Abstract
The electrochemical oxidation of β-nicotinamine adenine dinucleotide (NADH) was investigated at a glassy carbon electrode modified with carbon mesoporous materials (CMM). Due to the large surface area and electro-catalytic properties of CMM, the overpotential of the electrodes toward the oxidation of NADH is decreased by 595 mV in aqueous solution at neutral pH. The anodic peak currents increase steadily with the concentration of NADH in the range from 2 µM to 1.1 mM, the detection limit being 1.0 µM at pH 7.2 and a potential of +0.3 V vs. SCE. The apparent Michaelis-Menten constant is ∼21.5 μM. The results enable NADH to be sensed at a low potential and are promising with respect to the design of dehydrogenase-based amperometric biosensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
β-nicotinamide adenine dinucleotide (NADH) is an important coenzyme involved in metabolic processes, widely existing in the living cells. More than 300 enzymes have been identified as NADH-dependent dehydrogenases. Thus, the development of electrochemical biosensors for direct analysis of NADH have received considerable interests [1, 2]. Generally, the direct oxidation of NADH at the ordinary electrode occurs at a high overpotential [3] and is followed by the passivation of the electrode surface [4]. Therefore, how to reduce the oxidative overpotential has been a critical research topic. Modification of the electrode surface has been reported to be an effective method for amperometric analysis. Blaedel and his co-workers investigated that the oxygen-containing functionalities, such as hydroxyl, carbonyl, carboxyl and quinine, produced on carbon surfaces via the oxidative pretreatment were responsible for mediating the electrocatalytic oxidation process [5]. Thus, the electrodes modified with an o-quinone moiety as the mediator have been studied in detailed [6–10]. Besides these physical adsorption modification, continuing efforts have been made recently in order to explore the new matrices for the electrode surface modification to further reduce the overpotential of the electrochemical oxidation of NADH [11–18]. Musameh et. al. showed a direct detection of NADH at single-wall and mutil-wall carbon nanotube modified electrode at 0.3 V [12]. Raj et al. reported a novel carbon nanotubes polymer redox hybrid thin film for electrocatalytic sensing of NADH [15]. Additionally, a lay-by-layer assembled film based on chitosan/carbon nanotubes was designed to achieve the electrocatalytic oxidation of NADH [16].
Recently, the mesoporous materials possess a pore size in the range of 2–20 nm, high specific surface area and large pore volume, which have attracted increasing attention in various fields such as catalysis and electrochemistry [19, 20]. The application of mesoporous materials as matrices for the development of sensors in biological electroanalysis has been increasingly studied [21]. Ju and his coworkers used titanium-containing MCM-41 modified glassy carbon electrode in NADH oxidation reaction and achieved a low potential detection of NADH at 0.28 V (vs. SCE) [21]. You et al. reported an electrode modified with bicontinuous gyroidal mesoporous carbon for electro-oxidation of NADH [22]. The mesoporous carbon materials, possessing both the properties of general mesoporous materials and the good conductivity of carbon-based materials, might contribute the multiple advantages in the eletrocatalytic oxidation of NADH.
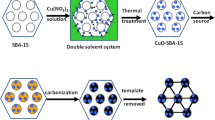
In this paper, the electrochemical oxidation of NADH was studied on a glassy carbon electrode modified with a carbon mesoporous material (CMM) that was easily synthesized using the ordered mesoporous silica SBA-15 as a template and sucrose as a carbon source [23, 24]. Such CMM has a larger surface area of 1430 m2 g−1, and the total pore volume and mesopore size are 1.28 cm3 g−1 and 4.2 nm, respectively. The CMM-modified electrodes exhibit a significant decrease in the overpotential of NADH oxidation.
Experimental
Chemicals and reagents
NADH (β-nicotinamide adenine dinucleotide, reduced form) and Nafion® perfluorinated ion-exchange resin, 5 wt.% solution were purchased from Sigma-Aldrich (www.sigmaaldrich.com). All other reagents were of analytical grade and used without further purification. The 0.05 M phosphate buffer solutions (PBS) were prepared by mixing the stock solution of NaH2PO4 and Na2HPO4. Deionized water was used in all experiments.
Preparation of CMM-modified electrode
A GC electrode (3 mm in diameter) was successively polished to a mirror finish with 0.3- and 0.05-μm alumina particles, rinsed thoroughly with deionized water, and successively sonicated in 1:1 nitric acid, acetone and deionized water. The CMM was synthesized by using SBA-15 mesoporous silica as a template and sucrose as a carbon source as reported previously [24]. Then the GC electrode was coated by casting an aliquot of 10 μL Nafion suspension uniformly dispersed with 0.5 mg mL−1 CMM samples and dried under ambient conditions.
Apparatus
Raman spectrum was obtained using a Dilor LabRAM-1B Raman microscope with 532.8 nm excitation. The electrochemical measurements were performed on a CHI 1030 Electrochemical Workstation (CHI, USA). A three-electrode system was set up, with a CMM modified GC electrode as the working electrode, a coiled platinum wire as the counter electrode, and a saturated calomel electrode (SCE) as the reference in an electrochemical glass cell containing 10 mL 0.05 M PBS at room temperature. A magnetic stirrer provided the convective transport during the amperometric measurement.
Results and discussion
Electrochemical oxidation of NADH at a CMM/GC electrode
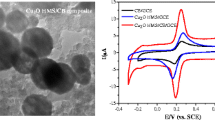
Figure 1 shows the electrochemical behaviours of CMM and NADH ranging from −0.5 V to 0.4 V at CMM modified GC electrode. As shown in curve a, a pair of surface peaks of CMM is at −0.18 V and −0.08 V, corresponding to the reduction and oxidation of the oxygen-containing groups on the CMM surface [25]. While in curve b, partly overlaid by the above redox peaks of CMM, the anodic peak of NADH at CMM/GC electrode can be observed at 0.10 V, indicating a substantial negative shift compared with 0.695 V at the bare GC electrode, and 0.3 ∼ 0.5 V at the electrodes modified with carbon nanotubes-related materials as reported [12, 26, 27]. The reason for the electrocatalytic oxidation of NADH by CMM might be related to the oxygen-rich groups such as hydroxyl and quinone on carbon surfaces, which can be used as mediators of electron communications [5–7, 10]. These oxygen-rich groups can be introduced during the CMM preparation, with the sulfuric acid used as the carbonization catalyst [24].
Additionally, the Raman spectrum of CMM exhibits the presence of D- (1315.08 cm−1) and G- (1585.86 cm−1) bands as shown in Fig. 2. The D-band is linked to breathing modes of six-fold aromatic rings, ascribing to atomically disordered carbon, while G-band due to the sp2 C-C stretching is the characteristic feature of ordered graphite carbon, revealing a well-defined graphitized structure of CMM [28]. Thus the higher relative intensity ratio of the D- and G-bands (ID/IG = 1.97) of CMM than that (ID/IG = 0.74) of carbon nanotubes [29] means CMM contains considerable edge-plane-like defective sites, providing CMM with the enhanced electrocatalytic activity and the minimizing passivation effects on NADH oxidation [30].
The influence of the scan rates on the electro-oxidation of NADH at the CMM/GC electrode is also investigated by cyclic voltammetry. The anodic peak current of 0.01 M NADH at the CMM/GC electrode increases linearly with the square root of the scan rate in the range from 0.02 V s−1 to 0.2 V s−1. This result suggests that the electrode reaction of NADH is a diffusion-controlled process.
Influence of the applied potentials and pH on the electrocatalytic oxidation of NADH
To optimize the electrocatalytic response of the CMM/GC electrode for NADH oxidation, the effect of the applied potentials on the amperometric response to the addition of 0.1 mM NADH was studied between 0 V and 0.6 V. A plot of potential versus current has a peak at 0.3 V (0.21 µA) and drops to 0.03 µA at 0.6 V. Hence, the potential of 0.3 V is selected to be used in the following experiments.
The electrochemical oxidation of NADH is a pH-dependent reaction. Thus, the amperometric response of the CMM/GC electrode to NADH was investigated with different pH values ranging from 5.6 to 8.0. The highest anodic current is obtained at pH 7.2 (0.21 µA) and drops to 0.07 µA at pH 8.0. Hence, the solution pH of 7.2 is the optimum and was used in the following experiments.
Electrochemical detection of NADH
Figure 3 shows the amperometric responses to successive additions of NADH into 5.0 mL 0.05 M PBS at CMM/GC electrodes under the optimized experimental conditions. The anodic peak current increases steadily with the increasing in the concentration of NADH. As shown in Fig. 4, the linear response range is from 2.0 × 10−6 to 1.1 × 10−3 M with a correlation coefficient of 0.995. The detection limit is 1.0 × 10−6 M with a signal-to-noise ratio of three (S/N = 3) and the sensitivity is (1.37 ± 0.03) μA mM−1. However, there is no response observed at the bare electrode for these concentration changes using this low-detection potential. The results show that such electrocatalysis at CMM-modified electrode could facilitate low-potential amperometric measurements of NADH. With the additions of NADH, the amperometric response current of CMM/GC biosensor increases and reaches a platform at the concentrations higher than 1.1 × 10−3 M gradually. It shows a Michaelis–Menten type process as being described by the Lineweaver-Burk equation [31]:
where \( K_M^{{app}} \) is the Michaelis-Menten constant, i cat the electrocatalytic current, C the bulk concentration of substrate, and i max the maximum current under optimized conditions. For the mesoporous carbon modified electrode, from the slope and the intercept of a double-reciprocal plot of the current and concentration of NADH (Fig. 4, inset), the apparent Michaelis–Menten constant (\( K_M^{{app}} \)) was calculated to be about 21.5 μM. Table 1 shows the comparison of this CMM/GC electrode with other NADH biosensors reported previously in the detection limit, linear range and apparent Michaelis–Menten constant. The CMM-modified electrode does not offer the best values currently, which will be further improved via adjusting the pore size and surface properties of the mesoporous carbon materials. The results show that the proposed method is an alternative strategy for electrocatalytic oxidation of NADH due to its simple procedure and fast response.
Conclusion
In this work, a two-dimensional mesoporous carbon modified glassy carbon electrode has been proposed for the electrocatalytic oxidation of NADH. A decrease of 595 mV in the overvoltage is observed at this CMM/GC electrode compared with the bare GC electrode in phosphate buffer solution under the optimal condition (pH 7.2). The CMM-modified electrodes show better electrochemical performance and electrocatalytic response to NADH with the concentrations in the range of 2.0 × 10−6 to 1.1 × 10−3 M with a detection limit of 1.0 × 10−6 M. This research might open up an approach for the development of amperometric biosensors based on NADH-dependent dehydrogenase and might be promising for the potential applications of biological analysis.
References
Katakis I, Domínguez E (1997) Catalytic electrooxidation of NADH for dehydrogenase amperometric biosensors. Microchim Acta 126:11–32
Gorton L, Dominguez E (2002) Electrocatalytic oxidation of NAD(P)H at mediator-modified electrodes. Rev Mol Biotechnol 82:371–392
Molroux J, Elving PJ (1978) Effects of adsorption, electrode material, and operational variables on the oxidation of dihydronicotinamide adenine dinucleotide at carbon electrodes. Anal Chem 50:1056–1062
Wang J, Angnes L, Martinez T (1992) Scanning tunneling microscopic probing of surface fouling during the oxidation of nicotinamide coenzymes. Bioelectrochem Bioenerg 29:215–221
Blaedel WJ, Jenkins RA (1976) Study of a reagentless lactate electrode. Anal Chem 48:1240–1247
Tse DCS, Kuwana T (1978) Electrocatalysis of dihydronicotinamide adenosine diphosphate with quinones and modified quinone electrodes. Anal Chem 50:1315–1318
Pariente F, Lorenzo E, Abruna HD (1994) Electrocatalysis of NADH oxidation with electropolymerized films of 3, 4-Dihydroxybenzaldehyde. Anal Chem 66:4337–4344
Zare HR, Golabi SM (1999) Electrocatalytic oxidation of reduced nicotinamide adenine dinucleotide (NADH) at a chlorogenic acid modified glassy carbon electrode. J Electroanal Chem 464:14–23
Dicu D, Munteanu FD, Popeseu IC, Gorton L (2003) Indophenol and O-quinone derivatives immobilized on zirconium phosphate for NADH electro-oxidation. Anal Lett 36:1755–1779
Lawrence NS, Wang J (2006) Chemical adsorption of phenothiazine dyes onto carbon nanotubes: Toward the low potential detection of NADH. Electrochem Commun 8:71–76
Raj CR, Ohsaka T (2001) Electrocatalytic sensing of NADH at an in situ functionalized self-assembled monolayer on gold electrode. Electrochem Commun 3:633–638
Musameh M, Wang J, Merkoci A, Lin YH (2002) Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem Commun 4:743–746
Chen J, Bao JC, Cai CX, Lu TH (2004) Electrocatalytic oxidation of NADH at an ordered carbon nanotubes modified glassy carbon electrode. Anal Chim Acta 516:29–34
Sha YF, Gao Q, Qi B, Yang XR (2004) Electropolymerization of azure B on a screen-printed carbon electrode and its application to the determination of NADH in a flow injection analysis system. Microchim Acta 148:335–341
Raj CR, Chakraborty S (2006) Carbon nanotubes-polymer-redox mediator hybrid thin film for electrocatalytic sensing. Biosens Bioelectron 22:700–706
Zhai XR, Wei WZ, Zeng JX, Gong SG, Yin J (2006) Layer-by-layer assembled film based on chitosan/carbon nanotubes, and its application to electrocatalytic oxidation of NADH. Microchim Acta 154:315–320
Merkoçi A (2006) Carbon nanotubes: exciting new materials for microanalysis and sensing. Microchim Acta 152:155–156
Mitsubayashi K, Nishio G, Sawai M, Kazawa E, Yoshida H, Saito T, Kudo H, Otsuka K, Takao M, Saito H (2008) A biochemical sniffer-chip for convenient analysis of gaseous formaldehyde from timber materials. Microchim Acta 160:427–433
Lee J, Yoon S, Hyeon T, Oh SM, Kim KB (1999) Synthesis of a new mesoporous carbon and its application to electrochemical double-layer capacitors. Chem Commun 2177–2178
Joo SH, Choi SJ, Oh I, Kwak J, Liu Z, Terasaki O, Ryoo R (2001) Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412:169–172
Dai ZH, Lu GF, Bao JC, Huang XH, Ju HX (2007) Low potential detection of NADH at titanium-containing MCM-41 modified glassy carbon electrode. Electroanalysis 19:604–607
You CP, Yan XW, Wang Y, Zhang S, Kong JL, Zhao DY, Liu BH (2009) ElectroElectrocatalytic oxidation of NADH based on bicontinuous gyroidal mesoporous carbon with low overpotential. Electrochem Commun 11:227–230
Zhao DY, Feng JL, Huo QS, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Jun S, Joo SH, Ryoo R, Kruk M, Jaroniec M, Liu Z, Ohsuna T, Terasaki O (2000) Synthesis of new, nanoporous carbon with hexagonally ordered mesostructure. J Am Chem Soc 122:10712–10713
Luo HX, Shi ZJ, Li NQ, Gu ZN, Zhuang QK (2001) Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode. Anal Chem 73:915–920
Tsai YC, Tsai MC, Chiu CC (2008) Self-assembly of carbon nanotubes and alumina-coated silica nanoparticles on a glassy carbon electrode for electroanalysis. Electrochem Commun 10:749–752
Deng CY, Chen JH, Chen XL, Mao CH, Nie Z, Yao SZ (2008) Boron-doped carbon nanotubes modified electrode for electroanalysis of NADH. Electrochem Commun 10:907–909
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095–14107
Zhou M, Guo JD, Guo LP, Bai J (2008) Electrochemical sensing platform based on the highly ordered mesoporous carbon-fullerene system. Anal Chem 80:4642–4650
Banks CE, Davies TJ, Wildgoose GG, Compton RG (2005) Electrocatalysis at graphite and carbon nanotube modified electrodes: edge-plane sites and tube ends are the reactive sites. Chem Commun 829–841
Kamin RA, Wilson GS (1980) Rotating-ring-disk enzyme electrode for biocatalysis kinetic-studies and characterization of the immobilized enzyme layer. Anal Chem 52:1198–1205
Zhou M, Shang L, Li BB, Huang LJ, Dong SJ (2008) The characteristics of highly ordered mesoporous carbons as electrode material for electrochemical sensing as compared with carbon nanotubes. Electrochem Commun 10:859–863
Zhang M, Smith A, Gorski W (2004) Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem 76:5045–5050
Zhu L, Zhai J, Yang R, Tian C, Guo L (2007) Electrocatalytic oxidation of NADH with Meldola’s blue functionalized carbon nanotubes electrodes. Biosens Bioelectron 22:2768–2773
Acknowledgements
This work was supported by the NSFC 20775016, Shanghai Leading Academic Discipline Project B108, B109 and Shuguang Project 06SG02.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., You, C., Zhang, S. et al. Electrocatalytic oxidation of NADH at mesoporous carbon modified electrodes. Microchim Acta 167, 75 (2009). https://doi.org/10.1007/s00604-009-0217-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-009-0217-4