focused on the use of modified CNTs. Modification of them with specific physicochemical properties can be achieved by chemical treatments to make CNTs that possess the best performance for metal ion removal [25–28].
This article describes the preparation of oxidized MWCNTs modified with 2-(5-bromo-2-pyridylazo)-5-(diethylamino)phenol, and the use of this sorbent for the separation and preconcentration of gold from different aqueous matrices. Finally, in order to reach a high enrichment factor, high sensitivity and low detection limit, the experimental parameters affecting the solid-phase extraction conditions were studied and optimized.
Experimental
Apparatus and reagents
A Varian Spectra AA 220 atomic absorption spectrometer (Victoria, Australia, http://www.varianinc.com) was used for the measuring of gold with a deuterium lamp background correction, equipped with graphite furnace (GTA-110 series). The following instrumental parameters were chosen: Analytical wavelength 242.8 nm, spectral bandwidth 1.0 nm, lamp current 4 mA, signal measured: peak height; sample volume 20 μL. The optimum temperatures program for GFAAS is given in Table S1 (Electronic Supplementary Material). The pH measurement was carried out using a Metrohm pH meter (Houston, TX, http://www.metrohm.com) model 827 with a combined pH glass electrode. A funnel-tipped glass tube (80 mm × 10 mm) was homemade and was used as a column for preconcentration.
All reagents used were of analytical reagent grade (Merck, Darmstadt, Germany, http://www.merckgroup.com). The Au (III) stock solution (1000 mg L−1) was prepared by dissolving appropriate amount of HAuCl4.4H2O into a 100.0 mL volumetric flask and diluting to the mark with deionized water. Working standard solutions of Au(III) were prepared freshly at various concentrations by diluting the stock standard solution with deionized water. A 0.05 % (w/v) solution of 2-(5-bromo-2-pyridylazo)-5-(diethylamino) phenol (5-Bromo-PADAP) was prepared by dissolving an appropriate amount of this chelating agent in ethanol. Buffer solution was prepared from 0.1 mol L−1 HAc-NaAc. MWCNTs with an average outer diameter of 3–20 nm, length of 1–10 μm and number of walls 3–15 were obtained from Plasma Chem. GmbH (Berlin, Germany, http://www.plasmachem.com). The solutions of other metals used for the interference study were obtained from the respective inorganic salts.
Preparation of modified MWCNTs
To remove amorphous carbon, raw MWCNTs were heated at 300 °C for 40 min. After cooling, they were refluxed with concentrated HNO3 for 1 h at 100 °C in order to oxidize the surface of them. Then, the oxidized MWCNTs were washed with distilled water until removing any excess of nitric acid (neutral pH of solution) and dried at room temperature [23].
2.0 g of oxidized MWCNTs and 25.0 mL of 0.05 % (w/v) 5-Bromo-PADAP in ethanol was placed in a 100 mL beaker and the mixture was stirred for 6 h. Afterward, modified MWCNTs were transferred onto a filter paper, washed with copious amount of distilled water and dried at room temperature.
Preparation of operational column
A glass column packed with 20 mg of modified MWCNTs sorbent was used as the operational column. The column could be used repeatedly for several times after washing with distilled water.
Preconcentration procedure
Initially, for column conditioning, deionized water was passed through the column. Then, 30.0 mL of gold solution (0.6 ng L−1) with pH = 6 (acetate buffer was used to adjust the pH) was passed through the column at a flow rate of 3.0 mL min−1. In this step, gold was adsorbed on modified MWCNTs. After that, the adsorbed gold was eluted from sorbent with 3.0 mL of HCl (2.0 M) and then with 2.0 mL thiourea (1.0 M) at a flow rate of 1.5 mL min−1. Finally, eluted solution was automatically injected by the autosampler into the graphite tube and then the absorbance of gold was measured compared with a blank.
Results and discussion
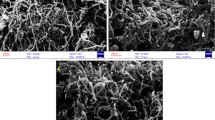
The composition and morphology of the sorbent were characterized by Fourier transform infrared (FT-IR) spectroscopy and scanning electron microscopy (SEM). Figure 1 displays the FT-IR spectra of raw MWCNTs (a) and oxidized MWCNTs (b). The peaks at 1718 cm−1 and 1458 cm−1 in the spectrum of oxidized MWCNTs can be assigned to C=O stretch and O–H bend, respectively. These functional groups improve the dispersive behavior of oxidized MWCNTs in aqueous solutions. The peaks at 2360 cm−1 and 2343 cm−1 due to asymmetrical and symmetrical C–H stretching vibrations. The great peak at 3447 cm−1 can be attributed to OH group. The SEM images at various magnifications revealed that the sorbent has a porous structure (Fig. 2).
To compare the tendency of oxidized MWCNTs and modified MWCNTs for the adsorption of gold, the experiments were done by the preconcentration procedure and showed that although, oxidized MWCNTs have a low tendency for the adsorption of gold (less than 60 %), they are not selective for the separation of gold in contamination matrices. Therefore, the oxidized MWCNTs are not a suitable sorbent for the separation of gold. On the other hand, gold percent recoveries for modified MWCNTs are higher than 98 %. Moreover, modified MWCNTs are selective for the separation of gold. Therefore, for further experiments, modified MWCNTs were chosen as sorbent.
In order to obtain highly sensitive and reproducible results, experimental parameters affecting the preconcentration of gold such as, type and volume of eluent solution, sample pH, flow rates of sample and eluent, sorption capacity and breakthrough volume were studied and optimized.
Type and volume of eluent
To desorption gold ions from modified MWCNTs, several solvents such as, tetrahydrofuran (THF), hydrochloric acid, thiourea, hydrochloric acid and then thiourea, thiourea and then hydrochloric acid, 1-propanol, ethanol and acetone were examined. The results showed that the best recovery was achieved when hydrochloric acid and then thiourea was used as eluent. Then, various concentration and volume of hydrochloric acid and thiourea were checked. By increasing the eluent volume, desorption was increased but enrichment factor was decreased. Finally, it was found that 3.0 mL of HCl (2.0 M) and then with 2.0 mL thiourea (1.0 M) was sufficient to elute the gold ions from modified MWCNTs.
The sample pH
The pH of the sample solution is an important factor affecting the formation of complexes with sufficient hydrophobicity and subsequent extraction. In this study, 5-Bromo-PADAP was used as a complexing agent for gold ions to produce a chelate that is extractable. Because the production of this chelate is pH-dependent, SPE was performed at different pH values in the 2.5–10.0 range by HNO3 (0.1 M) and NaOH (0.1 M). The results in Fig. 3 reveal that the absorbance was nearly constant in the pH range of 4.5–8.5. Accordingly, a pH = 6 was selected for subsequent optimization studies and real sample analysis.
The sample and eluent flow rate
The flow rate of the sample through the column is one of the factors affecting the length of time needed for the formation of gold-5-Bromo-PADAP chelates and adsorbing on the sorbent. Hence, sample solutions of 30 mL were passed through the column with flow rates in the range of 0.1–4.0 mL min−1. It was observed that the recovery did not change until 3.5 mL min−1 and after that it was decreased because gold ions have not sufficient time to adsorb completely. Therefore, a flow rate of 3.0 mL min−1 was selected for further experiments.
The effect of eluent flow rate on desorption of gold from the sorbent surface was studied in the range of 0.1–4.0 mL min−1. Based on the results shown in Fig. 4, flow rate of 1.5 mL min−1 was chosen in subsequent work.
Sorption capacity and breakthrough volume
The sorption capacity is the maximum gold quantity taken up by 1.0 g of modified MWCNTs. In order to determine the sorption capacity, 20 mg of modified MWCNTs sorbent were subjected to several loadings with 15 mL sample solution and then, followed by the determination of retained Au(III) using GFAAS. The maximum capacity was 4.15 mg Au(III) per gram of modified MWCNT sorbent.
To evaluate the multiple uses, the sorbent was subjected to several loadings with the sample solution and subsequent elution. It was found that adsorption properties of the sorbent remained constant after 40 cycles of sorption and desorption.
Under optimum conditions, the breakthrough volume of the method was studied by solutions containing 3.0 ng of gold in 200, 400, 600, 800, 1000 and 1200 mL of water were passed through the columns, the gold(III) was quantitatively retained in all cases below 1000 mL. Consequently, by considering the final elution volume of 5.0 mL of eluent, and a breakthrough volume of 1000 mL, an enrichment factor of 200 was achievable.
Interferences
In order to evaluate the selectivity of this method, the interference of various elements was investigated. The interference was due to the competition of other metal ions for co-extraction with gold ion. In these experiments, solutions containing 0.6 ng L−1 of gold(III) and the interfering ions were treated according to the recommended procedure. The tolerance limits of the coexisting ions, defined as the largest amount making the recovery of gold(III) less than 95 %. Table 1 clearly indicates that most of the tested ions do not influence the gold determination hence gold is not only preconcentrated successfully but also separated effectively from the other matrix.
Analytical performance of the method
Precision, linearity and limits of detection (LOD) were investigated under the chosen experimental conditions. The intra-day and inter-day relative standard deviations (RSDs) for seven replicate measurements of 0.6 ng L−1 Au were ±3.8 % and 4.7 %, respectively. It was indicating good precision of the method. The batch-to-batch reproducibility measured by three replicate analyses from 0.6 ng L−1 Au solution was ±5.2 %. The calibration curve was linear from 0.2 ng L−1 to 6.0 ng L−1 in initial solution or 0.04 to 1.2 ng mL−1 in final solution with a correlation coefficient (R 2) of 0.9998 and the equation of the calibration curve was A = 0.2707C + 0.0007, where A is the absorbance value of the eluent and C (ng mL−1) is the concentration of gold(III). The LOD was 31 pg L−1 based on three times the standard deviation of the blank solution measurements (n = 8) in original solution. Also, the limit of quantification (LOQ) was 0.04 ng mL−1.
Applications of the method
Several water samples, including; tap water, well water and wastewater were analyzed according to the method. The results are shown in Table 2. In all cases, the spiked recoveries confirmed the reliability of this method.
Also the method was applied to human hair (man) as a different matrix. The human hair sample was immersed in acetone for 30 min, then washed with water and dried. After that, 0.5 g of hair sample was digested by 30.0 mL of a mixture solution of concentrated HClO4 and HNO3 (1:8 v/v) at 15 °C. Then, the solution was heated for 1 h at 100 °C until dried. Several drops of 50 % sulfuric acid were added to the residue and heated to 50 °C until the solution was dried. Then 40 mL water was added to the dried residue and transferred to a 100 mL volumetric flask, and diluted to the mark with deionized water. A 30 mL aliquot of this solution was used for analysis. The results are given in Table 2.
Human urine (man) is another material that was analyzed. Three volume of urine samples (each volume was 25 mL and total volume was 75 mL) were added to a 100 mL glass baker and after that, a mixture solution of concentrated HClO4 and HNO3 (1:2 v/v) was transferred into the baker. This solution was heated for 30 min at 100 °C until dried. Then 30 mL water was added to the dried residue and transferred to a 100 mL volumetric flask, and diluted to the mark with deionized water. A 30 mL aliquot of this solution was used for analysis.
In order to confirm the validity of the procedure in standard reference material, the method has been applied to the determination of the content of gold in MA-1b reference gold ore. For this purpose, 30 mg of reference material was digested by 30.0 mL of a mixture solution of concentrated HNO3, HCl and HF (1:4:2 v/v) at 70 °C until dried. Then 30 mL water was added to the dried residue and transferred to a 100 mL volumetric flask, and diluted to the mark with deionized water. A 30 mL aliquot of this solution was used for analysis. The results are shown in Table 3. The results found were in good agreement with the certified value. Thus, these results confirm that the procedure is independent from matrix interferences.
Comparison with other methods
A comparison of the method with other preconcentration procedures is given in Table 4 [13, 29–34]. As seen from the data in this table, the method using modified MWCNTs has high enrichment factor and relatively low LOD, wider linear range and lower RSD that it is comparable or better than ones reported elsewhere.
Conclusion
In this work, the adsorption behavior of gold on modified multi-walled carbon nanotubes was studied. As a sorbent, oxidized CNTs modified with 2-(5-bromo-2-pyridylazo)-5-(diethylamino) phenol exhibits low detection limit and high enrichment factor for preconcentration of Au(III) in different matrices with acceptable accuracy and precision. The preparation of this sorbent is simple which can be used several times without a marked loss in sorption capacity. Therefore, due to the possibility of multiple uses of the sorbent, this method is economical. The obtained results show that this method is suitable to determine gold ion by GFAAS in several types of natural water, hair samples and certified reference materials at ultra-trace levels.
References
Dulski TR(1999) Trace elemental analysis of metals. Marcel Dekker Inc, P99
Moawed EA, El-Shahat MF (2013) Synthesis, characterization of low density polyhydroxy polyurethane foam and its application for separation and determination of gold in water and ores samples. Anal Chim Acta 788:200–207
Medved J, Bujdos M, Matus P, Kubova J (2004) Determination of trace amounts of gold in acid-attacked environmental samples by atomic absorption spectrometry with electrothermal atomization after preconcentration. Anal Bioanal Chem 379:60–65
El-Shahawi MS, Bashammakh AS, Bahaffi SO (2007) Chemical speciation and recovery of gold(I, III) from wastewater and silver by liquid–liquid extraction with the ion-pair reagent amiloride mono hydrochloride and AAS determination. Talanta 72:1494–1499
Tajik S, Taher MA (2011) New method for microextraction of ultra-trace quantities of gold in real samples using ultrasound-assisted emulsification of solidified floating organic drops. Microchim Acta 173:249–257
Konecna M, Komarek J (2007) Utilization of electrodeposition for electrothermal atomic absorption spectrometry determination of gold. Spectrochim Acta B 62:283–287
Soylak M, Saracoglu S, Divrikli U, Elci L (2005) Coprecipitation of heavy metals with erbium hydroxide for their flame atomic absorption spectrometric determinations in environmental samples. Talanta 66:1098–1102
Afzali D, Ghaseminezhad S, Taher MA (2010) Separation and preconcentration of trace amounts of gold(III) ions using modified multi-walled carbon nanotube sorbent prior to flame atomic absorption spectrometry determination. J AOAC Int 93:1287–1292
Pyrzynska K (2012) Sorbent materials for separation and preconcentration of gold in environmental and geological samples. Anal Chim Acta 741:9–14
Yu M, Sun D, Huang R, Tian W, Shen W, Zhang H, Xua N (2003) Determination of ultra-trace gold in natural water by graphite furnace atomic absorption spectrophotometry after in situ enrichment with thiol cotton fiber. Anal Chim Acta 479:225–231
Mysoedova GV, Mokhodoeva OB, Kubrakova IV (2007) Trends in sorption preconcentration combined with noble metal determination. Anal Sci 23:1031–1039
Elci L, Sahan D, Basaran A, Soylak M (2007) Solid phase extraction of gold(III) on Amberlite XAD-2000 prior to its flame atomic absorption spectrometric determination. Environ Monit Assess 132:331–338
El-Shahawi MS, Bashammakh AS, Al-Sibaai AA, Orief MI, Al-Shareef FM (2011) Solid phase preconcentration and determination of trace concentrations of total gold (I) and/or (III) in sea and wastewater by ion pairing impregnated packed column prior flame atomic absorption spectrometry. Int J Miner Process 100:110–115
Wang H, Bao C, Li F, Kong X, Xu J (2010) Preparation and application of 4-amino-4′-nitro azobenzene modified chitosan as a selective adsorbent for the determination of Au(III) and Pd(II). Microchim Acta 168:99–105
Yin CY, Aroua MK, Daud WMAW (2007) Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep Purif Technol 52:403–415
Bystrzejewski M, Pyrzynska K (2013) Enhancing the efficiency of AuCl4 − ion removal from aqueous solution using activated carbon and carbon nanomaterials. Mater Chem Phys 141:454–460
Merkoci A (2006) Carbon nanotubes in analytical sciences. Microchim Acta 152:157–174
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58
Behzadi M, Mirzaei M, Daneshpajooh M (2014) Carbon nanotubes/poly-ortho-aminophenol composite as a new coating for the headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. Anal Methods 6:9234–9241
Taghizadeh M, Asgharinezhad AA, Samkhaniany N, Tadjarodi A, Abbaszadeh A, Pooladi M (2014) Solid phase extraction of heavy metal ions based on a novel functionalized magnetic multi-walled carbon nanotube composite with the aid of experimental design methodology. Microchim Acta 181:597–605
Alothman ZA, Habila M, Yilmaz E, Soylak M (2012) Solid phase extraction of Cd(II), Pb(II), Zn(II) and Ni(II) from food samples using multi-walled carbon nanotubes impregnated with 4-(2-thiazolylazo)resorcinol. Microchim Acta 177:397–403
Behzadi M, Noroozian E, Mirzaei M (2013) Preparation and application of carbon nanotubes/poly(o-toluidine) composite fibers for the headspace solid-phase microextraction of benzene, toluene, ethylbenzene, and xylenes. J Sep Sci 36:3550–3557
Behzadi M, Noroozian E, Mirzaei M (2014) Electropolymerization of carbon nanotubes/poly-ortho-aminophenol nanocomposite on a stainless steel fiber for the solid-phase microextraction of phthalate esters. RSC Adv 4:50426–50434
Zhou Q, Xing A, Zhao K (2014) Simultaneous determination of nickel, cobalt and mercury ions in water samples by solid phase-extraction using multi-walled carbon nanotubes as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. J Chromatogr A 1360:76–81
Fazelirad H, Taher MA (2014) Preconcentration of ultra-trace amounts of iron and antimony using ion pair solid phase extraction with modified multi-walled carbon nanotubes. Microchim Acta 181:655–662
Ghaedi M, Montazerozohori M, Rahimi N, Nejati Biysreh M (2013) Chemically modified carbon nanotubes as efficient and selective sorbent for enrichment of trace amount of some metal ions. J Ind Eng Chem 19:1477–1482
Behbahani M, Bagheri A, Amini MA, Sadeghi O, Salarian M, Najafi F, Taghizadeh M (2013) Application of multi-walled carbon nanotubes modified by diphenylcarbazide for selective solid-phase extraction of ultra-traces Cd(II) in water samples and food products. Food Chem 141:48–53
Peng H, Zhang N, He M, Chen B, Hu B (2015) Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino) propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 131:266–272
Dobrowolski R, Kuryło M, Otto M, Mroz A (2012) Determination of gold in geological materials by carbon slurry sampling graphite furnace atomic absorption spectrometry. Talanta 99:750–757
Liang P, Zhao E, Ding Q, Du D (2008) Multi-walled carbon nanotubes microcolumn preconcentration and determination of gold in geological and water samples by flame atomic absorption spectrometry. Spectrochim Acta Part B 63:714–717
Manzoori JL, Abdolmohammad-Zadeh H, Amjadi M (2007) Simplified cloud point extraction for the preconcentration of ultra-trace amounts of gold prior to determination by electrothermal atomic absorption spectrometry. Microchim Acta 159:71–78
Kagaya S, Zakata D, Yoshimori T, Kanbara T, Tohda K (2010) A sensitive and selective method for determination of gold(III) based on electrothermal atomic absorption spectrometry in combination with dispersive liquid–liquid microextraction using dicyclohexylamine. Talanta 80:1364–1370
Ashkenani H, Taher MA (2012) Use of ionic liquid in simultaneous microextraction procedure for determination of gold and silver by ETAAS. Microchem J 103:185–190
Fazelirad H, Taher MA, Nasiri-Majd M (2014) GFAAS determination of gold with ionic liquid, ion pair based and ultrasound-assisted dispersive liquid–liquid microextraction. J Anal At Spectrom 29:2343–2348
Morzan E, Piano O, Stripeikis J, Tudino M (2012) Evaluation of quartz tubes as atomization cells for gold determination by thermospray flame furnace atomic absorption spectrometry. Spectrochim Acta Part B 77:58–62
Juvonen J, Lakomaa T, Soikkeli L (2005) Determination of gold and the platinum group elements in geological samples by ICP-MS after nickel sulphide fire assay: difficulties encountered with different types of geological samples. Talanta 58:595–603
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 44 kb)
Rights and permissions
About this article
Cite this article
Moghaddam, F.H., Taher, M.A., Behzadi, M. et al. Modified carbon nanotubes as a sorbent for solid-phase extraction of gold, and its determination by graphite furnace atomic absorption spectrometry. Microchim Acta 182, 2123–2129 (2015). https://doi.org/10.1007/s00604-015-1550-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1550-4