Abstract
Purpose
Inflammation-based markers predict long-term outcomes of various malignancies. We investigated the relationship between these markers and the long-term survival in obstructive colorectal cancer (OCRC) patients with self-expandable metallic colonic stents (SEMSs) who subsequently received curative surgery.
Methods
We retrospectively analyzed 72 consecutive pathological stage II and III OCRC patients between 2013 and 2019. The prognostic significance of the prognostic nutritional index (PNI), neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet–lymphocyte ratio (PLR) was evaluated.
Results
The overall survival (OS), cancer-specific survival, and disease-free survival (DFS) were significantly shorter in the PNI < 35 group than in the PNI ≥ 35 group (p = 0.006, p < 0.001, and p = 0.003, respectively), and multivariate analyses revealed the PNI to be the only inflammation-based marker independently associated with the survival. A PNI < 35 was significantly associated with an elevated CA 19–9 level (p = 0.04) and longer postoperative hospital stay (p = 0.03). Adjuvant chemotherapy was also significantly associated with the OS (p = 0.040) and DFS (p = 0.011) in multivariate analyses.
Conclusion
The results showed that the PNI was a potent prognostic indicator. For OCRC patients, both systemic inflammation and the nutrition status seem to be important for predicting the prognosis, and administering adjuvant chemotherapy was very important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a leading cause of death worldwide, accounting for an estimated 9.6 million deaths in 2018, and colon cancer was the second-most common cause of cancer-related deaths according to the World Health Organization statistics [1]. Although recent advances in chemotherapy regimens that include cytotoxic drugs and biologic agents have prolonged the survival of patients with advanced colorectal cancer (CRC) [2], the results remain unsatisfactory, and further studies are required to understand the disease and improve the outcome.
Accumulating evidence suggests that progression of cancer is dependent not solely on the tumor features but also on the systemic inflammatory response and nutritious status of the host. Inflammation is considered one of the hallmarks of cancer [3], and malnutrition manifested as hypoalbuminemia is associated with poor long-term outcomes [4]. Inflammation-based markers are easily calculated from the routinely measured laboratory results and are considered to reflect the systemic inflammatory response and nutritious status of the host. Furthermore, these markers have been shown to be significantly associated with the short- and long-term outcomes of various malignancies [5].
Intestinal obstruction is a common presenting symptom of CRC with an incidence as high as 30% [6]. Obstructive CRC (OCRC) accounts for 85% of colonic emergencies [7], often requiring surgical intervention accompanied by a high morbidity and stoma rate. Intestinal decompression using a self-expandable metallic colonic stent (SEMS) as “a bridge to surgery” is now considered an attractive alternative. Decompression allows for bowel preparation, medical stabilization with correction of dehydration and electrolyte abnormalities, and optimization of comorbid illnesses, which theoretically improves patients’ inflammatory and nutritious statuses, resulting in a reduced morbidity and stoma rate compared to emergency surgery [8, 9].
Inflammation-based markers have been shown to be independent prognostic factors in various groups of CRC patients [10,11,12,13,14,15,16]. However, the prognostic value of these markers in OCRC patients is unknown. In the present study, we investigated the prognostic value of several inflammation-based markers, namely the prognostic nutritional index (PNI), neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet–lymphocyte ratio (PLR), in OCRC patients who had an SEMS inserted and subsequently received curative surgery.
Methods
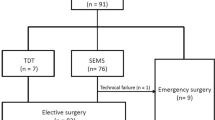
We retrospectively analyzed 72 consecutive pathological stage II and III OCRC patients who had an SEMS inserted as “a bridge to surgery” at Sendai City Medical Center between 2013 and 2019. Eligible patients had total or subtotal malignant colonic obstruction characterized by the following symptoms and findings: (1) obstructive symptoms, such as abdominal pain, fullness, vomiting, and constipation; (2) contrast-enhanced computed tomography (CT) findings of colorectal tumor with dilation of proximal bowel; and (3) severe stricture or obstruction demonstrated by contrast enema and colonoscopy. Patients were excluded if there were signs of peritonitis, perforation, or other serious complications demanding urgent surgery. Patients with benign disease, distant metastasis, positive surgical margin, and invasion from a non-colonic malignancy were excluded from the study. There were no patients with chronic inflammation. None of the patients had received neoadjuvant chemoradiation therapy.
Insertion of the SEMS was performed by endoscopists. A guidewire was introduced across the neoplastic stenosis under endoscopic and fluoroscopic guidance. A Niti-S colonic stent (TaeWoong Medical, Gimpo-si, Korea) was deployed over the wire and through the scope without balloon dilatation. The colon proximal to the stenosis was evaluated by water-soluble contrast enema, and a colonoscopic examination was performed after the surgery.
All patients subsequently underwent curative surgical resection. Postoperative complications were classified according to the Clavien–Dindo classification [17]. Pathological tumor staging was done according to the AJCC cancer staging manual (7th edition) [18]. Colonic lesions proximal to the splenic flexture were defined as right-sided tumors.
The primary endpoint of the study was the long-term outcome, which was defined as the overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS). The OS was measured from the date of the surgery to the date of death from any cause, and the CSS was measured until death from recurrent cancer. The DFS was measured from the date of surgery to the date of disease recurrence.
Laboratory tests were performed within 4 days before the surgery, and the PNI was calculated using the following formula: 10 × serum albumin value (g/dl) + 0.005 × total lymphocyte count in the peripheral blood [10]. The NLR, LMR, and PLR were calculated by directly dividing the neutrophil count by the lymphocyte count, the lymphocyte count by the monocyte count, and the platelet count by the lymphocyte count, respectively [19].
Continuous variables were presented as the mean ± standard deviation (SD) and assessed using Student’s t test. Associations between the PNI status and clinicopathological parameters were evaluated in a cross-table using Fisher’s exact test. The cut-off values for inflammation-based markers were determined using a receiver operating characteristic (ROC) curve analysis with the OS as an end-point. The cut-off value was defined using the most prominent point on the ROC curve (Youden index = maximum [sensitivity − (1 − specificity)]), and the area under the ROC (AUROC) curve was also calculated.
Survival curves were generated according to the Kaplan–Meier method and were analyzed by the log-rank test. A multivariate analysis was performed using the Cox proportional hazards backward regression model. Factors shown to have a p value of < 0.1 in the univariate analysis were included in the multivariate analysis. The T stage, N stage, venous invasion, and lymphatic invasion were incorporated into the analysis as potential confounding factors.
EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statical Computing, Vienna, Austria), was used for the statistical analyses, and differences with p values < 0.05 were considered significant [20].
Results
The characteristics of the 72 patients are summarized in Table 1. There were 41 men and 31 women. The mean age of the patients was 71.0 years (range 37–90), and the median follow-up time was 24 months (range 1–80). The mean interval between SEMS insertion and the surgery was 17.6 days (range 5–46), and the mean postoperative hospital stay was 19.2 days (range 8–77).
Regarding SEMS insertion, the rates of technical success (defined as correct placement) and clinical success (defined as resolution of occlusive symptoms) were 100% and 98.6%, respectively. Drainage-related complications were observed in two cases. One patient complained of mild abdominal pain after SEMS insertion, and another with inadequate drainage required insertion of a transanal decompression tube for additional drainage.
Patients were administered parenteral nutrition to meet the nutritious requirements when needed. Forty-eight patients (66.7%) were able to resume a normal diet after drainage. Non-steroidal anti-inflammatory drugs (NSAIDs)—including aspirin and steroids—were not used before the surgery. Information on other medications with anti-inflammatory properties, such as statins and histamine type 2 receptor antagonists (H2RA), during the perioperative period was not available.
Sixty-four patients (88.9%) underwent curative resection with primary anastomosis. Stoma was created in eight patients, including three diverting stomas. Laparoscopic surgery was performed in 26 cases, and conversion to open procedure was required in 4 cases because of severe adhesion in 3 and a tumor with direct invasion to the bladder in 1. There were five major postoperative complications of Clavien–Dindo grade ≥ 3, including one in-hospital death secondary to anastomotic leakage. Adjuvant chemotherapy was administered for 35 cases (49%). The reasons for not administering the chemotherapy were an advanced age in 16 (46%) followed by patients’ preference in 7 (20%).
The median PNI was 39.4 (range 24.3–51.3). An ROC curve analysis revealed the optimal cut-off value for PNI to be 35, which provided a sensitivity of 83%, a specificity of 56%, and an AUROC of 0.60. The optimal cut-off values for the NLR, LMR, and PLR were 2.6, 5.6, and 20, respectively (Online Resource 1).
Kaplan–Meier survival curves showed that the OS, CSS, and DFS were significantly shorter in the PNI < 35 group than in the PNI ≥ 35 group (p = 0.006, p < 0.001, and p = 0.003, respectively; Fig. 1), and the PNI was the only inflammation-based marker associated with the survival in this study. Not receiving adjuvant chemotherapy was significantly associated with a poor OS and DFS (p = 0.011 and p = 0.028, respectively; Fig. 2).
Survival curves of 72 pathological stage II and III obstructive colorectal cancer patients who underwent endoscopic stenting as a bridge to surgery according to the preoperative PNI value. The overall survival (a), cancer-specific survival (b), and disease-free survival (c) were significantly shorter in the PNI < 35 group than in the PNI ≥ 35 group
The relationship between the PNI status and clinicopathological parameters of the 72 patients is shown in Table 2. A PNI < 35 was significantly associated with elevated CA 19–9 levels (p = 0.04) and a longer postoperative hospital stay (25.0 days vs. 17.6 days, p = 0.03). Other clinicopathological factors, postoperative complications, and the interval between the SEMS insertion and the surgery were comparable between the groups.
Regarding the OS, univariate analyses revealed the PNI (p = 0.013) and adjuvant chemotherapy (p = 0.036) to be significant prognostic factors. An age over 70 years was marginally significant (p = 0.056). In a multivariate analysis with these three factors and potential confounding factors of T stage, N stage, venous invasion, and lymphatic invasion, a PNI < 35 (hazard ratio [HR] = 5.72, 95% confidence interval [CI] 1.30–25.15, p = 0.021) and not receiving adjuvant chemotherapy (HR = 8.97, 95% CI 1.11–72.61, p = 0.040) were identified as independent poor prognostic factors (Table 3).
Regarding the CSS, a PNI < 35 was the only prognostic factor in a univariate analysis and remained so even after adjusting for potential confounding factors in a multivariate analysis (HR = 11.06, 95% CI 2.02–60.74, p = 0.006; Table 4).
Regarding the DFS, a PNI < 35, not receiving adjuvant chemotherapy, and CA 19–9 ≥ 37 were identified as significant prognostic factors in a univariate analysis. A multivariate analysis with the potential confounding factors showed that a PNI < 35 (HR = 2.98, 95% CI 1.14–7.75, p = 0.026), not receiving adjuvant chemotherapy (HR = 3.97, 95% CI 1.38–11.40, p = 0.011), and CA 19–9 ≥ 37 (HR = 5.60, 95% CI 1.63–19.26, p = 0.006) were significant poor prognostic factors (Table 5).
Discussion
The TNM staging system is a validated staging system that is used routinely in patient assessments as well as in treatment decision-making. However, it is also recognized that the clinical course of patients varies considerably, even when categorized in the same stage, underscoring the need for another means of stratifying patients. Molecular parameters, such as the microsatellite instability status and BRAF/RAS status, have been shown to serve as both prognostic indicators and surrogate markers of drug efficacy [2, 21]. Although these parameters have received considerable attention and are included in the treatment decision tree for advanced disease [22], the tests are expensive and not always performed for every patient. Inflammation-based markers are calculated from standard laboratory results and have been shown to have prognostic value in various malignancies [5]. They are simple and easy to measure without extra cost, which facilitates their incorporation into daily practice.
Emergency surgery is usually indicated for patients with OCRC, which is associated with increased morbidity and mortality. It often results in multiple-stage surgery with the creation of temporary or permanent stoma. The created stoma has been reported to be permanent in up to 40% of cases and significantly diminishes the patient’s quality of life (QOL) [23]. Furthermore, emergency surgery might result in oncologically suboptimal resection [24]. Endoscopic decompression can convert emergency surgery into elective single-stage surgery. Because of concerns about short-term complications and the long-term survival, SEMSs were originally used with palliative intent [25], but recently, they have been used as a bridge to curative surgery. Recent meta-analyses showed that the long-term outcomes of SEMS implantation were comparable to those with emergency surgery when used as a bridge to surgery [7, 26] and as palliative therapy [27]. Moreover, the incidence of local and distant recurrence was not significantly different [7, 26]. Compared to patients who underwent drainage with a transanal decompression tube, no statistically significant differences were observed concerning recurrence patterns or the long-term survival [28].
In the present study, we investigated the relationship between inflammation-based markers and long-term outcomes in OCRC patients who had an SEMS inserted and subsequently received curative surgery. Our results showed that the preoperative PNI was an independent prognostic factor for the OS, CSS, and DFS. The PNI, originally proposed by Onodera et al. [29], is determined by the serum albumin level and peripheral lymphocyte count and is considered to reflect the immuno-nutritious status of the patient. Albumin reflects the nutritional status and is also a non-specific marker of inflammation, chronic disease, and the fluid status [30]. Hypoalbuminemia was shown to be associated with the survival [4]. Lymphocytes have an antitumor effect, and a low lymphocyte count is reportedly associated with a preexisting immuno-suppressed condition as well as a poor long-term survival [31]. The PNI was shown to correlate with the OS of various malignancies, such as esophagus cancer [32], gastric cancer [33], and pancreatic cancer [34]. Furthermore, the PNI was reported to be an independent prognostic factor in various groups of CRC patients, including those who underwent curative surgery [10,11,12,13,14], those who received liver resection for curative intent [15], and those with distant metastases [16]. To our knowledge, this was the first study to assess the PNI in OCRC patients with an SEMS as a bridge to curative surgery, and a multivariate analysis revealed the PNI to be a prognostic factor independent of the TNM stage. If the risk identified by the TNM stage is attributed to the tumor characteristics, the risk identified by the PNI might be attributed to the systemic environment of the host. Therefore, calculating the PNI might be equally as important as assessing TNM stage in the evaluation and management of patients.
The PNI is a continuous value with no standard cut-off value. Previous studies have used various cut-off values ranging from 35 to 49.6 and different statistical techniques to determine the values [10,11,12,13,14,15,16]. In the present study, an ROC curve analysis identified 35 as an optimal cut-off value. Nozoe et al. showed that the OCRC was associated with a low PNI among surgically treated CRC patients [11], which partly explained the low median PNI score as well as the low cut-off value used in the present study. A low PNI score might suggest that OCRC patients tend to have more systemic inflammation and malnutrition than non-obstructive CRC patients.
Although optimizing the preoperative immuno-nutritious status to improve the long-term outcomes seems an intriguing concept, studies on this topic have been scarce due to the difficulty in evaluating patients and developing appropriate intervention strategies. The effects of preoperative nutritional intervention on the long-term outcomes have largely been unknown. Buijs et al. [35] showed that perioperative arginine supplementation significantly improved the long-term survival of malnourished head and neck cancer patients. Aspirin, other NSAIDs, statins, and H2RA are agents with anti-inflammatory properties [36]. NSAIDs have been shown to reduce systemic inflammation and the CRP level, and daily aspirin was shown to reduce the incidence as well as mortality and recurrence rates of CRC [37,38,39]. A meta-analysis revealed that statin use both before and after a CRC diagnosis was associated with a reduced all-cause mortality and cancer-specific mortality [40]. The adjuvant use of H2RA, especially cimetidine, resulted in a statistically significant improvement in the OS of CRC patients [41]. A study in cancer-associated cachexia showed that the combination of a progestational agent, eicosapentaenoic acid, L-carnitine, and thalidomide significantly improved the Glasgow prognostic score (GPS) [42]. These agents may be potential candidates for improving the immuno-nutritious status. We previously showed that the preoperative change in the modified Glasgow prognostic score (mGPS) after SEMS insertion was significantly associated with the OS and CSS [43]. Lee et al. [44] measured the PNI before and after preoperative chemoradiation therapy for advanced rectal cancer, and the difference in the PNI was found to be an independent prognostic factor for the DFS and CSS. These results suggest that preoperative nutritional and medical interventions to improve the immuno-nutritious status might result in an improved long-term survival and that inflammation-based markers might serve as suitable indicators of the status.
In previous studies with cohorts of more than 500 CRC patients, the PNI was associated with the age, TMN stage (especially, the T stage), tumor size, location, histologic grade, CEA, CA 19–9, postoperative complications, and postoperative hospital stay [10,11,12,13,14]. In the present study, the PNI was significantly associated with the CA 19–9 level and postoperative hospital stay but not with other clinicopathological parameters. This might be due to the small number of cases and the different background characteristics among patients in this study.
The NLR, LMR, and PLR are other inflammation-based markers and were associated with the long-term outcomes of CRC patients in previous studies, but the results were somewhat inconsistent [12, 19]. In the present study, only the PNI showed prognostic value. The NLR, LMR, and PLR are all calculated using components in peripheral white blood cells and are considered to reflect the systemic inflammation status, whereas the PNI concomitantly reflects the nutritious status by incorporating the albumin value. To estimate the prognosis of OCRC patients, both the systemic inflammation and nutrition status seem important. Further studies will be required to elucidate the underlying mechanisms.
Receiving adjuvant chemotherapy was significantly associated with the OS and DFS in this study. Obstruction was considered as one of the poor prognostic features [22] for which adjuvant chemotherapy was indicated. However, adjuvant chemotherapy was administered to only half of the patients in this study, mainly because of advanced age and the patients’ preference. Adjuvant chemotherapy was not strongly recommended for stage II CRC by the Japanese guideline until 2019 [45], which might also have affected the decision. In fact, adjuvant chemotherapy was administered significantly less frequently for stage II patients in the present study than for stage III patients X (12 of 36 and 23 of 36 for stage II and III patients, respectively; p = 0.02). The results of the present study indicated that administering adjuvant chemotherapy was important for improving the long-term outcomes of OCRC patients.
Utilizing PNI and other inflammation-based markers as a guide for adjuvant chemotherapy seemed an appealing strategy, but previous studies have shown conflicting results. Peng et al. [46] demonstrated that a low PNI was independently associated with a poor prognosis in stage III colon cancer patients, and only patients with a low PNI showed an improved OS and DFS when treated with 6–8 cycles of XELOX adjuvant chemotherapy compared to those who received < 6 cycles. In the high-PNI group, the duration of chemotherapy was not associated with the OS. In contrast, in an analysis of stage III CRC patients, adjuvant chemotherapy for mGPS 1 and 2 patients, who exhibited a poor survival, did not result in an improved survival; while, the therapy did improve the survival in mGPS 0 patients [47]. Ihara et al. [48] showed that the PNI and GPS calculated before oxaliplatin-containing adjuvant chemotherapy were associated with the DFS in Stage III CRC patients. The relative dose intensity of oxaliplatin was significantly correlated with the PNI, which might have resulted in a low therapeutic effect in patients with a low PNI. These results suggest that inflammation-based markers might be most effectively used to identify patients with a high risk of recurrence stemming from a poor immuno-nutritious status, and actively improving their status might result in an improved long-term survival, partly through the administration of adjuvant chemotherapy as planned. Further studies are warranted to elucidate the relationship between the risk identified by inflammation-based markers and the efficacy of adjuvant chemotherapy.
This study was limited by the small sample size and retrospective, non-randomized design as well as its single-institution setting. The median follow-up time was too short to draw definitive conclusions. The patients were OCRC cases who underwent endoscopic stenting as a bridge to surgery, representing a unique subset of CRC patients; so, the results must be interpreted with caution.
In summary, the result of the present study showed that the preoperative value of the inflammation-based marker, the PNI, was an independent prognostic factor of the OS, CSS, and DFS in OCRC patients who had an SEMS inserted and subsequently received curative surgery. Furthermore, adjuvant chemotherapy was significantly associated with the OS and DFS in a multivariate analysis. For OCRC patients, both systemic inflammation and the nutritious status seem to be important for predicting the prognosis, and administering adjuvant chemotherapy was also very important. Further studies with a large sample size and longer observation period are warranted.
References
The Global Cancer Observatory. https://gco.iarc.fr/ Accessed 17 Jan 2020.
Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426–34.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: systematic review and meta-analysis. Sci Rep. 2017;7:16717.
McCullough JA, Engledow AH. Treatment options in obstructed left-sided colonic cancer. Clin Oncol (R Coll Radiol). 2010;22:764–70.
Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, et al. Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22:497–504.
Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, et al. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;86:416–26.
Allievi N, Ceresoli M, Fugazzola P, Montori G, Coccolini F, Ansaloni L. Endoscopic stenting as bridge to surgery versus emergency resection for left-sided malignant colorectal obstruction: an updated meta-analysis. Int J Surg Oncol. 2017;2017:2863272.
Noh GT, Han J, Cho MS, Hur H, Min BS, Lee KY, et al. Impact of the prognostic nutritional index on the recovery and long-term oncologic outcome of patients with colorectal cancer. J Cancer Res Clin Oncol. 2017;143:1235–42.
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda T, Matsukuma A, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today. 2012;42:532–5.
Akgül Ö, Çetinkaya E, Yalaza M, Özden S, Tez M. Prognostic efficacy of inflammation-based markers in patients with curative colorectal cancer resection. World J Gastrointest Oncol. 2017;9:300–7.
Yang Y, Gao P, Chen X, Song Y, Shi J, Zhao J, et al. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysis. Oncotarget. 2016;7:58543–52.
Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58:1048–57.
Zhao Y, Deng Y, Peng J, Sui Q, Lin J, Qiu M, et al. Does the preoperative prognostic nutritional index predict survival in patients with liver metastases from colorectal cancer who underwent curative resection? J Cancer. 2018;9:2167–74.
Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol. 2015;21:12410–20.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139:220–31.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–75.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Colon Cancer, Version 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 20 Jan 2020.
Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127–38.
Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg. 2012;149:e3–10.
Dohomoto M. New method-endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507–12.
Ceresoli M, Allievi N, Coccolini F, Montori G, Fugazzola P, Pisano M, et al. Long-term oncologic outcomes of stent as a bridge to surgery versus emergency surgery in malignant left side colonic obstructions: a meta-analysis. J Gastrointest Oncol. 2017;8:867–76.
Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558–E56767.
Sato R, Oikawa M, Kakita T, Okada T, Oyama A, Abe T, et al. Comparison of the long-term outcomes of the self-expandable metallic stent and transanal decompression tube for obstructive colorectal cancer. Ann Gastroenterol Surg. 2019;3:209–16.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients (in Japanese). Nihon Geka Gakkai zasshi. 1984;85:1001–5.
Ballmer PE. Causes and mechanisms of hypoalbuminaemia. Clin Nutr. 2001;20:271–3.
Iseki Y, Shibutani M, Maeda K, Nagahara H, Tamura T, Ohira G, et al. The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today. 2017;47:743–54.
Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002;28:396–400.
Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–3.
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–74.
Buijs N, van Bokhorst-de van der Schueren MA, Langius JA, Leemans CR, Kuik DJ, Vermeulen MA, et al. Perioperative arginine-supplemented nutrition in malnourished patients with head and neck cancer improves long-term survival. Am J Clin Nutr. 2010;92:1151–6.
Park JH, McMillan DC, Horgan PG, Roxburgh CS. The impact of anti-inflammatory agents on the outcome of patients with colorectal cancer. Cancer Treat Rev. 2014;40:68–77.
Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601.
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–50.
Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–12.
Li Y, He X, Ding Y, Chen H, Sun L. Statin uses and mortality in colorectal cancer patients: an updated systematic review and metaanalysis. Cancer Med. 2019. https://doi.org/10.1002/cam4.2151.
Deva S, Jameson M. Histamine type 2 receptor antagonists as adjuvant treatment for resected colorectal cancer. Cochrane Database Syst Rev. 2012;8:CD007814.
Mantovani G, Macciò A, Madeddu C, Serpe R, Massa E, Dessì M, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–11.
Sato R, Oikawa M, Kakita T, Okada T, Abe T, Yazawa T, et al. Preoperative change of modified Glasgow prognostic score after stenting predicts the long-term outcomes of obstructive colorectal cancer. Surg Today. 2020;50:232–9.
Lee YJ, Kim WR, Han J, Han YD, Cho MS, Hur H, et al. Prognostic impact of immunonutritional status changes during preoperative chemoradiation in patients with rectal cancer. Ann Coloproctol. 2016;32:208–14.
Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207–39.
Peng J, Zhang R, Zhao Y, Wu X, Chen G, Wan D, et al. Prognostic value of preoperative prognostic nutritional index and its associations with systemic inflammatory response markers in patients with stage III colon cancer. Chin J Cancer. 2017;36:96.
Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg. 2016;263:326–36.
Ihara K, Yamaguchi S, Shida Y, Fujita J, Matsudera S, Kikuchi M, et al. Nutritional status predicts adjuvant chemotherapy outcomes for stage III colorectal cancer. J Anus Rectum Colon. 2019;3:78–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to declare.
Ethical statements
The protocol for this research project was approved by the Ethics Committee of the institution (#2019-0008), and it conforms to the provisions of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sato, R., Oikawa, M., Kakita, T. et al. The prognostic value of the prognostic nutritional index and inflammation-based markers in obstructive colorectal cancer. Surg Today 50, 1272–1281 (2020). https://doi.org/10.1007/s00595-020-02007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02007-5