Abstract
Purposes
The indication of endoscopic (laparoscopic and thoracoscopic) hepatic resection (HR) has been expanded in the past decade because of its low invasiveness. However, the indications of endoscopic HR and radiofrequency ablation (RFA) have not yet been determined.
Methods
Among the 906 patients hospitalized for the initial treatment of hepatocellular carcinoma (HCC) between 2000 and 2017, 77 underwent endoscopic partial HR (E-pHR), and 94 underwent endoscopic RFA (E-RFA). We compared the short- and long-term outcomes between the E-pHR and E-RFA groups.
Results
The patients in the E-RFA group were characterized primarily by an impaired liver function. Among the patients with liver damage B or C, the overall survival (OS) in the E-pHR group was significantly worse than in the E-RFA group (3-year OS: 36% vs. 82%, p = 0.003).
Conclusion
E-RFA may be recommended for the initial treatment of HCC in patients with a severely impaired liver function. However, E-pHR should be avoided as the initial treatment of HCC in such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common cancer and the second-most common cause of cancer death worldwide [1, 2]. Many treatments are now available for HCC, including hepatic resection (HR); local ablation therapy, such as radiofrequency ablation (RFA), microwave coagulation (MCT), percutaneous ethanol injection (PEI) and cryoablation; liver transplantation; transcatheter hepatic arterial chemoembolization (TACE); molecular-targeted drugs and radiotherapy [3,4,5,6,7,8]. Laparoscopic HR was first reported in 1991 [9], leading to the consensus of minor hepatectomy as the standard treatment for HCC in 2008 [10]. While the superiority of laparoscopic HR is still being debated, this approach is considered to have theoretical advantages over open HR because the laparoscope allows for better exposure with a magnified view, and the pneumoperitoneum pressure reduces hepatic vein bleeding from the cut surface [11]. Furthermore, the recommendation of the second International Consensus Conference on Laparoscopic Liver Resection includes a difficulty scoring system for selecting patients to ensure patient safety [12].
HR, RFA and liver transplantation are therapeutic options for the treatment of HCC within the Milan criteria with curative intent. Although the recommendations for liver transplantation have not changed [13], HR and RFA should remain the first options for curative treatment of HCC because liver transplantation requires suitable donors, high cost and waiting period [14]. HR or RFA is recommended for HCCs with a diameter of ≤ 3 cm in patients with a relatively good liver functional reserve, according to the guidelines established by the American Association for the Study of the Liver Disease [15], the European Association for the Study of the Liver [16] and Japanese evidence-based guidelines [17, 18]. RFA is now being recognized as an alternative therapy for small HCC tumors (≤ 3 cm) because it is efficient, has a low associated mortality rate and is much less invasive than HR [19, 20]. Although several studies have examined the differences between HR and RFA [21,22,23,24,25,26] and the differences between open HR and laparoscopic HR [27,28,29,30], few reports have compared laparoscopic HR with laparoscopic RFA [31, 32].
In the present study, we compared the short- and long-term outcomes of endoscopic, including laparoscopic and thoracoscopic, HR and RFA as an initial treatment for HCC within the Milan criteria.
Methods
Patients
From January 2000 to March 2017, 2948 patients were hospitalized and treated for HCC at the Department of Gastroenterological Surgery, Kumamoto University Hospital, Kumamoto, Japan. Of these, 906 (30.7%) were treated for initial HCC with curative intent, including 632 with HR and 274 with local ablation therapy (Supplementary Fig. 1). The inclusion criterion of the endoscopic partial HR (E-pHR) group was laparoscopic or thoracoscopic partial hepatectomy for HCC within the Milan criteria. The exclusion criteria were open HR (n = 504), conversion from an endoscopic to an open procedure (n = 2), endoscopic anatomical HR (n = 36), being outside of the Milan criteria (n = 7) and combined with RFA (n = 6). Ultimately, 555 patients were excluded from this group for not meeting the inclusion criterion.
The inclusion criterion of the endoscopic RFA group (E-RFA) was laparoscopic or thoracoscopic RFA for HCC within the Milan criteria. The exclusion criteria were open or percutaneous RFA (n = 164) and being outside of the Milan criteria (n = 3).
Ultimately, 77 patients (14.5%) were included in the E-pHR group, and 94 patients (34.7%) were included in the E-RFA group. The scores of the liver damage classification were based on the criteria of the Liver Cancer Study Group of Japan [33, 34]. Difficulty scores were calculated based on the tumor location, tumor size, extent of liver resection, proximity to major vessels and the liver function [35]. The scores of the extent of liver resection and proximity to major vessels in the E-RFA group were defined as zero.
Treatment modalities
The surgical procedure was selected based on the tumor location, extent of the tumor, parenchymal liver function and patients’ general condition, as described previously [18]. In principal, HR was considered the first-choice treatment for patients with a good liver functional reserve. The reasons for performing RFA rather than HR included an unsuitable tumor location that required major hepatectomy, an insufficient liver function, a high operative risk associated with the general condition and the patient’s refusal to undergo HR.
Laparoscopic HR
For cases of partial HR, an endoscopic approach was typically selected as the first choice. For laparoscopic HR, patients were placed in the dorsal or semi-left lateral position according to the tumor location. A 12-mm port for the laparoscope was inserted at the umbilical portion, and additional 3 or 4 ports of 12 mm in size were inserted according to the tumor location. Laparoscopic HR was performed as follows: a Harmonic scalpel (Ethicon Endo-Surgery, USA) was used for the transection of the capsule and superficial parenchyma in the clamp-crushing method, and a Cavitron ultrasonic surgical aspirator (CUSA) was used for the transection of deeper parenchyma. During transection of the liver parenchyma, large intrahepatic glissonean pedicle or hepatic veins were exposed, encircled, and divided after clipping by a Hem-o-Lok® (Teleflex Medical, Japan) or Liga-clip® (Ethicon Endo-Surgery). The Pringle maneuver was routinely used except for in cases when the hepatoduodenal ligament could not be encircled because of severe adhesion.
Thoracoscopic HR
Thoracoscopic HR was selected when the tumor was located near the hepatic dome [36]. Patients were placed in the semi- or full-left lateral position according to the tumor location. For a thoracoscopic approach, left single-lung ventilation is required to maintain the working space of the right thoracic cavity. A 12-mm port for the thoracoscopy was inserted at the midaxillary line between the fourth and seventh ribs, and additional 2 or 3 ports of 12 mm in size were inserted according to the tumor location. After ultrasonography through the diaphragm, the diaphragm was incised using the vessel sealing system EnSeal® (Ethicon Endo-Surgery), with direct observation made at the surface of the diaphragm to avoid injuring the blood vessels. Transection was performed via the same method as laparoscopic HR. At the end of the procedure, the incised diaphragm was sutured using non-absorbable thread.
RFA procedures
When a patient’s tumor was not close to the liver surface and was detected by percutaneous ultrasonography, percutaneous ultrasonography-guided RFA was selected. When the tumor was located at the liver surface, adjacent to other organs that required retraction, or undetectable by percutaneous ultrasonography, RFA was performed laparoscopically or with laparotomy. When the tumor was located near the hepatic dome and was undetectable by percutaneous ultrasonography, thoracoscopic RFA was selected [36, 37]. When multiple tumors were detected, percutaneous and laparoscopic or thoracoscopic RFA were sometimes combined, as appropriate.
For tumor ablation, as previously described [20], an electrode with a 2- to 3-cm exposed tip (Radionics, Burlington, MA, USA) connected to a 500-kHz RF Generator (Radionics) was used. A tip temperature of 10–20 °C was maintained by a chilled saline solution infusion via a peristaltic pump. After electrode insertion into the lesion, we gradually increased the power to 60 W in a 2-cm-long needle or 80 W in a 3-cm-long needle at 20 W/min. After ablation exposure, we stopped the pump and measured the temperature of the needle tip. To achieve an accurate and wide tumor margin, we ablated not only the tumor nodule but also the area surrounding the tumor, especially if the target nodule was > 2 cm in diameter. Enhanced computed tomography (CT) was performed 7 days after RFA to evaluate the ablated region in all patients. Complete ablation was defined as the absence of contrast enhancement within the entire tumor. The procedure was repeated if a remaining unablated tumor remnant was suspected.
Follow-up
After treatment, all patients underwent regular follow-up examinations of their serum alpha-fetoprotein (AFP), lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) and des-γ-carboxy prothrombin (DCP) levels, and ultrasonography (US) and enhanced computed tomography (CT) or magnetic resonance imaging (MRI) were performed every 2–4 months to detect any intrahepatic recurrence or distant metastasis, as described previously [18, 38]. When tumor recurrence was confined to the remnant liver, various treatment modalities were selected, including repeat HR, RFA, TACE, chemotherapy with sorafenib, or a combination of these methods, according to the remnant liver function and the tumor size, number and location.
Statistical analyses
Continuous variables are expressed as means ± standard deviation (SD) and were compared using the Mann–Whitney U test. Categorical variables were compared using the χ2 test. Any death that occurred in the hospital after E-HR or E-RFA was recorded as a mortality. Grade II or higher complications according to the Clavien–Dindo classification were recorded as morbidities [39]. The overall survival (OS) and recurrence-free survival (RFS) curves were generated by the Kaplan–Meier method and compared by the log-rank test. We subjected variables that exhibited a p value of < 0.05 in a univariate analysis to a multivariate analysis using the Cox proportional hazards model. All analyses were performed using the JMP® 13.2.1 software program (SAS, Cary, NC, USA). A p value of < 0.05 was considered significant.
Results
Patients’ clinicopathological characteristics
The clinical characteristics of the E-pHR (n = 77) and the E-RFA (n = 94) groups are summarized in Table 1. Compared with the E-pHR group, the patients in the E-RFA group were characterized primarily by increased serum concentrations of total bilirubin; a decreased serum concentration of albumin, platelet count and prothrombin activity; and an impaired indocyanine green retention rate at 15 min (ICG-R15) and uptake ratio of the liver to the liver plus heart at 15 min (LHL15), as determined by 99mTc-galactosyl human serum albumin (GSA) scintigraphy. These findings suggested that the liver function was impaired in the E-RFA group.
The tumor-related factors and the surgical factors of the two groups are also summarized in Table 1. There were no significant differences in the tumor markers, tumor size, or tumor number between the two groups. There were no significant differences in the difficulty score between the two groups (3.4 vs. 3.8, p = 0.068). RFA was performed via a laparoscopic approach in 65 patients and a thoracoscopic approach in 29 patients. The median operating time was 317 (range 144–660) min in the E-pHR group, which was significantly longer than those in the E-RFA group (median, 188; range 27–375) min, p < 0.0001). The median amount of blood loss was 249 g in the E-pHR group, which was significantly larger than those in the E-RFA group (55 g, p < 0.0001). Red blood cell (RBC) transfusion was performed in 3 patients (3.9%) in the E-pHR group, whereas it was not performed at all in the E-RFA group. Local recurrence was observed in 0 patients in the HR group versus 9 patients (9.6%) in the RFA group (p = 0.0008).
Prognostic factors for the OS and RFS in the whole cohort
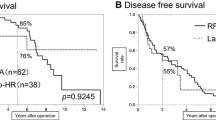
The median follow-up time of our series was 32.8 months. The survival curves related to the OS and RFS are illustrated in Fig. 1. The 5-year OS rates of the E-pHR and the E-RFA groups were 85% and 76%, respectively (p = 0.064). The 5-year RFS rates of the E-pHR and the E-RFA groups were 46% and 11%, respectively (p < 0.0001).
The univariate analysis revealed the following as poor prognostic factors for the OS: ≥ 67 years of age, Alb < 3.7 g/dl and RBC transfusion (Table 2). The multivariate analysis revealed that RBC transfusion (hazard ratio 38.7, p = 0.002) was the independent poor prognostic factor for OS (Table 2). For the RFS, ≥ 67 years of age, HBs-Ag negativity, HCV-Ab positivity, Alb < 3.7 g/dl, platelet count < 10 × 104/μl, LHL15 < 0.9, multiple tumors, E-RFA, Blood loss ≤ 150 g and RBC transfusion were identified as poor prognostic factors in the univariate analysis (Table 3). The multivariate analysis showed that RBC transfusion (hazard ratio 10.3, p = 0.004), multiple tumors (hazard ratio 2.05, p = 0.015), Alb < 3.7 g/dl (hazard ratio 2.32, p < 0.0001) were independent poor prognostic factors for the RFS (Table 3).
A subgroup analysis according to the liver damage classification
Among the patients with liver damage A, the OS and RFS in the E-pHR group were significantly better than in the E-RFA group (3-year OS: 93% vs. 82%, p = 0.18, Fig. 2a, and 3-year RFS: 60% vs. 26%, p = 0.0003, Fig. 2b, respectively). In contrast, among the patients with liver damage B or C, the OS in the E-pHR group was significantly worse than in the E-RFA group (3-year OS: 36% vs. 82%, p = 0.003, Fig. 2c). However, the RFS was similar between the two groups (3-year RFS: 21% vs. 27%, p = 0.69, Fig. 2d).
The long-term outcomes in patients who underwent endoscopic hepatic resection and endoscopic radiofrequency ablation for hepatocellular carcinoma according to the liver damage classification. a, b The overall (a) and recurrence-free survival (b) in patients with liver damage A. c, d The overall (c) and recurrence-free survival (d) in patients with liver damage B or C. E-pHR endoscopic partial hepatic resection, E-RFA endoscopic radiofrequency ablation
Among the patients with liver damage A, a univariate analysis revealed that male gender and E-RFA were poor prognostic factors for the OS (Table 4). In the multivariate analysis, E-RFA (hazard ratio 2.56, p = 0.026) was identified as independent poor prognostic factors for the OS (Table 4). In contrast, among the patients with liver damage B or C, a univariate analysis revealed that E-pHR and RBC transfusion were poor prognostic factors for the OS (Table 5). In the multivariate analysis, only RBC transfusion (hazard ratio 28.7, p = 0.001) was identified as an independent poor prognostic factor for the OS (Table 5).
Discussion
The current study investigated the short- and long-term outcomes of E-pHR and E-RFA for patients with initial HCC within the Milan criteria. The results showed that E-RFA resulted in a better long-term outcome in patients with liver damage B or C than E-pHR, despite the fact that the E-RFA group contained patients with a poorer liver functional reserve.
Although several reports have recently examined the prognoses after HR compared with those after RFA, the results are controversial [21, 23, 26, 40]. Furthermore, few reports have compared endoscopic HR with endoscopic RFA. A previous report described the therapeutic advantages of HR in patients with a single nodule and preserved liver function compared to ablation therapy through a laparoscopic approach [31]. However, that study did not take into consideration the background liver function, and the possible indications of HR and RFA have thus remained unclear [26].
Previous studies compared the long-term outcomes between HR and ablation therapy, including RFA, for HCC according to the background liver function [26]. Hasegawa et al. reported that HR was recommended for solitary lesions and HCC of 2–3 cm in size in patients with liver damage B according to data collected from a Japanese nationwide survey [26]. Utsunomiya et al. also reported that HR resulted in a significantly better prognosis than RFA in case of Japan Integrated Staging (JIS) score [41] “1” and “2”, including Child–Pugh A/Stage III or Child–Pugh B/Stage II in non-B/non-C HCC patients [40]. However, our previous study in 104 HCC patients with liver damage B showed that RFA produced comparable outcomes to HR, and a subgroup analysis revealed that the OS with RFA was significantly better in patients with a significantly impaired liver function than the OS with HR [42]. In the current study, although there were no significant differences in the OS between the two groups, the E-RFA group had a significantly poorer prognosis than the E-pHR group among patients with liver damage A, whereas the E-RFA group had a significantly better prognosis than the E-pHR group among patients with liver damage B or C. Given that the E-RFA patients in the present study had a poorer liver functional reserve than the E-pHR group, which only included patients with partial hepatectomy, our results suggest that E-RFA may be recommended as a treatment option for HCC patients with an impaired liver functional reserve when anatomical resection is not required. However, the reasons for the worse outcome of E-pHR in patients with liver damage B or C than in others are unclear. One possible reason is that surgical invasion due to HR in patients with an impaired liver function may lead to further impairment of the remnant liver, subsequent limitation of adding surgical intervention and a worsened survival. Further studies in a larger cohort will be necessary to resolve these issues.
In the current study, the RFS in the E-RFA group was significantly worse than in the E-HR group, possibly because of more advanced cirrhotic changes in the background liver. The local recurrence rate in the RFA group was 9.6%. Previous studies have reported the local recurrence rates of HCC after RFA to be 9.7–15.0% at 1 year and 19–27% at 3 years [43, 44]. Of note, Hori et al. reported that the cumulative local recurrence rate at 3 years reached 50% after percutaneous RFA for surface HCC [43]. In contrast, 2 meta-analyses reported that the rate of local recurrence after open HR ranged from 4.0–4.8% [21, 45]. One indication of E-RFA at our institution is basically for tumors located on the surface of the liver, to avoid cancer cell dissemination to the peritoneal cavity. As a result, the local recurrence rate after RFA has been kept relatively low, and tumor dissemination has not been observed. These findings and the comparable RFS rate compared with E-pHR in patients with liver damage B or C suggest that E-RFA may be an alternative to HR in patients with an impaired liver function.
Multivariate analyses in the current study revealed that RBC transfusion was an independent poor prognostic factor for the OS in the whole cohort as well as in patients with liver damage B/C. No patients with liver damage A received RBC transfusion. In addition, only patients who underwent E-pHR received RBC transfusion. It is well known that blood transfusion is significantly associated with adverse clinical outcomes for HCC patients undergoing surgery, including short- and long-term outcomes [46, 47]. These findings suggested that surgeons should reduce blood loss during surgery and avoid RBC transfusion, especially in patients with a severely impaired liver function.
The retrospective data analysis and small sample size from a single institution are the main limitations of this study. In addition, considerable bias may be present due to patients’ selection and the choices of their treatment. Indeed, the patients in the E-RFA group were characterized primarily by an impaired liver function, although the tumor factors were comparable to those of the E-pHR group. However, despite their impaired liver function, the survival after surgery was significantly better in the E-RFA group than in the E-pHR group among the patients with liver damage B or C. Larger cohort studies or randomized control studies are needed to confirm the results of this study.
In conclusion, E-RFA resulted in a better survival than E-HR in HCC patients with liver damage B or C. E-RFA may therefore be considered as an alternative in select patients, especially those with a severely impaired liver function. However, E-pHR should be avoided for the initial treatment of HCC in such patients.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386386.
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–8.
Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589–94.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Meng MB, Cui YL, Lu Y, She B, Chen Y, Guan YS, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol. 2009;92:184–94.
Imai K, Beppu T, Chikamoto A, Mima K, Okabe H, Hayashi H, et al. Salvage treatment for local recurrence of hepatocellular carcinoma after local ablation therapy. Hepatol Res. 2014;44:E335–E34545.
Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology (Baltimore, MD). 2015;61:1579–90.
Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–8.
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250:825–30.
Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281–9.
Cho JY, Han HS, Wakabayashi G, Soubrane O, Geller D, O'Rourke N, et al. Practical guidelines for performing laparoscopic liver resection based on the second international laparoscopic liver consensus conference. Surg Oncol. 2018;27:A5–9.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, MD). 2011;53:1020–2.
Duan C, Liu M, Zhang Z, Ma K, Bie P. Radiofrequency ablation versus hepatic resection for the treatment of early-stage hepatocellular carcinoma meeting Milan criteria: a systematic review and meta-analysis. World J Surg Oncol. 2013;11:190.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology (Baltimore, MD). 2005;42:1208–36.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51.
Imai K, Beppu T, Chikamoto A, Doni K, Okabe H, Hayashi H, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res. 2013;43:853–64.
Liu JG, Wang YJ, Du Z. Radiofrequency ablation in the treatment of small hepatocellular carcinoma: a meta analysis. World J Gastroenterol. 2010;16:3450–6.
Nitta H, Nakagawa S, Kaida T, Arima K, Higashi T, Taki K, et al. Pre-treatment double- or triple-positive tumor markers are predictive of a poor outcome for patients undergoing radiofrequency ablation for hepatocellular carcinoma. Surg Today. 2017;47:375–84.
Zhou Y, Zhao Y, Li B, Xu D, Yin Z, Xie F, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78.
Ito T, Tanaka S, Iwai S, Takemura S, Hagihara A, Uchida-Kobayashi S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case-control study with propensity score matching. Hepatol Res. 2016;46:565–74.
Lai EC, Tang CN. Radiofrequency ablation versus hepatic resection for hepatocellular carcinoma within the Milan criteria—a comparative study. Int J Surg. 2013;11:77–80.
Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802.
Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–66.
Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724–9.
Martin RC 2nd, Mbah NA, St Hill R, Kooby D, Weber S, Scoggins CR, et al. Laparoscopic versus open hepatic resection for hepatocellular carcinoma: improvement in outcomes and similar cost. World J Surg. 2015;39:1519–26.
Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721–7.
Franken C, Lau B, Putchakayala K, DiFronzo LA. Comparison of short-term outcomes in laparoscopic vs open hepatectomy. JAMA Surg. 2014;149:941–6.
Jiang B, Yan X, Zhang JH. Meta-analysis of laparoscopic versus open liver resection for hepatocellular carcinoma. Hepatol Res. 2018;48:635–63.
Santambrogio R, Barabino M, Bruno S, Mariani N, Maroni N, Bertolini E, et al. Surgical resection vs. ablative therapies through a laparoscopic approach for hepatocellular carcinoma: a comparative study. J Gastrointest Surg. 2017;22:650–60.
Yazici P, Akyuz M, Yigitbas H, Dural C, Okoh A, Aydin N, et al. A comparison of perioperative outcomes in elderly patients with malignant liver tumors undergoing laparoscopic liver resection versus radiofrequency ablation. Surg Endosc. 2017;31:1269–74.
Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884–92.
Nanashima A, Sumida Y, Abo T, Shindou H, Fukuoka H, Takeshita H, et al. Modified Japan Integrated Staging is currently the best available staging system for hepatocellular carcinoma patients who have undergone hepatectomy. J Gastroenterol. 2006;41:250–6.
Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745–53.
Yamao T, Imai K, Yamashita YI, Umezaki N, Tsukamoto M, Kitano Y, et al. Thoracoscopic surgery for hepatocellular carcinoma located in the hepatic dome: technical aspect and oncological results. Asian J Endosc Surg. 2019. https://doi.org/10.1111/ases.12755.
Doni K, Beppu T, Ishiko T, Chikamoto A, Hayashi H, Imai K, et al. Endoscopic radiofrequency ablation in elderly patients with hepatocellular carcinoma. Anticancer Res. 2015;35:3033–40.
Imai K, Beppu T, Nakayama Y, Ishiko T, Horino K, Komori H, et al. Preoperative prediction of poorly differentiated components in small-sized hepatocellular carcinoma for safe local ablation therapy. J Surg Oncol. 2009;100:121–6.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, et al. Nationwide study of 4741 patients with non-B non-C hepatocellular carcinoma with special reference to the therapeutic impact. Ann Surg. 2014;259:336–45.
Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, et al. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology (Baltimore, MD). 2004;40:1396–405.
Yamao T, Imai K, Yamashita YI, Kaida T, Nakagawa S, Mima K, et al. Surgical treatment strategy for hepatocellular carcinoma in patients with impaired liver function: hepatic resection or radiofrequency ablation? HPB Off J Int Hepatol Pancreat Biliary Assoc. 2018;20:244–50.
Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977–81.
Park W, Chung YH, Kim JA, Jin YJ, Lee D, Shim JH, et al. Recurrences of hepatocellular carcinoma following complete remission by transarterial chemoembolization or radiofrequency therapy: focused on the recurrence patterns. Hepatol Res. 2013;43:1304–12.
Li L, Zhang J, Liu X, Li X, Jiao B, Kang T. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2012;27:51–8.
Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS ONE ONE. 2013;8:e64261.
Xun Y, Tian H, Hu L, Yan P, Yang K, Guo T. The impact of perioperative allogeneic blood transfusion on prognosis of hepatocellular carcinoma after radical hepatectomy: a systematic review and meta-analysis of cohort studies. Medicine. 2018;97:e12911.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Masayo Tsukamoto and co-authors declare that we have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsukamoto, M., Imai, K., Yamashita, Yi. et al. Endoscopic hepatic resection and endoscopic radiofrequency ablation as initial treatments for hepatocellular carcinoma within the Milan criteria. Surg Today 50, 402–412 (2020). https://doi.org/10.1007/s00595-019-01903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01903-9