Abstract
We describe a novel magnetic metal-organic framework (MOF) for the preconcentration of Cd(II) and Pb(II) ions. The MOF was prepared from the Fe3O4-pyridine conjugate and the copper(II) complex of trimesic acid. The MOF was characterized by IR spectroscopy, elemental analysis, SEM and XRD. A Box-Behnken design through response surface methodology and experimental design was used to identify the optimal parameters for preconcentration. Extraction time, amount of magnetic MOF and pH value were found to be critical factors for uptake, while type, volume, concentration of eluent, and elution time are critical in the elution step. The ions were then determined by FAAS. The limits of detection are 0.2 and 1.1 μg L−1 for Cd(II), and Pb(II) ions, respectively, relative standard deviations are <4.5% (for five replicates at 50 μg L−1 of Cd(II) and Pb(II) ions), and the enrichment capacity of the MOF is at around 190 mg g−1 for both ions which is higher than the conventional Fe3O4-pyridine material. The magnetic MOF was successfully applied to the rapid extraction of trace quantities of Cd(II) and Pb(II) ions in fish, sediment, and water samples.

Schematic illustration of synthesized magnetic MOF-pyridine nanocomposite
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal ions are toxic pollutants which exist in wastewaters and are concerning for industries and environmental organizations all over the world. Most of these pollutants ions are so dangerous and very toxic for human health. Thus determination of trace amounts of heavy metals is often a major task for the analytical chemists, as it is a good tool for identification and monitoring of toxicants in environmental samples. Among existing heavy metals of the environment, cadmium monitoring is very vital since cadmium concentrations in the environment are increasing dramatically [1, 2]. Cadmium exposure can be linked to diseases associated with aging such as osteoporosis, prostate, and pancreatic cancer [3, 4]. Lead is one of the most toxic and hazardous elements in human health, because it can cause detrimental effect on metabolic processes of human beings [5], and it has been proven to be a carcinogenic agent. Various instrumental techniques, including electrothermal atomic absorption spectrometry (ETAAS) [6], inductively coupled plasma-optical emission spectrometry (ICP-OES) [7], flame atomic absorption spectrometry (FAAS) [8], inductively coupled plasma-mass spectrometry (ICP-MS) [9], and total reflection XRF-spectrometry [10] have been used for the determination of heavy metals. However, the heavy metals concentration level in environmental samples is fairly low and complexity of matrices is a main problem; thus preconcentration techniques are often required [11]. Different procedures such as: liquid-liquid extraction (LLE) [12], cloud point extraction [13], chemical precipitation [14], ion exchange [15], and solid phase extraction (SPE) have been developed for extraction and preconcentration of heavy metals in natural matrices [16, 17].
Among mentioned methods, the most commonly used technique is solid phase extraction for preconcentration of heavy metal ions from environmental samples due to its simplicity, rapidity, minimal cost, and low consumption of reagents [18]. By advent of SPE, various diverse sorbents have been utilized such as magnetic nanoparticles [19], activated carbon [20], solid sulfur [21], Amberlite XAD-2 resin [22], cotton [23], and modified porous material [24]. Porous materials are defined as solids containing empty voids which can host other molecules. The fundamental feature of these materials is their porosity, the ratio between total occupied and empty space, the (average) size of the pores and the surface area. Typical values for the surface area of porous materials applied in technological processes range between 2000 and 8000 m2 g-1 [25]. Most important applications of such materials are the storage of small molecules and filtering . The Metal Organic Framework defining a nanoporous material can consist of either inorganic or organic materials. MOFs have shown high potential in gas storage, separation, chemical sensing and drug delivery [26, 27]. In general, the flexible and highly porous structure of MOFs allow guest species such as metal ions to diffuse into the bulk structure, and the shape and size of the pores lead to selectivity over the guests that may be adsorbed. These features make MOFs an ideal sorbent in solid phase extraction of heavy metals. However, there is little information about MOFs as an adsorbent [28, 29].
In this work the magnetic MOF Fe3O4–pyridine nanocomposite has been utilized as a novel adsorbent for fast separation and preconcentration of Cd(II) and Pb(II) ions in various matrixes. Magnetic sorbent was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR) and scanning electron microscopy (SEM). The magnetic property of sorbent causes rapid and easy separation of the new solid phase from the solution. The presence of pyridine group in the sorbent helps the new solid phase to have selectivity towards these heavy metals. A Box–Behnken design was used in order to finding the optimum conditions for the method through response surface methodology. Finally, the sorbent was used for preconcentration and determination of Cd(II), and Pb(II) ions in different real samples and satisfactory results were obtained.
Experimental
Reagents and solutions
All reagents of analytical grade (benzene-1,3,5-tricarboxylate (BTC), Cd(II), Pb(II) Cu(OAc)2.3H2O, FeCl2, FeCl3, Cu(NO3)2.3H2O, HCl, HNO3, K2SO4, NaOH, KCl, thiourea, 3-choloropropyltriethoxysilane, toluene and triethylamine, ethanol, DMF, toluene, methanol and acetone) were purchased from Merck (Darmstadt, Germany, www.merck-chemicals.com) or from Fluka (www.sigmaaldrich.com) and used without further purification. N-[2-(triethoxysilyl) propyl] isonicotinamide (TPI) was prepared according to previous reports [30]. Standard solution of 1000 mg L−1 of Cd(II) and Pb(II) were prepared. All solutions were prepared using double distilled water.
Instrumentation
AA-680 Shimadzu (Kyoto, Japan) flame atomic absorption spectrometer with a deuterium background corrector was used. Cadmium and lead hollow cathode lamps (HCL) were used as the radiation source with wavelengths of 228.8 and 283.3 nm, respectively. All measurements were carried out in an air/acetylene flame. The pH of solution was measured at 25 ± 1 °C with a digital WTW Metrohm 827 Ion analyzer (Herisau, Switzerland) equipped with a combined glass-calomel electrode. CHN analysis was performed on a Thermo Finnigan Flash EA112 elemental analyzer (Okehampton, UK). IR spectra were recorded on a Bruker IFS-66 FT-IR Spectrophotometer. High-angle X-ray diffraction patterns were obtained on a Philips-PW 17C diffractometer with Cu Kα radiation. Scanning electron microscopy (SEM) was performed by gently distributing the powder sample on the stainless steel stubs, using SEM (KYKY- 3200, Beijing, China) instrument.
Synthesis of magnetic sorbent
Synthesis of Fe3O4– pyridine
Fe3O4 nanoparticles were synthesized according to the previously reported procedure [16] and their formation was confirmed by X-ray powder diffraction. For Preparation of pyridine functionalized Fe3O4 nanoparticles, 1.0 g of Fe3O4 nanoparticles was suspended in 70 mL of toluene, afterward 1.0 g of TPI was added and the mixture was refluxed for 24 h (Fig. 1a). The solid phase was filtered, separated, and washed 3 times with 50 mL of ethanol in order to remove the impurities and then dried at room temperature. Functionalization of Fe3O4 nanoparticles was confirmed by IR spectroscopy, and thermal and elemental analysis.
Synthesis of Cu3 (BTC) 2 metal-organic framework
Cu3(BTC)2 MOF was synthesized according to the previously reported procedure [31]. A solution consisting 0.84 g of trimesic acid and 1.75 g of Cu(NO3)2.3H2O in 50 mL of ethanol was refluxed under vigorous stirring. After a few hours, a blue solid started to precipitate which was kept under reflux conditions for 48 h, and subsequently cooled at room temperature. The obtained blue powder was recovered by centrifugation, washed with water and ethanol respectively. Afterwards, the sample was dried under vacuum at 100 °C for 12 h and then kept under dry nitrogen until further use (sample A). The synthesized MOF was characterized by IR spectroscopy, CHN, SEM and XRD.
Synthesis of magnetic metal-organic framework nanocomposite
Magnetic metal-organic framework nanocomposite was synthesized according to the following procedure (Fig. 1b). 2.38 mmol trimesic acid (H3BTC) was dissolved in a mixture of DMF/ethanol solution (1:1, 80 mL). Thereafter 25 mL of ethanol solution containing 0.2 g Fe3O4-pyridine was prepared, and then10 mL (sample B) was added to the mixture under magnetic stirring, and it was heated to 70 °C. After the addition of 40 mL Cu(OAc)2. H2O (0.86 g, 4.31 mmol) aqueous solution to the reaction mixture, the reaction was carried out at 70 °C with stirring for a further 4 h. Finally, the product was isolated from the supernatant solution by magnetic decantation and washed with water (50 mL) and ethanol (10 mL × 3). Magnetic sorbent was characterized by IR spectroscopy, CHN, SEM and XRD.
Uptake and elution step
General procedure for preconcentration of Cd(II) and Pb(II) ions from aqueous solutions was performed in batch mode analysis. Uptakes were performed in test tubes containing 25 μg of Cd(II) and Pb(II) ions in 25 mL of double distilled water. According to a preliminary experimental design, the pH of solution was adjusted by drop wise addition of 1 mol L−1 sodium hydroxide and 1 mol L−1 hydrochloric acid; and then magnetic sorbent was added into the solutions. After that, the mixture was shaken for an appropriate time to extract Cd(II) and Pb(II) ions from the solution completely. Finally, the test tubes were exposed to a strong magnet (15 cm × 12 cm × 5 cm, 0.8 T), where permanent magnet in the wall causes the particles to aggregate on one side of the test tube. The enriched amounts of Cd(II) and Pb(II) ions were determined using FAAS from the concentration change of these ions in solution after adsorption. The instrument response was periodically checked with known Cd(II) and Pb(II) standard solutions. Extraction percentage for each ion was calculated from the following equation:
where CA and CB are initial and final concentrations (mg L−1) of each ion in the solution, respectively. In the elution step, 6 mL of 0.8 mol L−l EDTA in 0.01 mol L−1 NaOH solution was added to magnetic sorbent and shaken. The result mixture was again exposed to a strong magnet and the clear solution, containing the eluted Cd(II) and Pb(II) ions was introduced to FAAS in order to determine the amount of ions.
Experimental design methodology
To fully understand the effect of the experimental variables that significantly affect the extraction procedure, individual factors must be considered along with nonlinear effects and interaction terms. The chemometric approach has a rational experimental design, which allows the simultaneous variation of all experimental factors, reducing the required time and number of trials that ends in reducing the overall required costs. The Box–Behnken design (BBD) is probably the most widely used experimental design applied for fitting a second-order response surface. This cubic design is characterized by a set of points lying at the midpoint of each edge of a multidimensional cube and center point replicates whereas the ‘missing corners’ help the experimenter to avoid using the combined factor extremes. This property prevents a potential loss of data in those cases [32].
In this study the StatGraphics plus 5.1 software was used for the analysis of the experimental design data and calculation of the predicted responses.
Real sample pretreatment
This method was successfully used to determine Cd(II) and Pb(II) ions in tap, river, sea and mineral water, fish and sediment samples.
Water samples
River water sample was collected from Siahroud River (Gilan Province in North of Iran) and sea water sample from the Caspian Sea (North of Iran). The samples were filtered through nylon filters (Millipore) and were stored in cleaned polyethylene bottles. Tap water was taken from our research laboratory with no pretreatment. Before SPE procedure, the samples were adjusted to pH of 6.3 according to the optimized experimental conditions.
Fish and sediment samples
Fish and sediment samples were collected from the Persian Gulf (the south of Iran), and has several subsidiary creeks. The fish samples (platycephalus indicus) were placed in ice, transferred to the laboratory and stored at -20 °C prior to analysis. Fish samples were dissected with clean plastic knife and a part of the muscle was taken out quickly and was dried in an oven at 70 °C for 48 h. Analysis were performed according to the procedure by Yilmaz (2003) [33]. A porcelain mortar was used to grind dry tissues. 0.4 g of each sample was digested with 5 mL of concentrated HNO3 in Teflon beakers for 4 h at 100 °C. The content was filtered into a 25 mL standard volumetric flask and diluted up to 25 mL with double distillated water. Sediment samples were collected using Peterson grab sampler, stored in a plastic bag in ice, transferred to the laboratory and kept at −20 °C before analysis. 1.0 g of these samples was digested with 6 mL of HCl (37%) and 2 mL of HNO3 (65%) in a microwave digestion system. Digestion was carried out for 2 min at 250 W, 2 min off, 6 min at 250 W, 4 min at 400 W, 8 min at 550 W and then venting for 8 min. The residue resulted from the digestion was then diluted to 100 mL with double distilled water.
Results and discussion
Characterization studies
Spectra and elemental analysis
The FT-IR spectra of MOF, and magnetic MOF were recorded using KBr pellet method (Fig. 1s, Supporting Information). In the IR spectra of MOF absorptions due to C = O (1645 cm−1), C–H (2936 cm−1) corresponding to aromatic groups, and –O–C–O– (1552 and 1437 cm−1) were observed. In the IR spectra of magnetic MOF, the same absorptions were witnessed with a little shift that indicates the successful immobilization of Fe3O4–pyridine. In addition, absorptions due to N–H (3486 cm−1) was observed which confirms the immobilization of MOF by Fe3O4–pyridine. Furthermore, elemental analysis showed the presence of 0.252% N in the structure of magnetic MOF which emphasizes that Fe3O4–pyridine had been sufficiently immobilized in the structure of magnetic MOF (C: 55.923%, H: 7.512%, N: 0.252%, O: 16.024%).
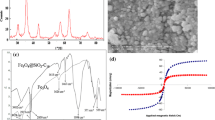
Scanning electron microscopy
To investigate the surface morphology of the MOF and magnetic sorbent, the samples were characterized by SEM (Fig. 2). The crystals of original [Cu3 (BTC)2(H2O)3]n sample are octahedral with a smooth surface and have an average size of 10 μm (Fig. 2a). However, surface of the magnetic MOF tends to be rougher after immobilization by Fe3O4-pyridine (Fig. 2b).
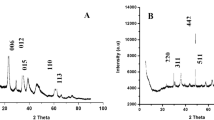
X-ray diffraction analysis
For further study, MOF and magnetic MOF-pyridine nanocomposite were investigated by XRD. All of the diffraction peaks for Fe3O4-pyridine immobilized samples showed that the sketch of the MOF crystal is well retained even after modification with Fe3O4-Pyridine, because all diffraction peaks of the immobilized MOF can readily be indexed to [Cu3(BTC)2(H2O)3]n (Fig. 2s, Supporting Information).
Optimization of the preconcentration procedure
Uptake step
The optimization step for uptake of metal ions on the magnetic sorbent was carried out using Box–Behnken design (BBD). Variables affecting the extraction efficiency were chosen: pH, amount of magnetic sorbent, and extraction time. Other parameters involved in the extraction were kept constant, especially the concentration of heavy metal ions (0.5 mg L−1). This design permitted the responses to be modeled by fitting a second-order polynomial, which can be expressed as the following equation:
where, x1, x 2, and x3 are the independent variables, β0 is an intercept, β1- β33 are the regression coefficients, and Y is the response (removal % or recovery %). The number of experiments (N) is defined by the expression below:
where K is the number of variables and Co is the number of center points [34]. In this study, K and Co were both set at 3, which meant that 15 experiments had to be done. The levels of the factors are listed in Table 1. The ANOVA results producing the Pareto chart of main and interaction effects are shown in Fig. 3a. The standard effect was estimated for computing the t-statistic for each effect. The vertical line on the plot shows statistically significant effects. The bar extracting beyond the line corresponds to the effects that are statistically significant at 95% confidence level [35]. Furthermore, the positive or negative sign (corresponding to a colored or colorless response) can be enhanced or reduced, respectively, when passing from the lowest to the highest level set for the specific factor. According to Pareto chart the pH of solution has the highest significantly positive effect on the extraction efficiency. The adsorption of heavy metal ions increases as the pH increases. This observation is due to the protonation of the active sites of the magnetic sorbent especially O and N atoms of pyridine group in acidic solutions. A proposed mechanism for uptake of Cd(II) and Pb(II) is shown in Fig. 3s (Supporting Information). The response surface methodology (RSM) (Fig. 3b) was applied to analysis simultaneous effects of time and pH variables on the response and the two-dimensional contour plot that displayed the interaction between independent variables. The adsorption efficiency of Cd(II) and Pb(II) ions increased along with the increase in pH and magnetic MOF amount. Extraction time, amount of magnetic MOF and pH of solution showed a positive and significant effect on the extraction efficiency. According to the overall results of the optimization study, the following experimental conditions were chosen: pH, 6.3; extraction time, 14 min; amount of magnetic sorbent, 30 mg.
a Pareto chart of the main effects in the BBD (uptake step). AA, BB and CC are the quadratic effects of the pH, extraction time and amount of polymer, respectively. AB, AC and BC are the interaction effects between pH and extraction time; pH and amount of polymer and extraction time and amount of polymer, respectively. b RSM obtained by plotting of pH vs magnetic MOF amount using the BBD
Selection of eluent
In this work several eluents including HCl, HNO3, K2SO4, NaOH, KCl, EDTA solution and mixtures of them were examined as the desorption solvent. Other factors were kept constant during the optimization (pH, 6.3; extraction time, 14 min; amount of magnetic sorbent, 30 mg; eluent volume, 5 mL; elution time, 25 min). Results showed that HCl, HNO3, and NaOH decompose the structure of magnetic sorbent but 0.01 mol L−1 NaOH increases the recovery without any structure decomposition. Based on the results, the best quantitative recovery was obtained with 0.01 mol L−1 NaOH in EDTA solution as the desorption solvent.
Elution step
Three factors were studied in desorption step using experimental design: eluent volume (mL), elution time (min), and concentration of eluent (mol L−l). In these conditions, a response surface design could be done without previously performing a screening design. The BBD was chosen because it requires the least number of experiments (15 run). The data obtained were evaluated by analysis of variance (ANOVA). The results of the experimental design were evaluated at 5% of significance and analyzed by standardized Pareto chart (Fig. 4). Based on BBD, volume and concentration of eluent showed a positive and significant effect on the recovery of heavy metal ions while elution time had a positive and non-significant effect. As Fig. 4 shows, eluent volume has the greatest influence. The RSM (Fig. 5) was applied to analyze simultaneous effects of eluent concentration and eluent volume variables on the responses. The two-dimensional contour plot (Fig. 4s, Supporting Information) displays the interaction between independent variables. The extraction efficiency of Cd(II) and Pb(II) ions increased along with the increase in eluent concentration and elution time. According to the overall results of the optimization study, the following experimental conditions were chosen: eluent volume, 6 mL; concentration of eluent, 0.8 mol L−l EDTA in 0.01 mol L−1 NaOH solution; and elution time, 16.5 min.
Pareto chart of the main effects in the BBD (elution step). AA, BB and CC are the quadratic effects of the eluent concentration, eluent volume and elution time, respectively. AB, AC and BC are the interaction effects between eluent concentration and eluent volume; eluent concentration and elution time; and eluent volume and elution time, respectively
Effect of breakthrough volume
For the analysis of real samples, the sample volume is one of the important parameters affecting the preconcentration factor. The breakthrough volume of sample solutions was investigated by dissolving 1 mg of each Cd(II) and Pb(II) in 25, 100, 250, 500, 750, 1000, 1100, 1200, 1300, 1400, and 1500 mL of distilled water and the SPE protocol was carried out. The results demonstrated that the dilution effect was not significant for sample volumes up to 1000 mL. Thus, the adsorbent enabling an enrichment factor of 167 was obtained for Cd(II) and Pb(II) ions.
Effect of the potentially interfering ions
To investigate the effect of potentially interfering ions found in natural samples, various metal ions were added to 100 mL of solution containing 0.01 mg Cd(II) and 0.01 mg Pb(II) ions. The degree of tolerance for some alkaline, alkaline earth, and transition metal ions are presented in Table 2. From the tolerance data, it can be seen that the high level of potentially interfering ions has no impact on the preconcentration of Cd(II) and Pb(II) ions at pH 6.3. So the method could be applied to determine these heavy metal ions in complicated matrix samples.
Enrichment capacity study
In order to investigate the enrichment capacity of magnetic sorbent a standard solution containing 100 mg L−1 of Cd(II) and Pb(II) ions was used. In order to evaluate the maximum enrichment capacity, the initial and equilibrium amounts of heavy metal ions were determined by FAAS. The maximum enrichment capacity is defined as the total amount of heavy metal ions adsorbed per gram of the sorbent. The obtained capacities for magnetic sorbent were found to be 186 and 198 mg g−1 for Cd(II) and Pb(II) ions, respectively.
Analytical performance of the method
Under the optimal conditions, calibration curves were constructed for the determination of Cd(II) and Pb(II) ions, according to the mentioned procedure. Linearity was within the range of 1–80 ng mL−1 for Cd(II) and 2.5–125 ng mL−1 for Pb(II) in initial solution. The correlation of determination (r2) was 0.9988 for Cd(II) and 0.9975 for Pb(II) ions. The limit of detection which is defined as LOD = 3Sb/m, where Sb is the standard deviation of 10 replicate blank signals and m is the slope of the calibration curve after preconcentration, for a sample volume of 500 mL, was found to be 0.2 ng mL−1 for Cd(II) and 1.1 ng mL−1 for Pb(II) ions, respectively. The precision of the method for a standard solution containing 50 ng mL−1 of heavy metal ions (n = 5) was evaluated as the relative standard deviation (RSD%) and was found to be 3.6 and 4.3%, for Cd(II) and Pb(II) ions respectively.
Validation of the method
The concentrations of Cd(II) and Pb(II) ions obtained by the current method were compared to the standard reference materials (NIST-1640A (Natural water)) and Ore Polymetallic gold Zidarovo-PMZrZ (206 BG 326)). As it can be seen in Table 3, good correlation was achieved between the estimated content by the present method and reference materials. Therefore, magnetic sorbent can be used as a reliable solid phase for the extraction and determination of Cd(II) and Pb(II) ions in various samples.
Determination of Cd(II) and Pb(II) ions in various real samples
Since natural samples have complex matrices, non-specific background absorption was caused by interfering species of the sample matrix. To reduce this undesirable effect, magnetic sorbent was applied for selective extraction of Cd(II) and Pb(II) ions in pH 6.3. Table 4 shows the Cd(II) and Pb(II) ions recoveries in various real samples which in all cases, were almost quantitative.
Conclusion
A simple, fast, reproducible, and selective solid-phase extraction procedure, based on metal-organic frameworks (magnetic MOF-pyridine nanocomposite), for determining Cd(II) and Pb(II) ions have been developed. In comparison with other solid-phases, magnetic sorbent has the advantages of high enrichment capacity, low limit of detection, and high enrichment factor (Table 1s, supporting information). Other advantages of this method are: (1) low time-consuming due to magnetically-assisted separation of the adsorbent, and higher surface area; therefore, satisfactory results can be achieved using less amounts of the adsorbents. Due to the relatively high preconcentration factor, trace amounts of heavy metals at ng mL−1 levels in high-volume samples can be quantified by magnetic sorbent.
References
Farooq U, Khan MA, Athar M, Kozinski JA (2011) Effect of modification of environmentally friendly biosorbent wheat (Triticum aestivum) on the biosorptive removal of cadmium(II) ions from aqueous solution. Chem Engin J 171:400–410
Afkhami A, Madrakian T, Ahmadi R, Bagheri H, Tabatabaee M (2011) Chemically modified alumina nanoparticles for selective solid phase extraction and preconcentration of trace amounts of Cd(II). Microchim Acta 175:69–77
Kalariya NM, Nair B, Kalariya DK, Wills NK, Kuijk FJGMV (2010) Cadmium-induced induction of cell death in human lens epithelial cells: Implications to smoking associated cataractogenesis. Toxicol Let 198:56–62
Guo W, Hu S, Xiao Y, Zhang H, Xie X (2010) Direct determination of trace cadmium in environmental samples by dynamic reaction cell inductively coupled plasma mass spectrometry. Chemospher 81:1463–1468
Gama EM, Lima AS, Lemos VA (2006) Preconcentration system for cadmium and lead determination in environmental samples using polyurethane foam/Me-BTANC. J Hazard Mater 136:757–762
Kiptoo JK, Ngila JC, Silavwe ND (2008) Solid-phase extraction of Zn(II), Cu(II), Ni(II) and Pb(II) on poly(vinyl chloride) modified with 3-ferrocenyl-3-hydroxydithioacrylic acid, and their subsequent determination by electrothermal atomic absorption spectrometry. Microchim Acta 160:211–218
Silva EL, dos Santos RP, Giné MF (2009) Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. J Hazard Mater 171:1133–1138
Tokalıoğlu S, Kartal S (2008) Synthesis and application of a new chelating resin functionalized with salicylaldoxime for the determination of Pb(II), Ni(II), Cu(II) and Mn(II) ions in water samples by flame atomic absorption spectrometry. Microchim Acta 162:87–92
Yin J, Jiang Z, Chang G, Hu B (2005) Simultaneous on-line preconcentration and determination of trace metals in environmental samples by flow injection combined with inductively coupled plasma mass spectrometry using a nanometer-sized alumina packed micro-column. Anal Chim Acta 540:333–339
Zawisza B, Sitko R (2007) Determination of Te, Bi, Ni, Sb and Au by X-ray fluorescence spectrometry following electro enrichment on a copper cathode. Spectrochim Acta B Atomic Spect 62:1147–1152
Duran C, Senturk HB, Elci L, Soylak M, Tufekci M (2009) Simultaneous preconcentration of Co(II), Ni(II), Cu(II), and Cd(II) from environmental samples on Amberlite XAD-2000 column and determination by FAAS. J Hazard Mater 162:292–299
Abe S, Fuji K, Sono T (1994) Liquid-liquid extraction of manganese(II), copper(II) and zinc(II) with acyclic and macrocyclic Schiff bases containing bisphenol A subunits. Anal Chim Acta 293:325–330
Afkhami A, Bahram M (2006) Cloud Point Extraction Simultaneous Spectrophotometric Determination of Zn(II), Co(II) and Ni(II) in Water and Urine Samples by 1-(2-Pyridylazo) 2-Naphthol Using Partial Least Squares Regression. Microchim Acta 155:403–407
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36:4757–4764
Bruno P, Caselli M, Gennaro G, Ielpo P, Ladisa T, Placentino CM (2006) Ion chromatography determination of heavy metals in airborne particulate with preconcentration and large volume direct injection. Chromatographia 64:537–542
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–177
ALOthman AA, Habila M, Yilmaz E, Soylak M (2012) Solid phase extraction of Cd(II), Pb(II), Zn(II) and Ni(II) from food samples using multiwalled carbon nanotubes impregnated with 4-(2-thiazolylazo) resorcinol. Microchim Acta 177:397–403
Tuzen M, Saygi KO, Soylak M (2008) Novel solid phase extraction procedure for gold(III) on Dowex M 4195 prior to its flame atomic absorption spectrometric determination. J Hazard Mater 156:591–595
Zhai Y, He Q, Han Q, Duan S (2012) Solid-phase extraction of trace metal ions with magnetic nanoparticles modified with 2,6-diaminopyridine. Microchim Acta 178:405–412
Sarrafi AHM, Konoz E, Khanmohammadi A (2012) Determination of some heavy metals in real samples by FAAS after preconcentration on 4-Nitro-2-(2-nitro-phenelazo)phenol modified activated carbon. Chem Sci Trans 1:530–537
Parham H, Pourreza N, Rahbar N (2009) Solid phase extraction of lead and cadmium using solid sulfur as a new metal extractor prior to determination by flame atomic absorption spectrometry. J Hazard Mater 163:588–592
Panahi HA, Mottaghinejad E, Badr AR, Moniri E (2011) Synthesis, characterization, and application of amberlite XAD-2-salicylic acid-iminodiacetic acid for lead removal from human plasma and environmental samples. J Appl Polym Sci 121:1127–1133
Faraji M, Yamini Y, Shariati S (2009) Application of cotton as a solid phase extraction sorbent for on-line preconcentration of copper in water samples prior to inductively coupled plasma optical emission spectrometry determination. J Hazard Mater 166:1383–1388
Ebrahimzadeh H, Asgharinezhad AA, Tavassoli N, Sadeghi O, Amini MM, Kamarei F (2012) Separation and spectrophotometric determination of very low levels of Cr(VI) in water samples by novel pyridine-functionalized mesoporous silica. Intern J Environ Anal Chem 92:509–521
Chae HK, Siberio-Perez DY, Kim J, Go Y, Eddaoudi M, Matzger AJ, O’Keeffe M, Yaghi OM (2004) A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 427:523–527
Hu YH, Zhang L (2010) Hydrogen storage in metal-organic frameworks. Adv Mater 22:E117–E130
Taylor-Pashow KML, Rocca JD, Xie ZG, Tran S, Lin WB (2009) Postsynthetic modifications of iron-carboxylate nanoscale metal-organic frameworks for imaging and drug delivery. J Am Chem Soc 131:14261–14263
Ke F, Qiu LG, Yuan Y-P, Peng F-M, Jiang X, Xie A-J, Shen Y-H, Zhu J-F (2011) Thiol-functionalization of metal-organic framework by a facile coordination-based postsynthetic strategy and enhanced removal of Hg2+ from water. J Hazard Mater 196:36–43
Bagheri A, Taghizadeh M, Behbahani M, Asgharinezhad AA, Salarian M, Dehghani A, Ebrahimzadeh H, Amini MM (2012) Synthesis and characterization of magnetic metal-organic framework (MOF) as a novel sorbent, and its optimization by experimental design methodology for determination of palladium in environmental samples. Talanta 99:132–139
Hoogboom J, Garcia PML, Otten MBJ, Elemans JAAW, Sly J, Lazarenko SV, Rasing T, Rowan AE, Nolte RJM (2005) Unable command layers for liquid crystal alignment. J Am Chem Soc 127:11047–11052
Hartmann M, Kunz S, Himsl D, Tangermann O, Ernst S, Wagener A (2008) Adsorptive separation of isobutene and isobutane on Cu3(BTC)2. Langmuir 24:8634–8642
Box GEP, Draper NR (1987) Empirical model building and response surfaces. John Wiley and Sons, NewYork
Yilmaz AB (2003) Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay. Turkey Environ Res 92:277–281
Kamarei F, Ebrahimzadeh H, Yamini Y (2010) Optimization of solvent bar microextraction combined with gas chromatography for the analysis of aliphatic amines in water samples. J Hazard Mater 178:747–752
StatGraphics Plus 5.1 for Windows, Statistical Graphic Crop., online manuals, 2001.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 155 kb)
Rights and permissions
About this article
Cite this article
Sohrabi, M.R., Matbouie, Z., Asgharinezhad, A.A. et al. Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim Acta 180, 589–597 (2013). https://doi.org/10.1007/s00604-013-0952-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-0952-4